Immunogenicity of a Bivalent Non-Purified Recombinant Vaccine against Botulism in Cattle
Abstract
:1. Introduction
2. Results
2.1. Recombinant Vaccine Formulations are Safe for Animal Use
2.2. Recombinant Vaccines Induce Protective Humoral Response in Cattle
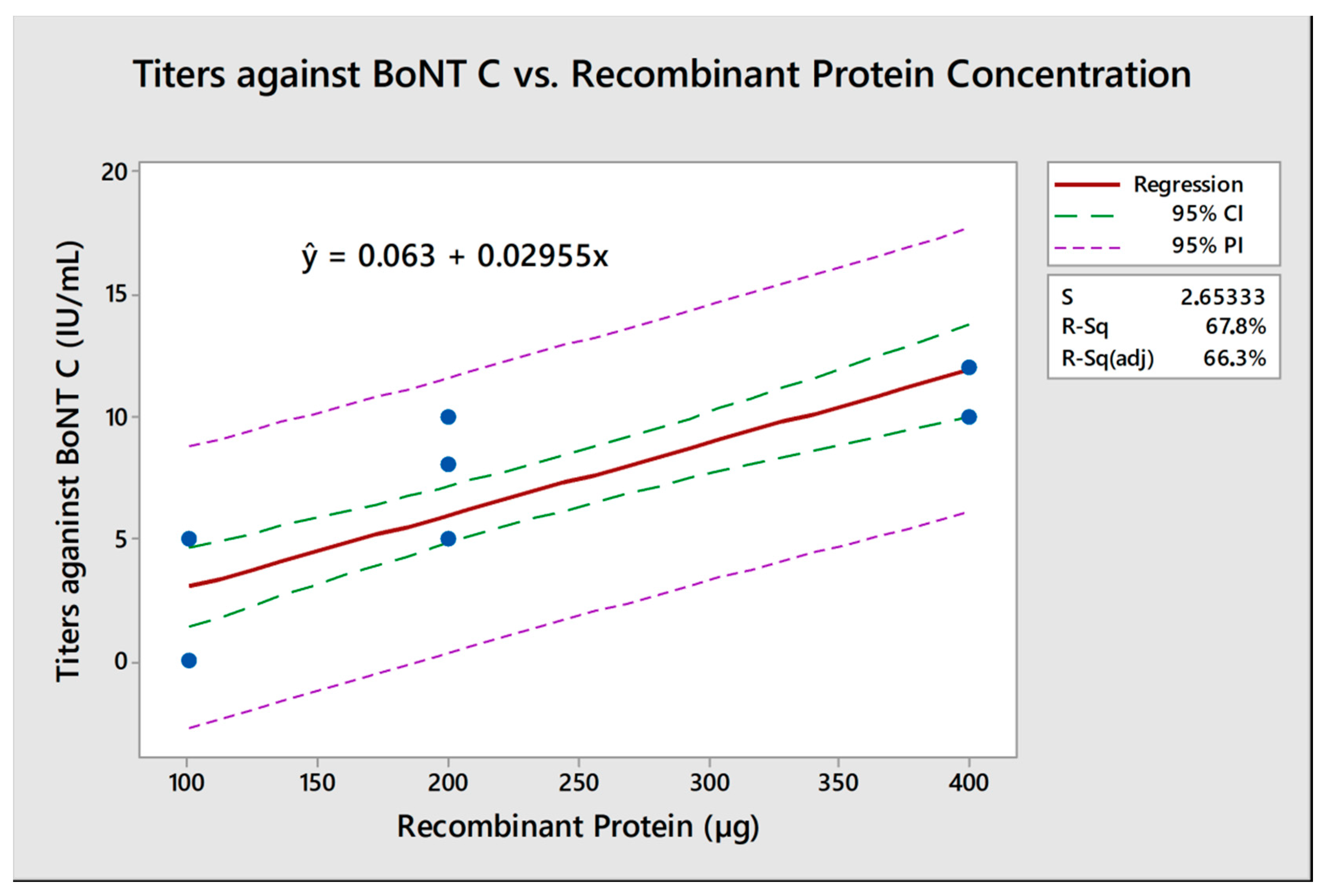
2.3. Recombinant Protein Concentration and Titers against Botulinum Neurotoxins (BoNTs) C and D Showed a Positive Linear Correlation
2.4. Longevity of the Immune Response in Cattle Increases with the Amount of Heavy-Chain BoNTs (HCBoNTs)
3. Discussion
4. Materials and Methods
4.1. Vaccine Formulation and Safety
4.2. Cattle Vaccination
4.3. Serum Neutralization Assay
4.4. Statistics Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Popoff, M.R.; Bouvet, P. Genetic characteristics of toxigenic Clostridia and toxin gene evolution. Toxicon 2013, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, M.R.; Tepp, W.H.; Przedpelski, A.; Pier, C.L.; Bradshaw, M.; Johnson, E.A.; Barbieri, J.T. Subunit vaccine against the seven serotypes of botulism. Infect. Immun. 2008, 76, 1314–1318. [Google Scholar] [CrossRef] [PubMed]
- Woudstra, C.; Brito, R.; Fonseca, A.A., Jr.; Silva, R.O.; Lobato, F.; Fach, P. Draft Genome Sequences of Five Brazilian Clostridium botulinum Group III Type D/C Strains. Genome Announc. 2017, 5, e00349-17. [Google Scholar] [CrossRef] [PubMed]
- Dutra, I.S.; Döbereiner, J.; Souza, A.M. Botulismo em bovinos de corte e leite alimentados com cama de frango. Pesqui. Veterinária Bras. 2005, 25, 115–119. [Google Scholar] [CrossRef]
- Souza, A.M.; Marques, D.F.; Döbereiner, J.; Dutra, I.S. Esporos e toxinas de Clostridium botulinum dos tipos C e D em cacimbas no Vale do Araguaia, Goiás. Pesqui. Veterinária Bras. 2006, 26, 133–138. [Google Scholar] [CrossRef]
- Martins, T.B.; Guizelini, C.C.; Paula, J.P.L.; Lemos, R.A.A.; Alcantara, L.O.B. O triunfo da morte: Mortandade de bois por botulismo em um confinamento de Mato Grosso do Sul. Pesqui. Vet. Bras. 2017, 37, 46–49. [Google Scholar]
- Mawhinney, I.; Palmer, D.; Gessler, F.; Cranwell, M.; Foyle, L.; Otter, A.; Payne, J.; Strugnell, B. Investigation of serology for diagnosis of outbreaks of botulism in cattle. Vet. J. 2012, 192, 382–384. [Google Scholar] [CrossRef] [PubMed]
- Steinman, A.; Galon, N.; Arazi, A.; Bar-Giora, Y.; Shpigel, N.Y. Cattle immune response to botulinum type D toxoid: Results of a vaccination study. Vaccine 2007, 25, 7636–7640. [Google Scholar] [CrossRef] [PubMed]
- Steinman, A.; Chaffer, M.; Elad, D.; Shpigel, N.Y. Quantitative analysis of levels of serum immunoglobulin G against botulinum neurotoxin type D and association with protection in natural outbreaks of cattle botulism. Clin. Vaccine Immunol. 2006, 13, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.O.S.; Oliveira Junior, C.A.; Gonçalves, L.A.; Lobato, F.C.F. Botulism in ruminants in Brazil. Ciência R. 2016, 46, 1411–1417. [Google Scholar] [CrossRef] [Green Version]
- Torii, Y.; Sugimoto, N.; Kohda, T.; Kozaki, S.; Morokuma, K.; Horikawa, Y.; Ginnaga, A.; Yamamoto, A.; Takahashi, M. Clinical Study of New Tetravalent (Type A, B, E, and F) Botulinum Toxoid Vaccine Derived from M Toxin in Japan. Jpn. J. Infect. Dis. 2017, 70, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Cunha, C.E.P.; Moreira, G.M.S.G.; Salvarani, F.M.; Neves, M.S.; Lobato, F.C.F.; Dellagostin, O.A.; Conceição, F.R. Vaccination of cattle with a recombinant bivalent toxoid against botulism serotypes C and D. Vaccine 2014, 32, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Otaka, D.Y.; Barbosa, J.D.; Moreira, C.; Ferreira, M.R.A.; Cunha, C.E.P.; Brito, A.R.S.; Donassolo, R.A.; Moreira, Â.N.; Conceição, F.R.; Salvarani, F.M. Humoral response of buffaloes to a recombinant vaccine against botulism serotypes C and D. Toxins 2017, 9, 297. [Google Scholar] [CrossRef] [PubMed]
- Stahl, C.; Unger, L.; Mazuet, C.; Popoff, M.; Straub, R.; Frey, J. Immune response of horses to vaccination with the recombinant Hc domain of botulinum neurotoxin types C and D. Vaccine 2009, 27, 5661–5666. [Google Scholar] [CrossRef] [PubMed]
- Arimitsu, H.; Lee, J.-C.; Sakaguchi, Y.; Hayakawa, Y.; Hayashi, M.; Nakaura, M.; Takai, H.; Lin, S.-N.; Mukamoto, M.; Murphy, T.; et al. Vaccination with Recombinant Whole Heavy Chain Fragments of Clostridium botulinum Type C and D Neurotoxins. Clin. Vaccine Immunol. 2004, 11, 496–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundeen, G.; Barbieri, J.T. Vaccines against Botulism. Toxins 2017, 9, 268. [Google Scholar] [CrossRef] [PubMed]
- Moreira, G.M.S.G.; Cunha, C.E.P.; Salvarani, F.M.; Gonçalves, L.A.; Pires, P.S.; Conceição, F.R.; Lobato, F.C.F. Production of recombinant botulism antigens: A review of expression systems. Anaerobe 2014, 28, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Heldens, J.G.M.; Patel, J.R.; Chanter, N.; ten Thij, G.J.; Gravendijck, M.; Schijns, V.E.J.C.; Langen, A.; Schetters, T.P.M. Veterinary vaccine development from an industrial perspective. Vet. J. 2008, 178, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Moreira, C.; da Cunha, C.E.P.; Moreira, G.M.S.G.; Mendonça, M.; Salvarani, F.M.; Moreira, Â.N.; Conceição, F.R. Protective potential of recombinant non-purified botulinum neurotoxin serotypes C and D. Anaerobe 2016, 40, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Brasil. Ministério da Agricultura. Instrução normativa n. 23. In Diário Of. da União; 2002. Available online: http://sistemasweb.agricultura.gov.br/sislegis/action/detalhaAto.do?method=visualizarAtoPortalMapa&chave=612413058 (accessed on 13 November 2016).
- USDA—United States Department of Agriculture Livestock and Poultry: World Markets and Trade. Available online: http://www.fas.usda.gov/data/livestock-and-poultry-world-markets-and-trade (accessed on 8 April 2017).
- Webb, R.P.; Smith, T.J.; Wright, P.M.; Guernieri, R.L.; Brown, J.L.; Skerry, J.C. Recombinant Botulinum Neurotoxin Hc Subunit (BoNT Hc) and Catalytically Inactive Clostridium botulinum Holoproteins (ciBoNT HPs) as Vaccine Candidates for the Prevention of Botulism. Toxins 2017, 9, 269. [Google Scholar] [CrossRef] [PubMed]
- Przedpelski, A.; Tepp, W.H.; Zuverink, M.; Johnson, E.A.; Pellet, S.; Barbieri, J.T. Enhancing toxin-based vaccines against botulism. Vaccine 2018, 36, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Moreira, G.M.S.G.; Salvarani, F.M.; da Cunha, C.E.P.; Mendonça, M.; Moreira, Â.N.; Gonçalves, L.A.; Pires, P.S.; Lobato, F.C.F.; Conceição, F.R. Immunogenicity of a Trivalent Recombinant Vaccine Against Clostridium perfringens Alpha, Beta, and Epsilon Toxins in Farm Ruminants. Sci. Rep. 2016, 6, 22816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, R.O.S.; Duarte, M.C.; Oliveira Junior, C.A.; de Assis, R.A.; Lana, A.M.Q.; Lobato, F.C.F. Comparison of humoral neutralizing antibody response in rabbits, guinea pigs, and cattle vaccinated with epsilon and beta toxoids from Clostridium perfringens and C. botulinum types C and D toxoids. Anaerobe 2018, 54, 19–22. [Google Scholar] [CrossRef] [PubMed]


| Treatment | Anti-BoNT Serotype C titers (IU/mL) | Anti-BoNT Serotype D titers (IU/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Animal | 100 µg | 200 µg | 400 µg | CV | PBS | 100 µg | 200 µg | 400 µg | CV | PBS | |
| Animal 1 | ND a | 8 | 10 | 5 | ND | 2 | 10 | 15 | 3 | ND | |
| Animal 2 | 5 | 10 | 10 | 5 | ND | ND | 10 | 15 | 2 | ND | |
| Animal 3 | ND | 8 | 12 | ND | ND | 2 | 10 | 15 | ND | ND | |
| Animal 4 | ND | 10 | 12 | 6 | ND | ND | 10 | 15 | 5 | ND | |
| Animal 5 | ND | 10 | 12 | 5 | ND | ND | 12 | 15 | ND | ND | |
| Animal 6 | 5 | 10 | 12 | 6 | ND | 4 | 10 | 15 | 4 | ND | |
| Animal 7 | ND | 8 | 10 | 5 | ND | 2 | 12 | 10 | 2 | ND | |
| Animal 8 | ND | 5 | 10 | ND | ND | 2 | 8 | 10 | 5 | ND | |
| Mean ± SD b | 1.25 ± 2.32 | 8.65 ± 1.77 | 11.0 ± 1.07 | 4.0 ± 2.51 | 0 | 1.5 ± 1.41 | 10.25 ± 1.28 | 13.75 ± 2.32 | 2.62 ± 2.0 | 0 | |
| Seroconversion Rate c | 25% | 100% | 100% | 75% | 62.5% | 100% | 100% | 75% | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, C., Jr.; Ferreira, M.R.A.; Da Cunha, C.E.P.; Donassolo, R.A.; Finger, P.F.; Moreira, G.M.S.G.; Otaka, D.Y.; De Sousa, L.A.; Barbosa, J.D.; Moreira, Â.N.; et al. Immunogenicity of a Bivalent Non-Purified Recombinant Vaccine against Botulism in Cattle. Toxins 2018, 10, 381. https://doi.org/10.3390/toxins10100381
Moreira C Jr., Ferreira MRA, Da Cunha CEP, Donassolo RA, Finger PF, Moreira GMSG, Otaka DY, De Sousa LA, Barbosa JD, Moreira ÂN, et al. Immunogenicity of a Bivalent Non-Purified Recombinant Vaccine against Botulism in Cattle. Toxins. 2018; 10(10):381. https://doi.org/10.3390/toxins10100381
Chicago/Turabian StyleMoreira, Clóvis, Jr., Marcos R. A. Ferreira, Carlos E. P. Da Cunha, Rafael A. Donassolo, Paula F. Finger, Gustavo M. S. G. Moreira, Denis Y. Otaka, Loise A. De Sousa, José D. Barbosa, Ângela N. Moreira, and et al. 2018. "Immunogenicity of a Bivalent Non-Purified Recombinant Vaccine against Botulism in Cattle" Toxins 10, no. 10: 381. https://doi.org/10.3390/toxins10100381
APA StyleMoreira, C., Jr., Ferreira, M. R. A., Da Cunha, C. E. P., Donassolo, R. A., Finger, P. F., Moreira, G. M. S. G., Otaka, D. Y., De Sousa, L. A., Barbosa, J. D., Moreira, Â. N., Salvarani, F. M., & Conceição, F. R. (2018). Immunogenicity of a Bivalent Non-Purified Recombinant Vaccine against Botulism in Cattle. Toxins, 10(10), 381. https://doi.org/10.3390/toxins10100381






