Analysis of Microcystins in Cyanobacterial Blooms from Freshwater Bodies in England
Abstract
1. Introduction
2. Results
2.1. Total Microcystins
2.1.1. Microcystin Occurrence
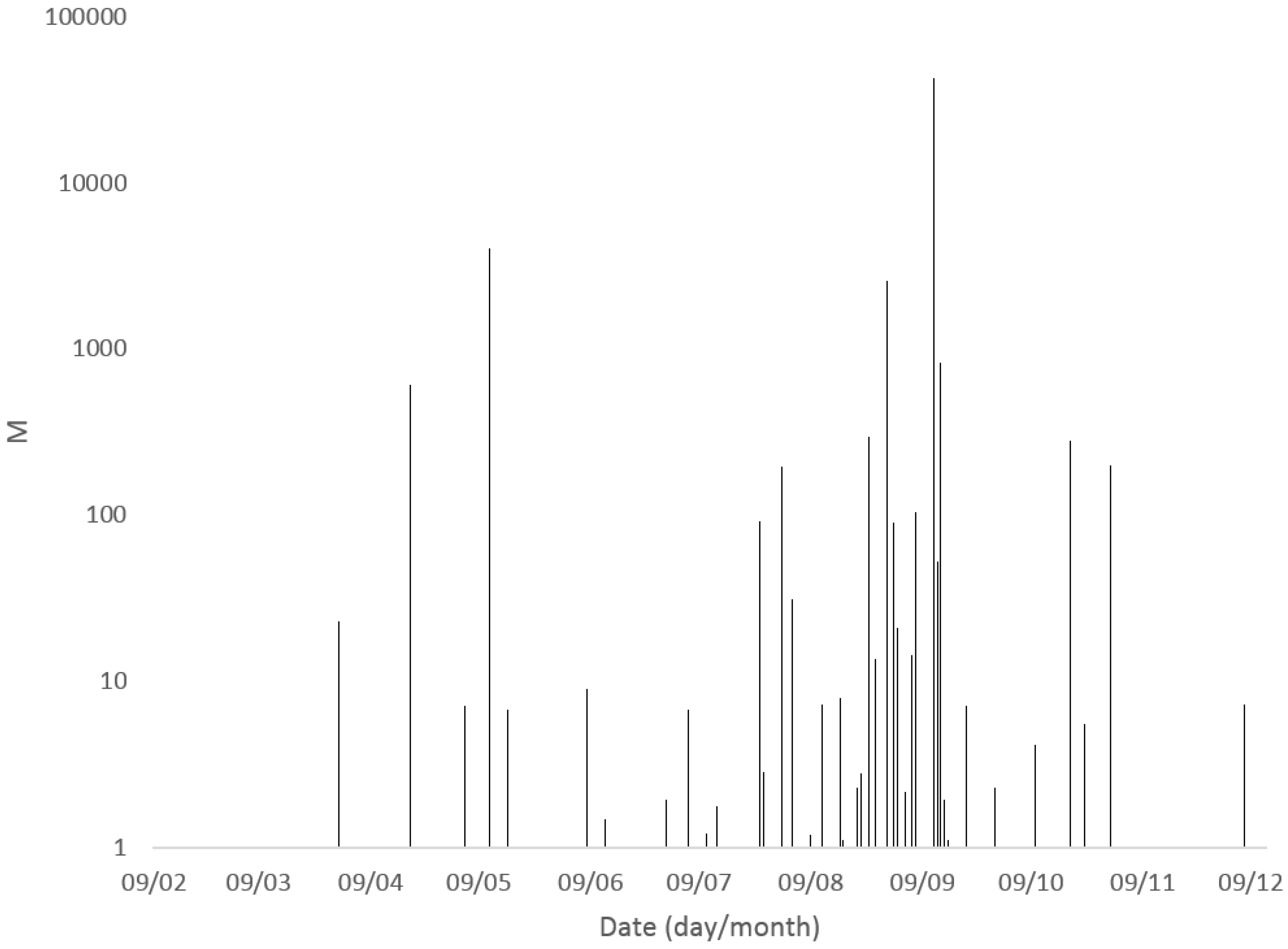
2.1.2. Seasonality
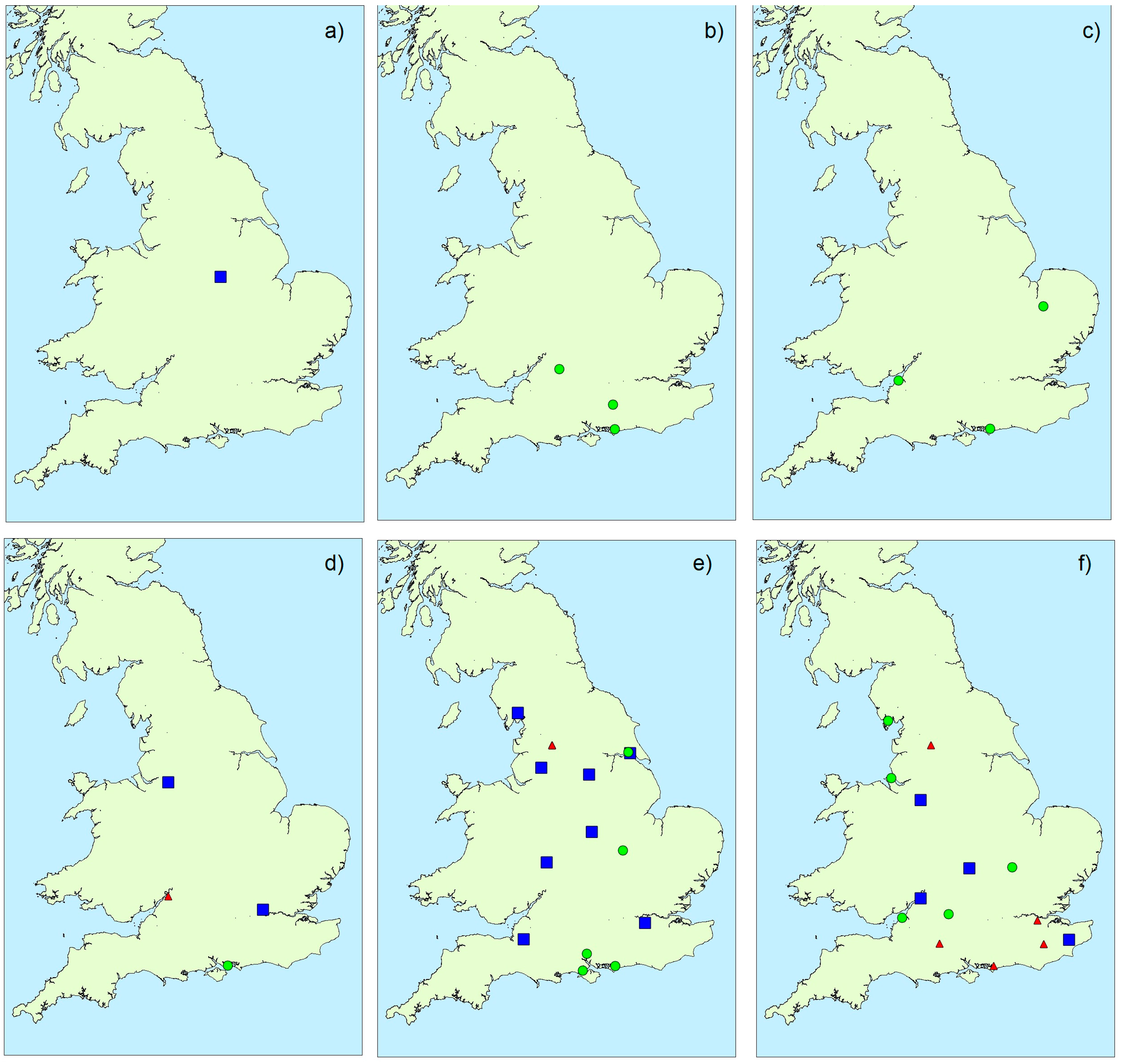
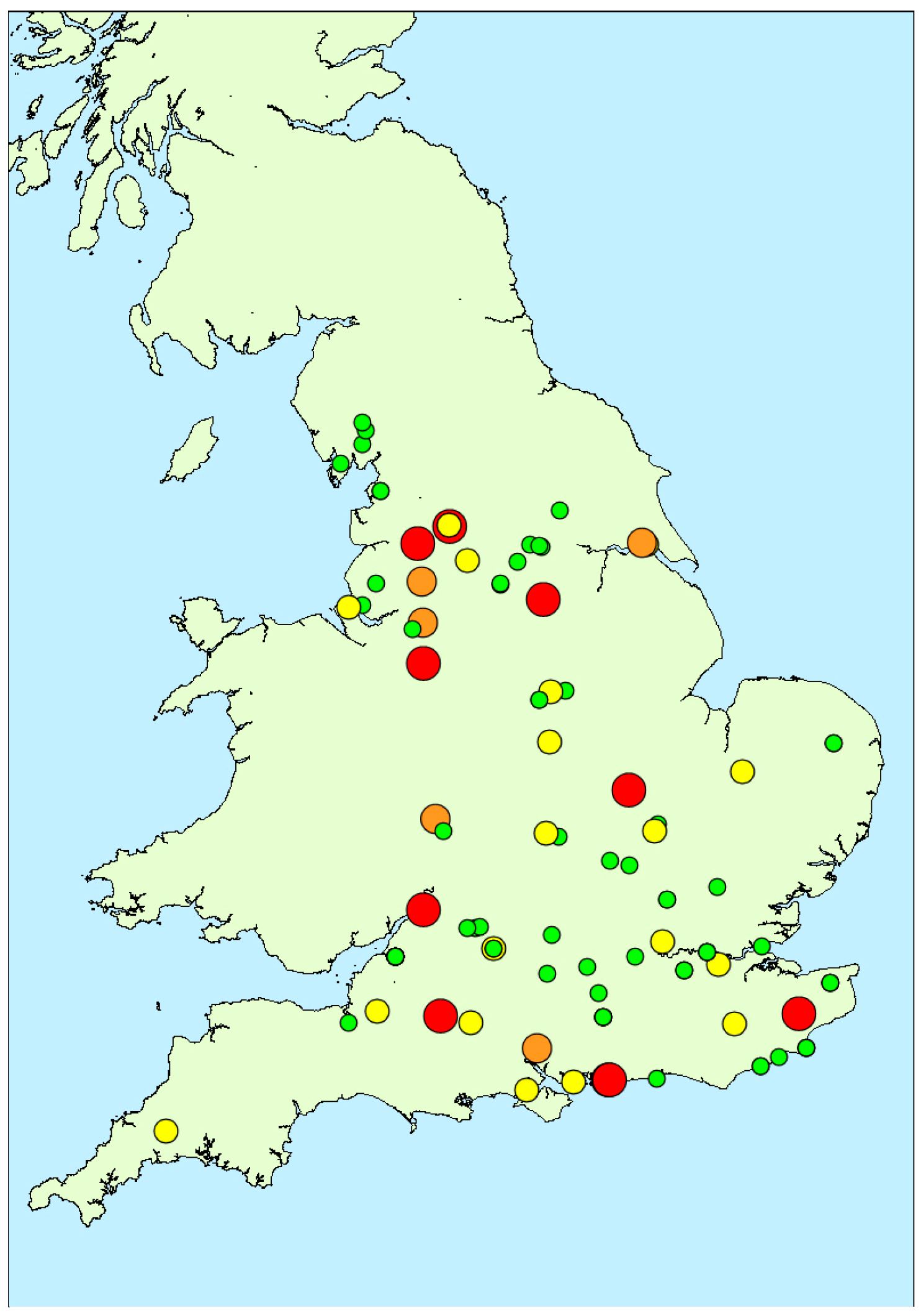
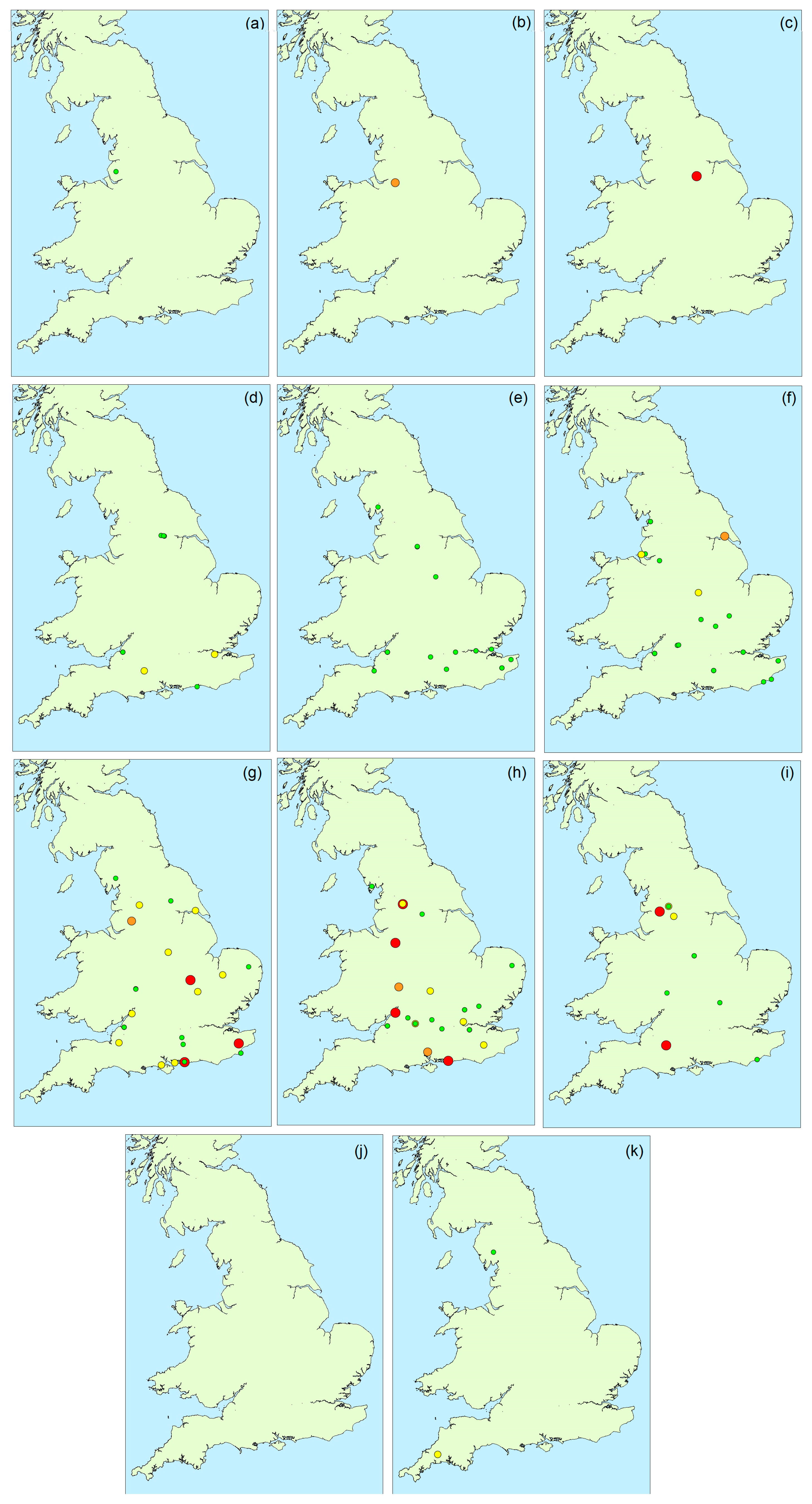
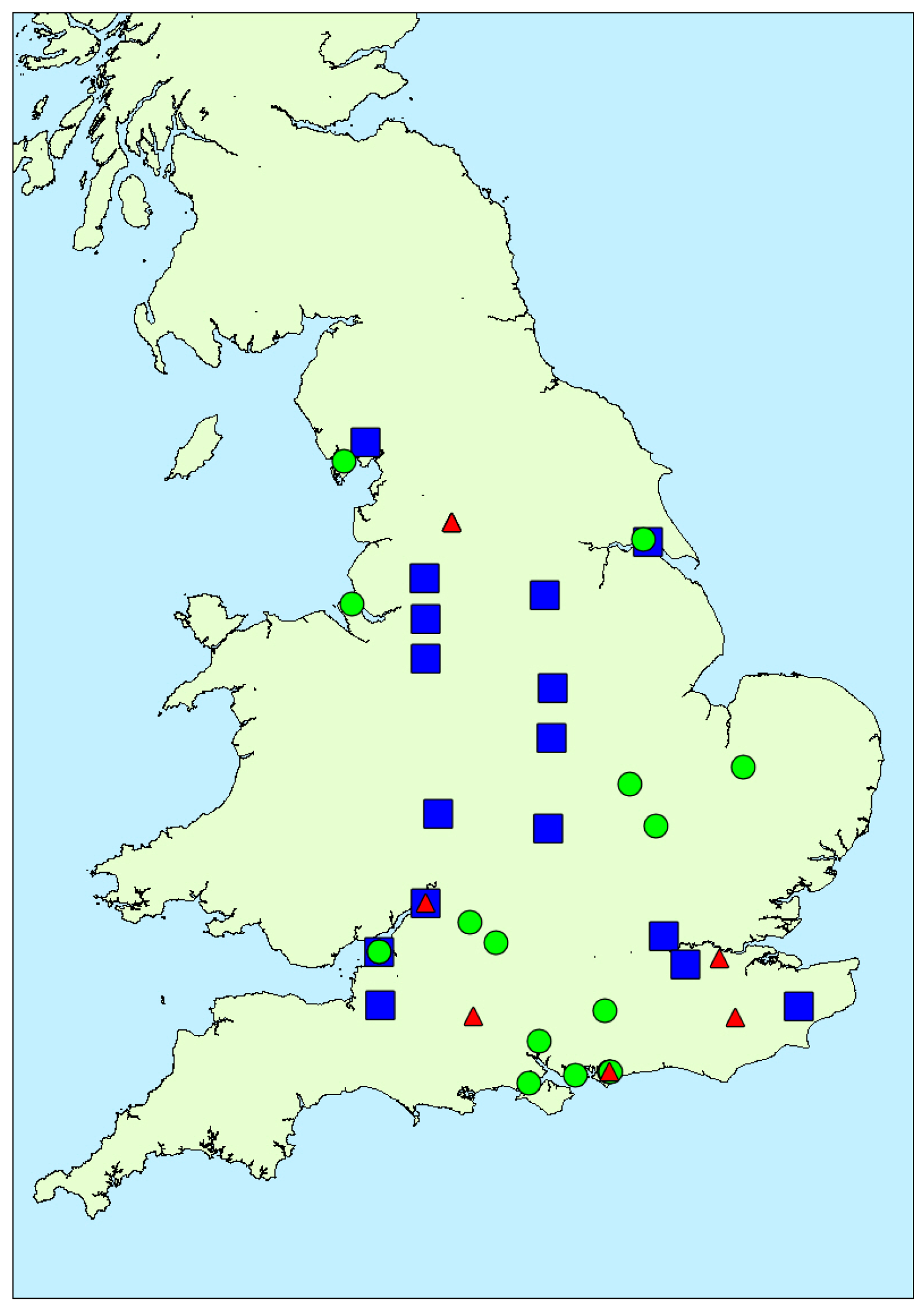
2.1.3. Spatial Variability
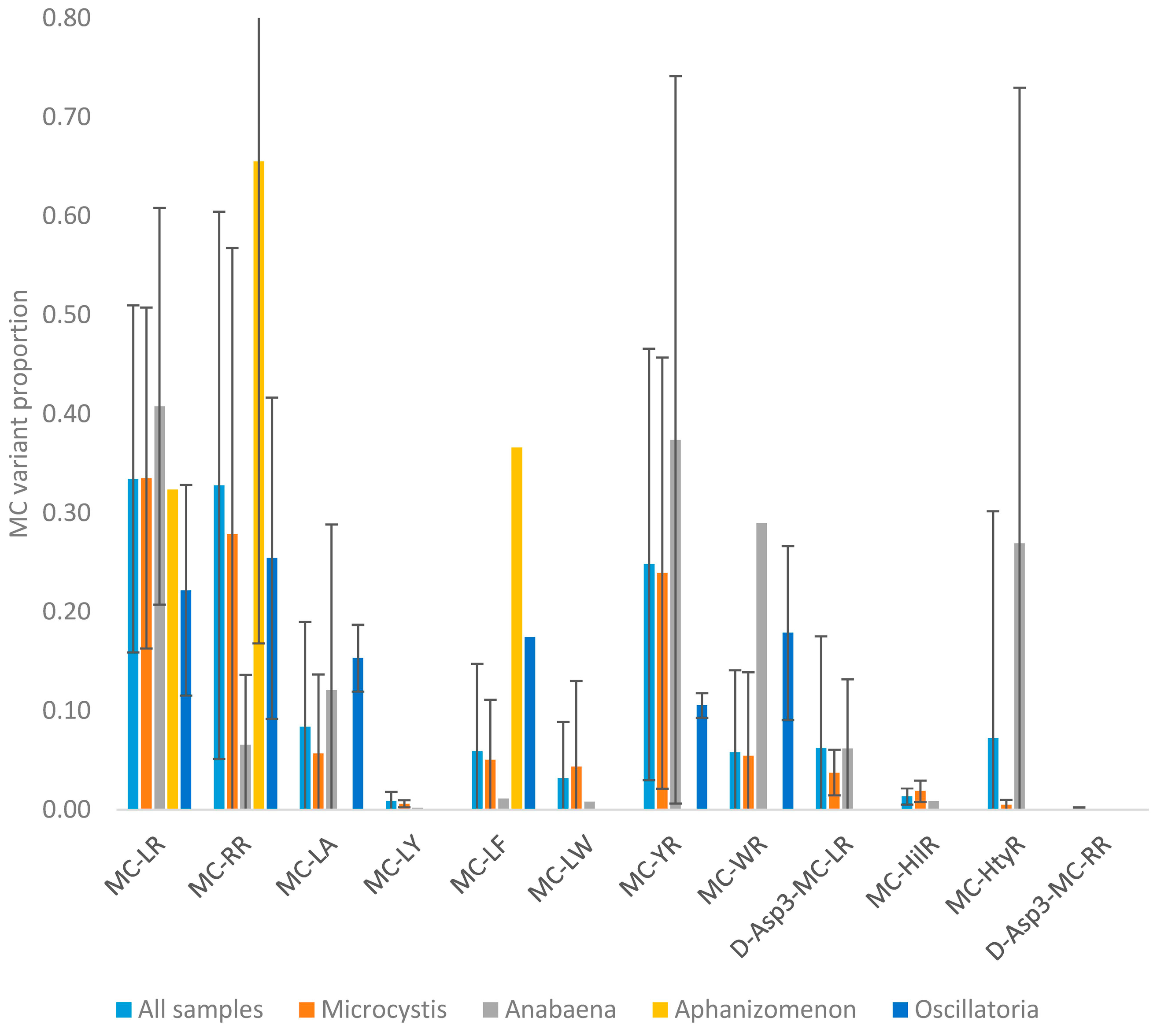
2.1.4. Taxa
2.1.5. Intra and Extra-Cellular Toxins
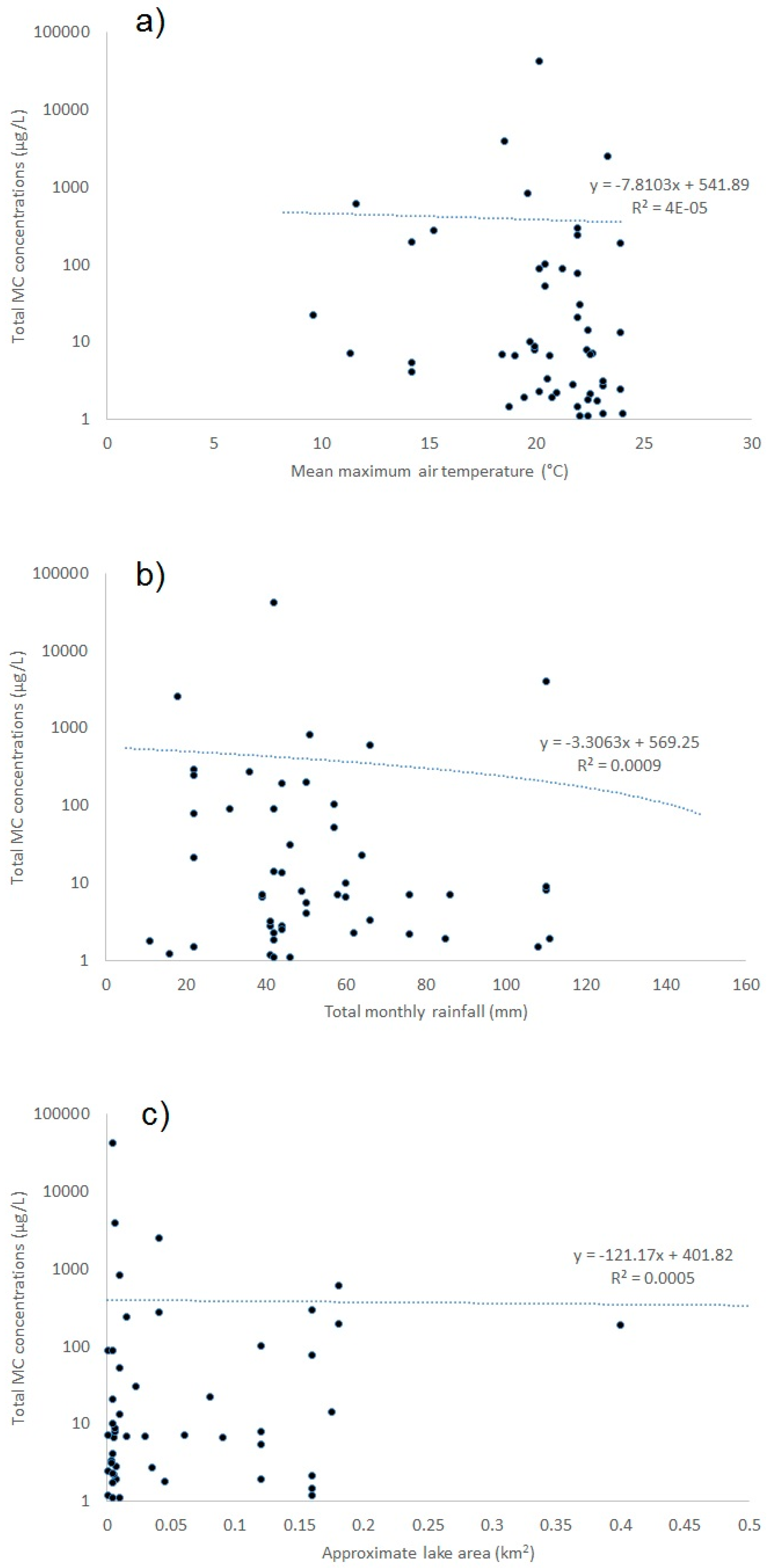
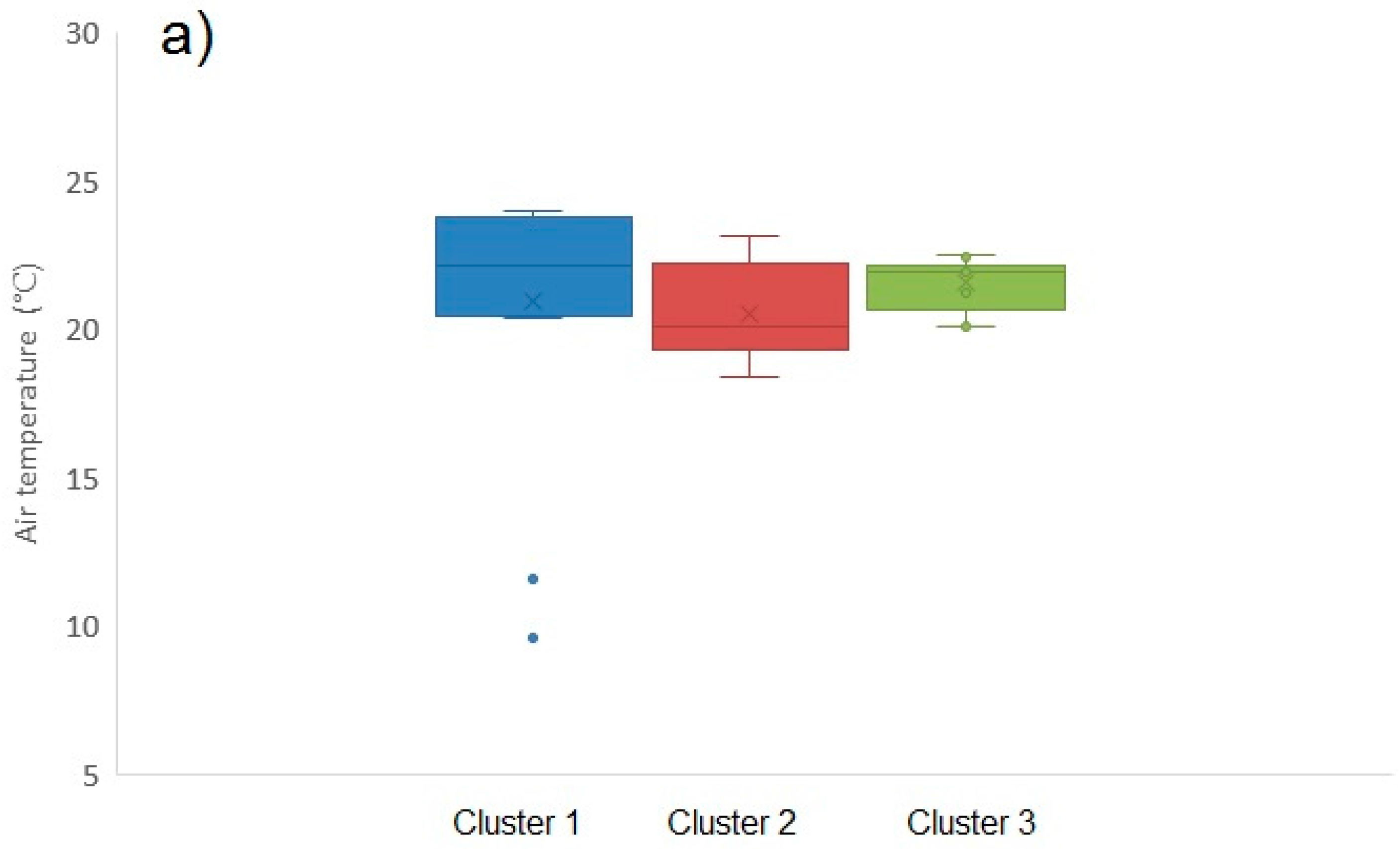
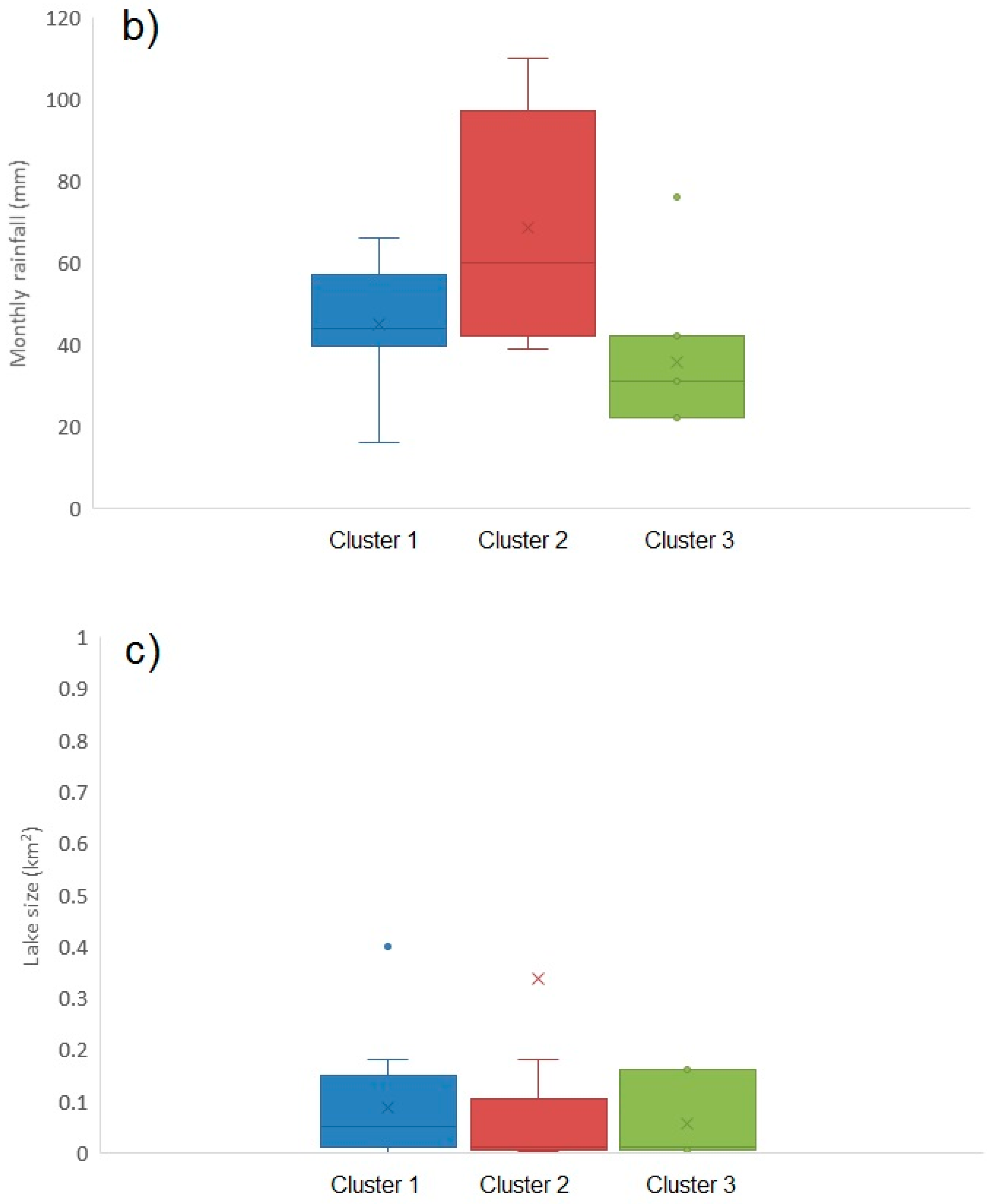
2.1.6. Environmental Influences
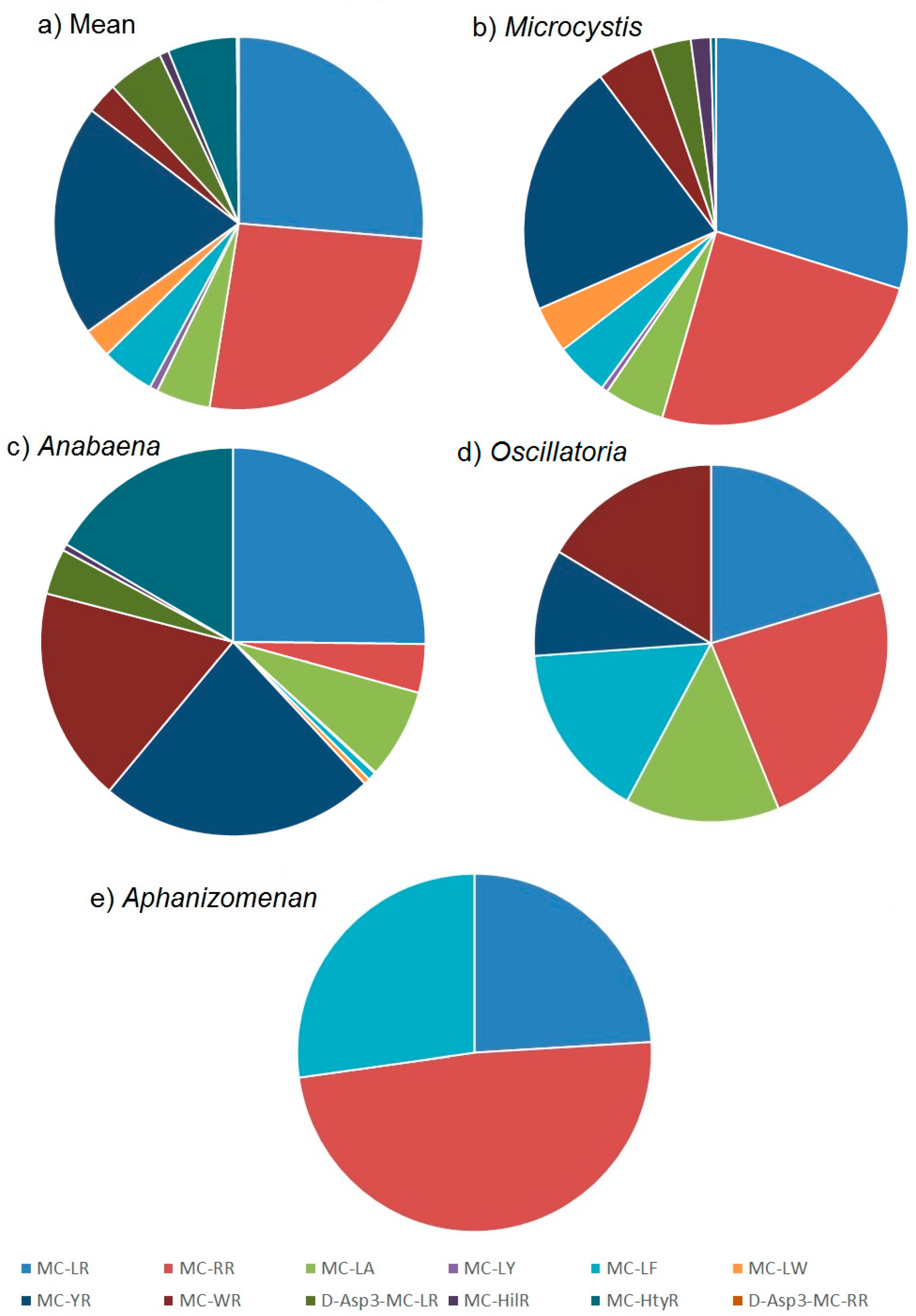
2.2. Toxin Profiles
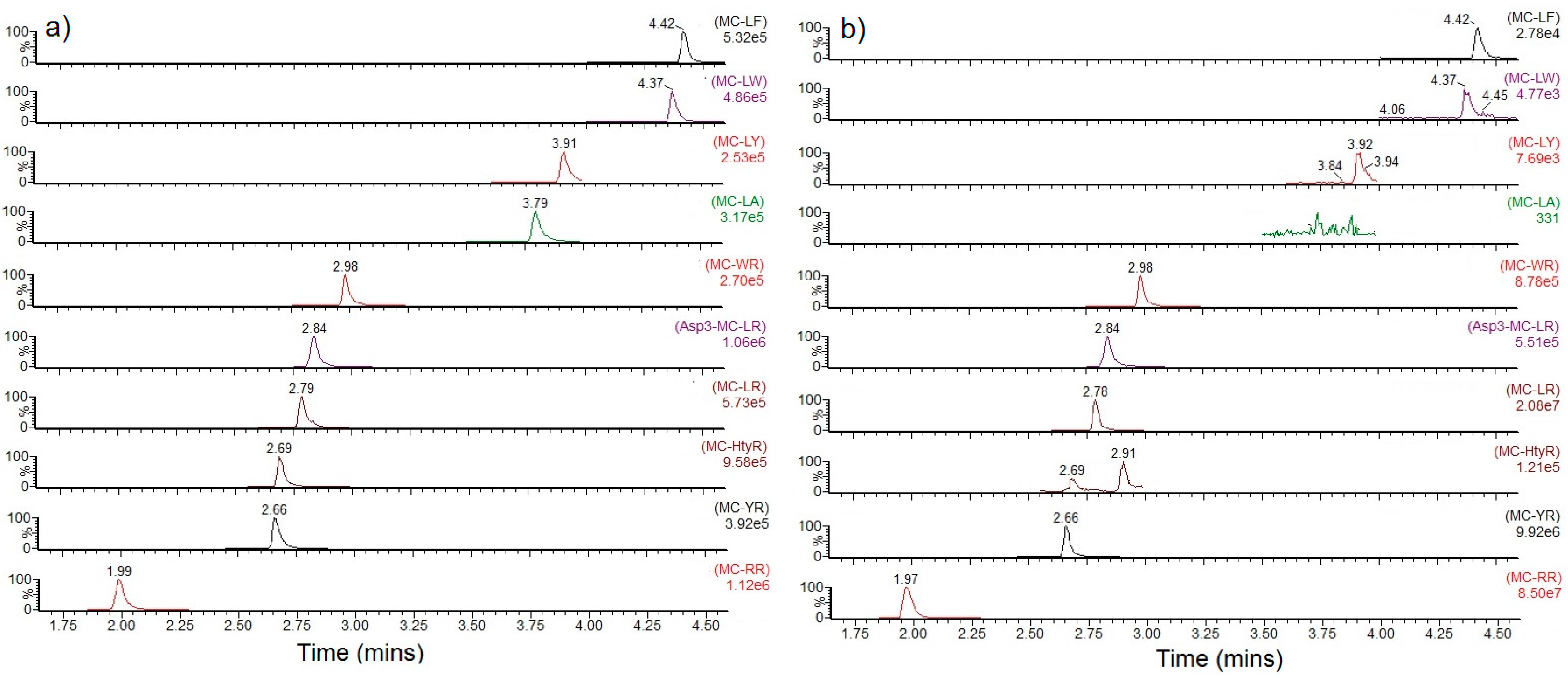
2.2.1. Mean Profiles
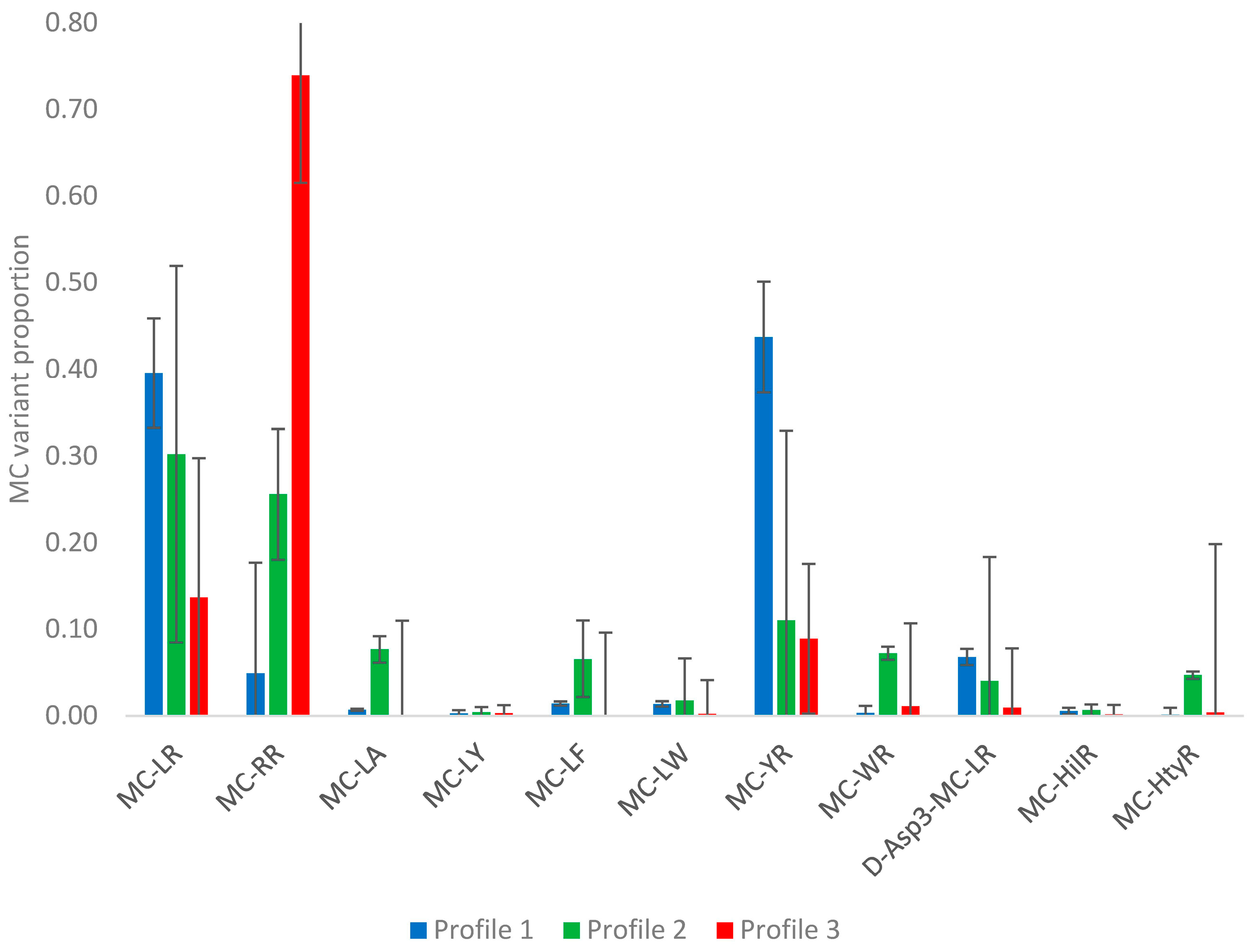
2.2.2. Profile Dependencies
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Water Sampling
4.3. Sample Analysis
4.3.1. Detection of Cyanobacteria
4.3.2. Water Sample Processing
4.3.3. UHPLC-MS/MS Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Sample | Date Collected | Grid Reference | Size (km2) | Type | Taxa | Scum | Threshold Exceeded | Total MCs (μg/L) | Total MCs (pg/Cell ‡) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 09/02/16 | SD4648304966 | 0.0024 | NL | M | N | N | 0.0 | 304 |
| 2 | 31/03/16 | SJ7500781064 | 0.08 | NL | Ap Os | Y | y | 22.7 | 45 |
| 3 | 20/04/16 | SK4764995980 | 0.18 | R | An | Y | y | 610.5 | 1221 |
| 4 | 05/05/16 | SU0415037183 | 0.03 | NL | Os | Y | y | 7.1 | 14 |
| 5 | 09/05/16 | ST5805477480 | 0.009 | NL | - | N | n | 0.0 | na |
| 6 | 09/05/16 | TQ1730003400 | 0.009 | NL | Os | N | n | 0.0 | 0.0 |
| P-1 | 12/05/16 | ST4637977160 | 0.006 | AL | An | N | Y | 4019.4 | na |
| P-2 | 12/05/16 | ST4637977160 | 0.006 | AL | An | Y | Y | 0.8 | na |
| P-3 | 12/05/16 | ST4637977160 | 0.006 | AL | M | N | N | 0.8 | na |
| P-4 | 12/05/16 | ST4637977160 | 0.006 | AL | M | Y | Y | 0.0 | na |
| 7 | 17/05/16 | ST5805477480 | 0.009 | NL | - | N | n | 0.0 | na |
| 9 | 17/05/16 | TQ5453672982 | 0.005 | NL | Os | N | n | 6.7 | 67,182 |
| 8 | 18/05/16 | TQ5453672982 | 0.005 | NL | Os | N | n | 0.0 | 0.0 |
| 10 | 23/05/16 | ST5805477480 | 0.009 | NL | - | N | n | 0.7 | na |
| 11 | 23/05/16 | SE3999828451 | 0.06 | NL | Pl | n | y | 0.0 | 0.0 |
| 12 | 23/05/16 | SE4710227289 | 0.09 | NL | Os | n | y | 0.0 | 0.0 |
| 13 | 23/05/16 | SE4535127829 | 0.18 | NL | Os | n | y | 0.0 | 0.0 |
| 14 | 03/06/16 | ST5805477480 | 0.009 | NL | - | N | n | 0.1 | na |
| 15 | 08/06/16 | ST5805477480 | 0.009 | NL | - | N | n | 0.0 | na |
| 16 | 08/06/16 | SK6175739286 | 0.4 | AL | Os An | N | y | 0.04 | 0.3 |
| P-5 | 08/06/16 | ST4637977160 | 0.006 | AL | M | N | N | 8.0 | 668 |
| P-6 | 08/06/16 | ST4637977160 | 0.006 | AL | M | Y | Y | 9.0 | 751 |
| 17 | 10/06/16 | TR0318143471 | 0.04 | NL | An | N | y | 0.0 | 0.0 |
| 18 | 12/06/16 | SE2221504615 | 0.09 | R | Pl | Y | y | 0.2 | 0.4 |
| 19 | 12/06/16 | SE2204504797 | 0.09 | R | Pl | Y | y | 0.1 | 0.3 |
| 21 | 13/06/16 | SD3754089840 | 5 | NL | An Os | Y | y | 1.5 | 3.0 |
| 20 | 14/06/16 | ST5805477480 | 0.009 | NL | - | N | n | 0.8 | na |
| 22 | 17/06/16 | TR0318143471 | 0.04 | NL | An | N | y | 0.0 | 1.7 |
| 23 | 21/06/16 | TQ4769380228 | 0.03 | AL | Ap Go | Y | y | 1.0 | 2.0 |
| 26 * | 23/06/16 | TQ8153583675 | 0.0012 | AL | An | Y | y | 0.0 | 0.0 |
| 29 | 24/06/16 | SU5071867008 | 0.0225 | NL | Ap | Y | y | 0.0 | 0.0 |
| 24 | 26/06/16 | ST5805477480 | 0.009 | NL | - | N | n | 0.0 | na |
| 30 | 26/06/16 | SU8478040480 | 0.16 | NL | M Gl | Y | y | 0.4 | 0.8 |
| 25 | 28/06/16 | ST5805477480 | 0.009 | NL | - | N | n | 0.0 | na |
| 27 | 28/06/16 | TQ4769380228 | 0.03 | AL | Ap | Y | y | 0.5 | 1.0 |
| 28 | 28/06/16 | TQ0390677601 | 0.01 | NL | An | Y | y | 0.6 | 1.2 |
| 31 | 30/06/16 | ST2975437518 | 0.0075 | AL | Os | Y | y | 1.9 | 3.9 |
| 32 | 30/06/16 | TR2268161365 | 0.004 | AL | Os An | Y | y | 0.1 | 0.2 |
| 33 | 04/07/16 | TQ4769380228 | 0.03 | AL | Ap | Y | y | 0.0 | 0.0 |
| 34 | 04/07/16 | ST5805477480 | 0.009 | NL | - | N | n | 0.0 | na |
| 35 | 06/07/16 | SK5215608209 | 0.09 | NL | An | Y | y | 6.7 | 13 |
| 43 † | 06/07/16 | TQ9163916682 | 0.0003 | NL | Gy | N | n | 0.0 | 0.0 |
| 36 | 07/07/16 | TR2268161365 | 0.004 | AL | Os An | N | n | 0.1 | 92 |
| 37 * | 08/07/16 | TL1775658154 | 0.03 | NL | An | Y | y | 0.0 | 0.3 |
| 38 | 11/07/16 | SU8478040480 | 0.16 | NL | M Gl | Y | y | 1.2 | 2.4 |
| 41 | 12/07/16 | SJ3791791961 | 0.0075 | NL | An | N | n | 0.9 | 18133 |
| 39 | 13/07/16 | ST5805477480 | 0.009 | NL | - | N | n | 0.0 | na |
| 40 | 14/07/16 | TR2268161365 | 0.004 | AL | An Os | Y | y | 1.8 | 13 |
| 42 | 14/07/16 | TQ4769380228 | 0.03 | AL | Ap | Y | y | 0.0 | 0.0 |
| 44 | 18/07/16 | ST5805477480 | 0.009 | NL | - | N | n | 0.0 | na |
| 45 | 18/07/16 | SD4881461153 | 0.0003 | NL | An | Y | y | 0.0 | 0.0 |
| 46 | 21/07/16 | ST5805477480 | 0.009 | NL | - | N | n | 0.0 | na |
| 47 | 24/07/16 | ST5805477480 | 0.009 | NL | - | N | n | 0.7 | na |
| 48 | 25/07/16 | SJ6844577250 | 0.125 | NL | M An | n | n | 0.1 | 10 |
| 52 | 25/07/16 | SU0686094628 | 0.24 | NL | Gl | y | y | 0.0 | 0.0 |
| 53 | 25/07/16 | SP5732550449 | 0.006 | NL | Ap | y | y | 0.0 | 0.0 |
| 50 | 26/07/16 | TA0848030328 | 0.0005 | AL | M | y | y | 90.9 | 182 |
| 54 | 26/07/16 | SD4881461153 | 0.0003 | NL | An | y | y | 0.0 | 0.0 |
| 55 * | 26/07/16 | TR0808521709 | 0.0008 | AL | M An | n | y | 0.5 | 0.1 |
| 49 | 27/07/16 | SP8896035736 | 0.09 | NL | Ap | y | y | 0.1 | 0.3 |
| 51 | 27/07/16 | SU8478040480 | 0.16 | NL | Gl | y | y | 0.0 | 0.0 |
| 56 | 27/07/16 | SJ2926290282 | 0.0075 | AL | M | n | n | 2.8 | 234 |
| 57 | 28/07/16 | SU0927095489 | 0.0024 | NL | An | n | y | 0.0 | 0.0 |
| 58 | 01/08/16 | ST5805477480 | 0.009 | NL | - | N | n | 0.0 | na |
| 59 | 01/08/16 | SP9990079600 | 0.4 | NL | M | Y | y | 193.6 | 387 |
| 60 | 01/08/16 | SE5809049247 | 0.0225 | NL | An | Y | y | 0.0 | 0.0 |
| 61 | 02/08/16 | TR0808521709 | 0.0008 | AL | M | N | y | 0.6 | 0.2 |
| 62 | 04/08/16 | TA1094628599 | 0.0036 | AL | An Os | N | y | 3.3 | 62 |
| 63 | 04/08/16 | SD7415406707 | 0.0225 | AL | Ap M | Y | y | 30.8 | 62 |
| 64 | 05/08/16 | SD4017798465 | 5 | An | Y | y | 0.2 | 0.3 | |
| 65 | 09/08/16 | TR0808521709 | 0.0008 | AL | M | N | n | 1.2 | 167 |
| 67 | 09/08/16 | TR0318143471 | 0.04 | NL | Ap | Y | y | 0.1 | 0.2 |
| 66 | 11/08/16 | ST5805477480 | 0.009 | NL | - | N | n | 0.0 | na |
| 68 | 12/08/16 | SK5282738907 | 0.06 | NL | An M | Y | y | 7.2 | 14 |
| 81 | 15/08/16 | SO8307960032 | 0.00125 | AL | An Os | N | y | 0.0 | 0.0 |
| 82 | 15/08/16 | SO8315359973 | 0.025 | AL | An Os | N | y | 0.0 | 0.0 |
| 80 | 17/08/16 | SO7499006989 | 0.12 | NL | Ap M An At | Y | y | 7.9 | 15.8 |
| 78 | 18/08/16 | ST5805477480 | 0.009 | NL | - | N | n | 0.0 | na |
| 85 | 18/08/16 | SD9089540319 | 0.004 | AL | M Go | N | y | 1.1 | 2.2 |
| 79 | 19/08/16 | TG2522007900 | 0.2 | NL | Ap M An | N | n | 0.1 | 1046 |
| 83 | 22/08/16 | TR0318143471 | 0.04 | NL | Ap | Y | y | 0.8 | 1.6 |
| 86 | 22/08/16 | ST4679744377 | 0.005 | AL | An M Os | Y | y | 2.3 | 4.5 |
| 84 | 23/08/16 | ST5805477480 | 0.009 | NL | - | N | n | 0.0 | na |
| 88 | 23/08/16 | SZ3777596540 | 0.035 | NL | M | Y | y | 2.8 | 5.6 |
| 87 | 25/08/16 | SD9089540319 | 0.004 | AL | M Go | Y | y | 10.0 | 20 |
| 89 | 25/08/16 | SU8478040480 | 0.16 | NL | Gl | Y | y | 0.0 | 0.0 |
| 90 | 25/08/16 | SU8201355304 | 0.09 | NL | Ap | Y | y | 0.0 | 0.0 |
| 99 | 25/08/16 | SU8728403186 | 0.16 | NL | M Ap | Y | y | 78.7 | 157 |
| 101 | 25/08/16 | SU8728403186 | 0.16 | NL | M | Y | y | 296.9 | 594 |
| 102 | 25/08/16 | SU8728403186 | 0.16 | NL | M Ap | N | y | 1.5 | 3.0 |
| 100 | 26/08/16 | SU8728403186 | 0.16 | NL | Ap | Y | y | 0.1 | 0.2 |
| 91 | 27/08/16 | TL1572653882 | 0.01 | NL | M | Y | y | 13.6 | 27 |
| 92 | 30/08/16 | TR0318143471 | 0.04 | NL | Ap M An | Y | y | 2561.0 | 5122 |
| 93 | 30/08/16 | TL6943690325 | 0.0009 | AL | M | Y | y | 2.5 | 5.0 |
| 97 | 30/08/16 | SU6663501299 | 0.0036 | NL | Ap Ps | N | y | 3.2 | 21 |
| 94 | 01/09/16 | SD9089540319 | 0.004 | AL | M Go | Y | y | 89.9 | 180 |
| 95 | 02/09/16 | ST5805477480 | 0.009 | NL | - | N | n | 0.0 | na |
| 98 | 02/09/16 | SU4410721883 | 0.004 | NL | M An | Y | y | 21.1 | 42 |
| 96 | 04/09/16 | TQ2097086918 | 0.16 | R | Ap | Y | y | 2.2 | 4.4 |
| 107 | 06/09/16 | SU1762682163 | 0.175 | NL | M | Y | y | 14.2 | 28 |
| 108 | 06/09/16 | SU1762682163 | 0.175 | NL | Ap | N | n | 0.0 | 0.0 |
| 103 | 07/09/16 | SO7499006989 | 0.12 | NL | M Ap | Y | y | 103.2 | 206 |
| 104 | 07/09/16 | TQ3403669313 | 0.0096 | NL | Ap | Y | y | 0.0 | 0.0 |
| 106 | 07/09/16 | SU5296391042 | 0.0024 | NL | At | N | y | 0.0 | 0.1 |
| 105 | 09/09/16 | SE3248717960 | 0.3 | NL | M Ap | Y | y | 0.3 | 0.6 |
| 111 | 12/09/16 | SU8779803166 | 0.015 | NL | M An | Y | y | 244.0 | 488 |
| 114 | 12/09/16 | SD9089540319 | 0.004 | AL | M Go | Y | y | 42,724.1 | 85,448 |
| 110 | 13/09/16 | SO8267761829 | 0.01 | AL | Ap An M | Y | y | 52.7 | 105 |
| 112 | 13/09/16 | TL538202 | 0.0075 | NL | An | Y | y | 0.2 | 0.5 |
| 115 | 13/09/16 | SU7483471048 | 0.015 | NL | An Ps Os | N | y | 0.0 | 0.0 |
| 118 | 13/09/16 | SU1762682163 | 0.175 | NL | Ap | Y | y | 0.2 | 0.3 |
| 109 | 14/09/16 | ST5805477480 | 0.009 | NL | - | N | n | 0.2 | na |
| 119 | 14/09/16 | SJ7493757095 | 0.01 | NL | An M | Y | y | 830.4 | 1661 |
| 122 | 14/09/16 | TL 23456 12000 | 0.01 | NL | M | N | n | 0.3 | 2706 |
| 116 | 14/09/16 | SU0158594707 | 0.045 | AL | M An | Y | y | 1.8 | 3.7 |
| 113 | 15/09/16 | SD2430078100 | 0.18 | R | Go M An | Y | y | 1.0 | 2.0 |
| 117 | 15/09/16 | SP4976352470 | 0.12 | R | Ap M An Os | Y | y | 1.9 | 3.9 |
| 120 | 16/09/16 | TQ3403669313 | 0.0096 | NL | Ap | Y | y | 1.1 | 2.2 |
| 123 | 19/09/16 | TL 23456 12000 | 0.01 | NL | M | N | n | 1.0 | 9926 |
| 121 | 20/09/16 | TG2522007900 | 0.2 | NL | Sn Os M Me | N | n | 0.0 | 0.0 |
| 125 | 21/09/16 | TQ6421836460 | 0.015 | NL | Os Go | Y | y | 7.1 | 14.2 |
| 124 | 22/09/16 | ST5805477480 | 0.009 | NL | - | N | n | 0.0 | 238 |
| 126 | 22/09/16 | TQ3403669313 | 0.0096 | NL | Ap | Y | y | 0.0 | 0.1 |
| 128 | 29/09/16 | SD9089540319 | 0.004 | AL | Go M | Y | y | 2.3 | 4.6 |
| 132 | 07/10/16 | TL0072233302 | 0.0015 | NL | An | Y | y | 0.3 | 5.7 |
| 133 | 10/10/16 | SD9089540319 | 0.004 | AL | Go M | Y | y | 4.1 | 8.3 |
| 129 | 11/10/16 | SO8745754176 | 0.0008 | AL | Os M | Y | y | 0.0 | 0.0 |
| 130 | 11/10/16 | SK4542133791 | 0.025 | NL | Pl | N | y | 0.0 | 0.0 |
| 131 | 11/10/16 | SK4542133791 | 0.025 | NL | Pl | N | y | 0.0 | 0.0 |
| 135 | 13/10/16 | TQ8074010542 | 0.002 | NL | Go Os Ly | N | y | 0.4 | 1.2 |
| 136 | 13/10/16 | TQ8032210477 | 0.004 | R | Go | N | y | 0.7 | 0.8 |
| 134 | 20/10/16 | ST8529642290 | 0.04 | NL | Go Ap Os | Y | y | 278.8 | 558 |
| 137 | 24/10/16 | SE0209118762 | 0.12 | R | Ap | Y | y | 5.5 | 11.0 |
| 138 | 26/10/16 | SD9089540319 | 0.004 | AL | M Ap | N | n | 0.5 | 309 |
| 139 | 31/10/16 | SD7146329903 | 0.18 | R | Go | Y | y | 198.8 | 398 |
| 140 | 07/12/16 | SX1799070900 | 0.001 | NL | M Os | Y | y | 7.2 | 14 |
| 141 | 13/12/16 | NY3750003300 | 3.2 | NL | Go | Y | y | 0.0 | 0.0 |
References
- Sivonen, K. Cyanobacterial toxins. In Encyclopedia of Microbiology, 3rd ed.; Schaechter, M., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2009; pp. 290–307. [Google Scholar]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- Henderson, R.; Chips, M.; Cornwell, N.; Hitchins, P.; Holden, B.; Hurley, S.; Parsons, S.A.; Wetherill, A.; Jefferson, B. Experiences of algae in UK waters: A treatment perspective. Water Environ. J. 2008, 22, 184–192. [Google Scholar] [CrossRef]
- Svirčev, Z.; Drobac, D.; Tokodi, N.; Mijović, B.; Codd, G.A.; Meriluoto, J. Toxicology of microcystins with reference to cases of human intoxications and epidemiological investigations of exposures to cyanobacteria and cyanotoxins. Arch. Toxicol. 2017, 91, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, W.W.; Azevedo, S.M.F.O.; An, J.S.; Molica, R.J.R.; Jochimsen, E.M.; Lau, S.; Rinehart, K.L.; Shaw, G.R.; Eaglesham, G.K. Human fatalities from cyanobacteria: Chemical and biological evidence for cyanotoxins. Environ. Health Perspect. 2001, 109, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Bláha, L.; Babica, P.; Maršálek, B. Toxins produced in cyanobacterial water blooms—Toxicity and risks. Interdiscip. Toxicol. 2009, 2, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Pearson, L.; Mihali, T.; Moffitt, M.; Kellmann, R.; Neilan, B. On the chemistry, toxicology and genetics of the cyanobacterial toxins, microcystin, nodularin, saxitoxin and cylindrospermopsin. Mar. Drugs 2010, 8, 1650–1680. [Google Scholar] [CrossRef] [PubMed]
- Howard, A.; McDonald, A.T.; Kneale, P.E.; Whitehead, P.G. Cyanobacterial (blue-green algal) blooms in the UK: A review of the current situation and potential management options. Prog. Phys. Geogr. 1996, 20, 53–61. [Google Scholar] [CrossRef]
- Howard, A.; Easthope, M.P. Application of a model to predict cyanobacterial growth patterns in response to climatic change at Farmoor reservoir, Oxfordshire, UK. Sci. Total Environ. 2002, 282–283, 459–469. [Google Scholar] [CrossRef]
- Miller, M.A.; Kudela, R.M.; Mekebri, A.; Crane, D.; Oates, S.C.; Tinker, M.T.; Staedler, M.; Miller, W.A.; Toy-Choutka, S.; Dominik, C.; et al. Evidence for a novel marine harmful algal bloom: Cyanotoxin (microcystin) transfer from land to sea otters. PLoS ONE 2010, 5, e12576. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, J.S.; Codd, G.A. A Review of Current Knowledge, Cyanobacterial Toxins (Cyanotoxins) in Water; Foundation for Water Research: Marlow, UK, 2014; p. 47. [Google Scholar]
- Hunter, P.D.; Hanley, N.; Czajkowski, M.; Mearns, K.; Tyler, A.N.; Carvalho, L.; Codd, G.A. The effect of risk perception on public preferences and willingness to pay for reductions in the health risks posed by toxic cyanobacterial blooms. Sci. Total Environ. 2012, 426, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Lawton, L.A.; Codd, G.A. Cyanobacterial (blue-green algal) toxins and their significance in UK and European waters. Water Environ. J. 1991, 5, 460–465. [Google Scholar] [CrossRef]
- Krokowski, J.T.; Lang, P.; Bell, A.; Broad, N.; Clayton, J.; Milne, I.; Nicolson, M.; Ross, A.; Ross, N. A review of the incidence of cyanobacteria (blue-green algae) in surface eaters in Scotland including potential effects of climate change, with a list of the common species and new records from the Scottish Environmental Protection Agency. Glasg. Nat. 2012, 25, 99–104. [Google Scholar]
- Krokowski, J.T.; Jamieson, J. A decade of monitoring and management of freshwater algae, in particular cyanobacteria in England and Wales. Freshw. Forum 2002, 18, 3–12. [Google Scholar]
- Zurawell, R.W.; Chen, H.; Burke, J.M.; Prepas, E.E. Hepatotoxic cyanobacteria: A review of the biological importance of microcystins in freshwater environments. J. Toxicol. Environ. Health Part B 2005, 8, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, J.; Fan, H.; Xie, P.; He, J. A review of neurotoxicity of microcystins. Environ. Sci. Pollut. Res. 2016, 23, 7211–7219. [Google Scholar] [CrossRef] [PubMed]
- Zegura, B. An overview of the mechanisms of microcystin-lr genotoxicity and potential carcinogenicity. Mi-Rev. Méd. Chem. 2016, 16, 1042–1062. [Google Scholar] [CrossRef]
- Codd, G.; Bell, S.; Kaya, K.; Ward, C.; Beattie, K.; Metcalf, J. Cyanobacterial toxins, exposure routes and human health. Eur. J. Phycol. 1999, 34, 405–415. [Google Scholar] [CrossRef]
- Turner, P.C.; Gammie, A.J.; Hollinrake, K.; Codd, G.A. Pneumonia associated with contact with cyanobacteria. BMJ 1990, 300, 1440–1441. [Google Scholar] [CrossRef] [PubMed]
- Codd, G.A.; Morrison, L.F.; Metcalf, J.S. Cyanobacterial toxins: Risk management for health protection. Toxicol. Appl. Pharmacol. 2005, 203, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, T.; Kagalou, I.; Stalikas, C.; Pilidis, G.; Leonardos, I.D. Assessment of microcystin distribution and biomagnification in tissues of aquatic food web compartments from a shallow lake and evaluation of potential risks to public health. Ecotoxicology 2012, 21, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Lawton, L.A.; Edwards, C.; Beattie, K.A.; Pleasance, S.; Dear, G.J.; Codd, G.A. Isolation and characterization of microcystins from laboratory cultures and environmental samples of Microcystis aeruginosa and from an associated animal toxicosis. Nat. Toxins 1995, 3, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Hitzfeld, B.C.; Hoger, S.J.; Dietrich, D.R. Cyanobacterial toxins: Removal during drinking water treatment, and human risk assessment. Environ. Health Perspect. 2000, 108 (Suppl. S1), 113–122. [Google Scholar] [CrossRef] [PubMed]
- Water UK. Water UK Technical Briefing Note: Blue Green Algal Toxins in Drinking Water; Water UK Position Paper; Water UK: London, UK, 2006. [Google Scholar]
- Guardian. Different Strokes: Open Water Swimming Takes UK by Storm. Article in Guardian Newspaper. 2015. Available online: https://www.theguardian.com/lifeandstyle/2015/aug/18/different-strokes-open-water-swimming-uk-event-lake-river-sea (accessed on 16 November 2017).
- OSS. The OSS Mission Statement. Outdoor Swimming Society. 2017. Available online: https://www.outdoorswimmingsociety.com/the-oss-mission-statement/ (accessed on 16 November 2017).
- Scottish Environment Protection Agency (SEPA). Improving Scotland’s Water Environment; SEPA State of the Environment Report; Scottish Environment Protection Agency: Glasgow, UK, 1999. [Google Scholar]
- Scottish Environment Protection Agency (SEPA). Eutrophication Assessment of Scottish Coastal, Estuarine and Inland Waters; Scottish Environment Protection Agency: Glasgow, UK, 2005. [Google Scholar]
- Scottish Government. Blue-Green Algae (Cyanobacteria) in Inland and Inshore Waters: Assessment and Minimisation of Risks to Public Health: Revised Guidance; Scottish Executive: Edinburgh, UK, 2007.
- Scottish Government. Cyanobacteria (Blue-Green Algae) in Inland and Inshore Waters: Assessment and Minimisation of Risk to Public Health; Scottish Executive: Edinburgh, UK, 2012.
- Environment Agency (EA). Monitoring Freshwater and Marine Algal Incidents, version 8; Unpublished Operational Instruction 906_08; Environment Agency Report; EA: Bristol, UK, 2016. [Google Scholar]
- World Health Organization (WHO). Guidelines for Safe Recreational Water Environments; Volume 1: Coastal and Fresh Waters; WHO: Geneva, Switzerland, 2003. [Google Scholar]
- Mekebri, A.; Blondina, G.J.; Crane, D.B. Method validation of microcystins in water and tissue by enhanced liquid chromatography tandem mass spectrometry. J. Chromatogr. Coruña 2009, 1216, 3147–3155. [Google Scholar] [CrossRef] [PubMed]
- Lawton, L.A.; Edwards, C.; Codd, G.A. Extraction and high-performance liquid chromatographic method for the determination of microcystins in raw and treated waters. Analyst 1994, 119, 1525–1530. [Google Scholar] [CrossRef] [PubMed]
- Lawton, L.A.; Chambers, H.; Edwards, C.; Nwaopara, A.A.; Healy, M. Rapid detection of microcystins in cells and water. Toxicon 2010, 55, 973–978. [Google Scholar] [CrossRef] [PubMed]
- McElhiney, J.; Lawton, L.A. Detection of the cyanobacterial hepatotoxins microcystins. Toxicol. Appl. Pharmacol. 2005, 203, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yang, Q.; Xie, P.; Deng, X.; Chen, J.; Dai, M. The role of cysteine conjugation in the detoxification of microcystin-lr in liver of bighead carp (aristichthys nobilis): A field and laboratory study. Ecotoxicology 2011, 21, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.D.; Waack, J.; Lewis, A.; Edwards, C.; Lawton, L. Development and single-laboratory validation of a UPLC-MS/MS method for quantitation of cyanotoxins in natural water, cyanobacteria, shellfish and algal supplement tablet powders. J. Chromatogr. B 2018. [Google Scholar] [CrossRef]
- Ueno, Y.; Nagata, S.; Tsutsumi, T.; Hasegawa, A.; Yoshida, F.; Suttajit, M.; Putsch, M.; Vasconcelos, V. Survey of microcystins in environmental water by a highly sensitive immunoassay based on monoclonal antibody. Nat. Toxins 1996, 4, 271–276. [Google Scholar] [CrossRef]
- Tsuji, K.; Setsuda, S.; Watanuki, T.; Kondo, F.; Nakazawa, H.; Suzuki, M.; Harada, K.-I. Microcystin levels during 1992–95 for lakes sagami and tsukui-japan. Nat. Toxins 1996, 4, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Nagata, S.; Tsutsumi, T.; Hasegawa, A.; Yoshida, F.; Ueno, Y.; Watanabe, M.F. Enzyme immunoassay for direct determination of microcystins in environmental water. J. AOAC Int. 1997, 80, 408–417. [Google Scholar]
- Greer, B.; McNamee, S.E.; Boots, B.; Cimarelli, L.; Guillebault, D.; Helmi, K.; Marcheggiani, S.; Panaiotov, S.; Breitenbach, U.; Akçaalan, R.; et al. A validated uplc–ms/ms method for the surveillance of ten aquatic biotoxins in european brackish and freshwater systems. Harmful Algae 2016, 55, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Sivonen, K.; Jones, G. Cyanobacterial toxins. In Toxic Cyanobacteria in Water: A Guide to Public Health Significance, Monitoring and Management; Chorus, I., Bertram, J., Eds.; The World Health Organization: London, UK, 1999; pp. 41–111. [Google Scholar]
- Chorus, I.; Bartram, J. Toxic Cyanobacteria in Water. A Guide to Their Public Health Consequences, Monitoring and Management; World Health Organization: Geneva, Switzerland, 1999. [Google Scholar]
- Casterlin, M.E.; Reynolds, W.W. Seasonal algal succession and cultural eutrophication in a north temperate lake. Hydrobiologia 1977, 54, 99–108. [Google Scholar] [CrossRef]
- Met Office. UK et Office, Reports Describing Climate Summaries for the UK. 2016. Available online: http://www.etoffice.gov.uk/cliate/uk/suaries/2016/septeber (accessed on 8 September 2017).
- Dittmann, E.; Meißner, K.; Börner, T. Conserved sequences of peptide synthetase genes in the cyanobacterium microcystis aeruginosa. Phycologia 1996, 35 (Suppl. S6), 62–67. [Google Scholar] [CrossRef]
- Chorus, I. Cyanotoxins, Occurrence, Causes, Consequences; Springer: Heidelberg, Germany, 2001; p. 357. [Google Scholar]
- Testai, E.; Buratti, F.M.; Funari, E.; Manganelli, M.; Vichi, S.; Arnich, N.; Biré, R.; Fessard, V.; Sialehaamoa, A. Review and Analysis of Occurrence, Exposure and Toxicity of Cyanobacteria Toxins in Food. EFSA Support. Publ. 2016, 13. [Google Scholar] [CrossRef]
- Davis, T.W.; Berry, D.L.; Boyer, G.L.; Gobler, C.J. The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of microcystis during cyanobacteria blooms. Harmful Algae 2009, 8, 715–725. [Google Scholar] [CrossRef]
- Dziallas, C.; Grossart, H.-P. Increasing oxygen radicals and water temperature select for toxic microcystis sp. PLoS ONE 2011, 6, e25569. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yu, Y.; Yang, Z.; Kong, F.; Zhang, T.; Tang, S. The dynamics of toxic and nontoxic microcystis during bloom in the large shallow lake, Lake Taihu, china. Environ. Monit. Assess. 2014, 186, 3053–3062. [Google Scholar] [CrossRef] [PubMed]
- Briand, E.; Yepremian, C.; Humbert, J.-F.; Quiblier, C. Competition between microcystin and non-microcystin producing Planktothrix agardhii (cyanobacteria) strains under different environmental conditions. Environ. Microbiol. 2008, 10, 3337–3348. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.W.; Orr, P.T.; Boyer, G.L.; Burford, M.A. Investigating the production and release of cylindrospermopsin and deoxy-cylindrospermopsin by cylindrospermopsis raciborskii over a natural growth cycle. Harmful Algae 2014, 31, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Willis, A.; Chuang, A.W.; Woodhouse, J.N.; Neilan, B.A.; Burford, M.A. Intraspecific variation in growth, morphology and toxin quotas for the cyanobacterium, cylindrospermopsis raciborskii. Toxicon 2016, 119, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Gaget, V.; Lau, M.; Sendall, B.; Froscio, S.; Humpage, A.R. Cyanotoxins: which detection technique for an optimum risk assessment? Water Res. 2017, 118, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Codd, G.A.; Beattie, K.A. Cyanobacteria (blue-green algae) and their toxins: Awareness and action in the United Kingdom. Public Health Lab. Serv. Microbiol. Dig. 1991, 8, 82–86. [Google Scholar]
- Zervou, S.-K.; Christophoridis, C.; Kaloudis, T.; Triantis, T.M.; Hiskia, A. New SPE-LC-MS/MS method for simultaneous determination of multi-class cyanobacterial and algal toxins. J. Hazard. Mater. 2017, 321, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Halinene, K.; Jokela, J.; Fewer, D.P.; Wahlsten, M.; Sivonen, K. Direct evidence for production of microcystins by anabaena strains from the baltic sea. Appl. Environ. Microbiol. 2007, 73, 6543–6550. [Google Scholar] [CrossRef] [PubMed]
- Pekar, H.; Westerberg, E.; Bruno, O.; Laane, A.; Persson, K.M.; Sundstrom, L.F.; Thim, A.-M. Fast, rugged and sensitive ultra high pressure liquid chromaotgrpahy tandem mass spectrometry method for analysis of cyanotoxins in raw water and drinking water—First findings of anatoxins, cylindrospermopsins and microcystin variants in Swedish source waters and infiltration ponds. J. Chromatogr. A 2016, 1429, 265–276. [Google Scholar] [PubMed]
- Met Office. Historical Data for UK Meteorological Stations. Published On-Line under Open Government Licence. 2017. Available online: https://data.gov.uk/dataset/historic-monthly-meteorological-station-data/resource/4c5c5210-f50d-4e0f-8af7-c5486e2b62da (accessed on 22 December 2017).
| Sample ID | Date Collected | Taxa | Total MCs (μg/L) | Total MCs (pg/Cell) * |
|---|---|---|---|---|
| 2 | 31/03/16 | Ap, Os | 22.7 | 45 |
| 3 | 20/04/16 | An | 611 | 1221 |
| P-1 | 12/05/16 | An | 4019 | na |
| 50 | 26/07/16 | M | 91 | 182 |
| 59 | 01/08/16 | M | 194 | 387 |
| 63 | 04/08/16 | Ap, M | 31 | 62 |
| 99 | 25/08/16 | Ap, M | 79 | 157 |
| 101 | 25/08/16 | M | 297 | 594 |
| 92 | 30/08/16 | An, Ap, M | 2561 | 5122 |
| 94 | 01/09/16 | Go, M | 89.9 | 180 |
| 98 | 02/09/16 | An, M | 21 | 42 |
| 103 | 07/09/16 | Ap, M | 103 | 206 |
| 111 | 12/09/16 | An, M | 244 | 488 |
| 114 | 12/09/16 | Go, M | 42724 | 85448 |
| 110 | 13/09/16 | An, Ap, M | 53 | 105 |
| 119 | 14/09/16 | An, Ap, M | 830 | 1661 |
| 134 | 20/10/16 | Ap, Go, Os | 279 | 558 |
| 139 | 31/10/16 | Go | 199 | 398 |
| Total Samples | M | An | Ap | Os | Go | Pl | Gl | At | Ly | Ps | Sn | Me | Gy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 48 | 40 | 33 | 25 | 14 | 5 | 5 | 2 | 1 | 2 | 1 | 1 | 1 | |
| No. MC detected | 43 | 22 | 20 | 13 | 13 | 1 | 2 | 1 | 1 | 1 | 0 | 0 | 0 |
| No. MC > 2 μg/L | 27 | 9 | 12 | 8 | 8 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| No. MC > 20 μg/L | 13 | 7 | 8 | 2 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| No. MC > 100 μg/L | 7 | 4 | 4 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turner, A.D.; Dhanji-Rapkova, M.; O’Neill, A.; Coates, L.; Lewis, A.; Lewis, K. Analysis of Microcystins in Cyanobacterial Blooms from Freshwater Bodies in England. Toxins 2018, 10, 39. https://doi.org/10.3390/toxins10010039
Turner AD, Dhanji-Rapkova M, O’Neill A, Coates L, Lewis A, Lewis K. Analysis of Microcystins in Cyanobacterial Blooms from Freshwater Bodies in England. Toxins. 2018; 10(1):39. https://doi.org/10.3390/toxins10010039
Chicago/Turabian StyleTurner, Andrew D., Monika Dhanji-Rapkova, Alison O’Neill, Lewis Coates, Adam Lewis, and Katy Lewis. 2018. "Analysis of Microcystins in Cyanobacterial Blooms from Freshwater Bodies in England" Toxins 10, no. 1: 39. https://doi.org/10.3390/toxins10010039
APA StyleTurner, A. D., Dhanji-Rapkova, M., O’Neill, A., Coates, L., Lewis, A., & Lewis, K. (2018). Analysis of Microcystins in Cyanobacterial Blooms from Freshwater Bodies in England. Toxins, 10(1), 39. https://doi.org/10.3390/toxins10010039






