An Appetite for Modernizing the Regulatory Framework for Protein Content Claims in Canada
Abstract
:1. Introduction
2. Summary of Current Regulatory Frameworks for Protein Content Claims in Canada and the USA
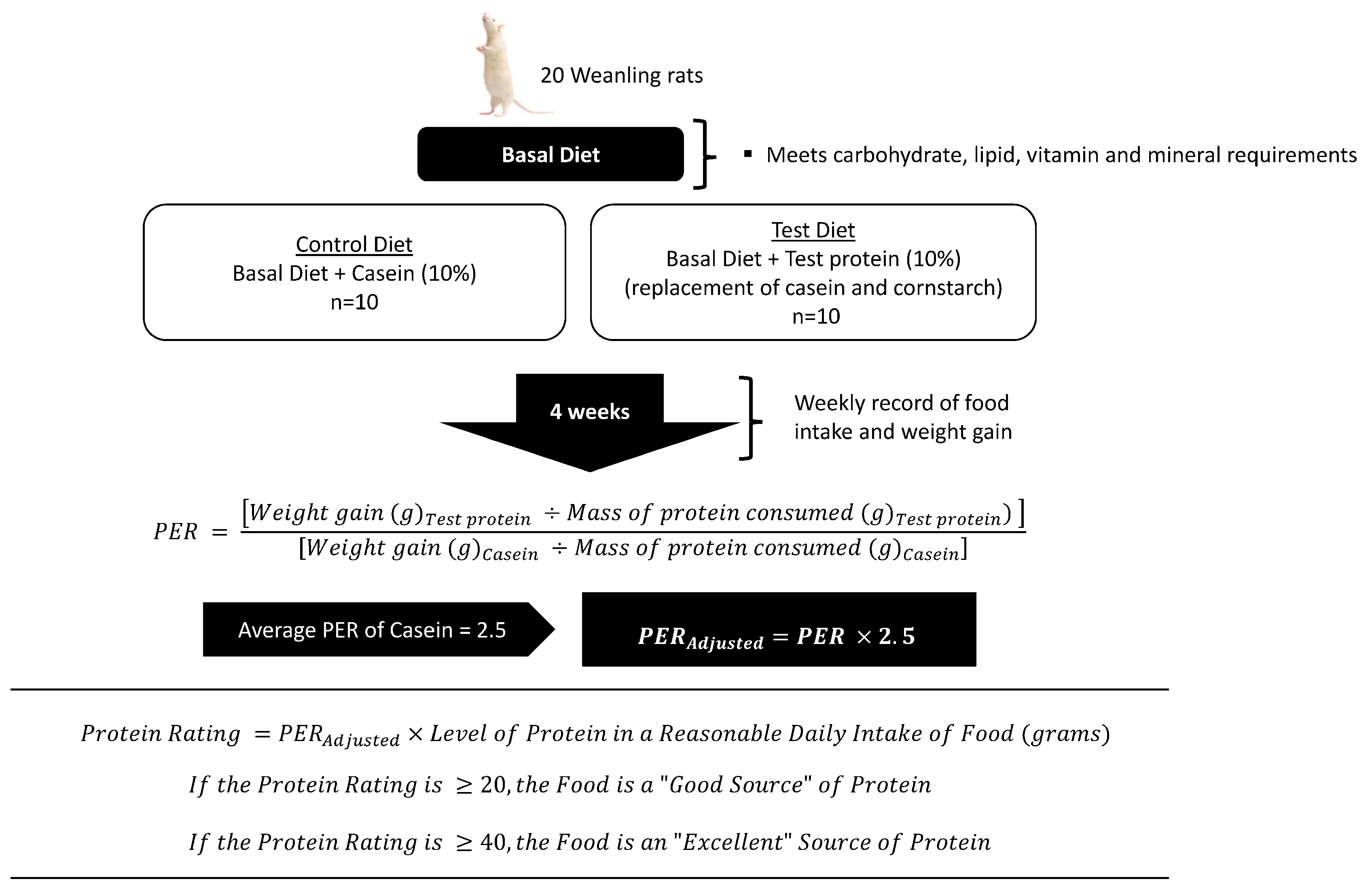
2.1. Summary of the PER and Protein Rating Methodologies for Supporting Protein Content Claims in Canada
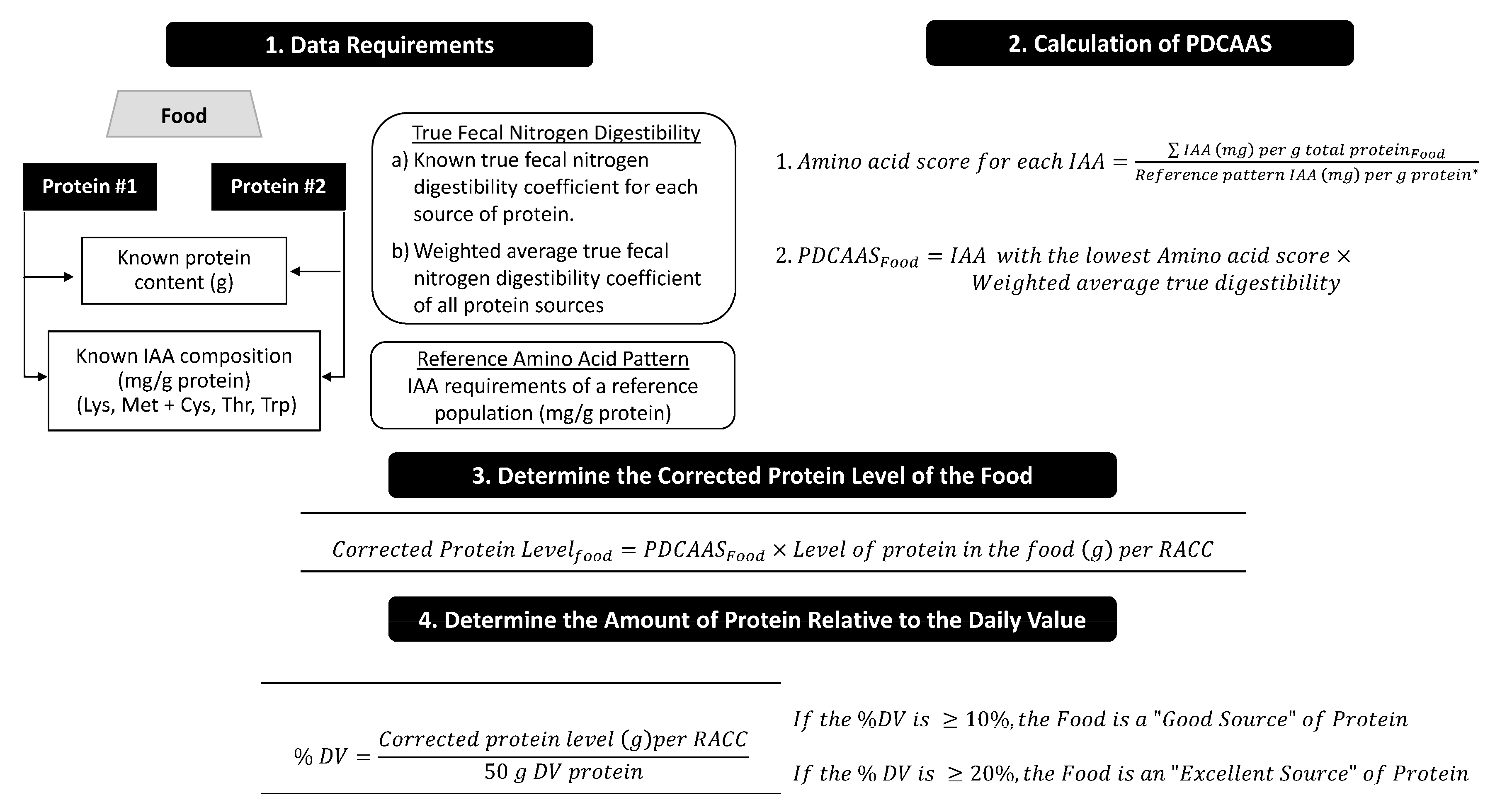
2.2. Summary of the PDCAAS Methodology for Supporting Protein Content Claims in the USA
3. Current Challenges Associated with using the PER and Protein Rating System to Support Protein Content Claims in Canada
3.1. Challenge 1: Methodological Assumptions of the PER
3.2. Challenge 2: PER Values Are Not Additive and Unestablished for New Foods
3.3. Challenge 3: The PER Is Combined with the RDI for Generating the Protein Rating of a Food
4. Proposed Regulatory Frameworks for Modernizing Regulations for Protein Content Claims in Canada
4.1. Option 1 (Preferred Option): Permit Protein Content Claims Made in Food Labeling and Advertising in Canada to Be Based on the Absolute Protein Content
4.2. Option 2 (Less Preferred): Adoption of PDCAAS as the Official Method of Protein Quality Assessment as Support for Protein Content Claims in Food Labeling and Advertising in Canada
- Adopt PDCAAS as the official methodology for assessing the quality of the protein in foods sold in Canada.
- Adopt 50 g as the DV for protein in Canada. This is the same threshold used in the USA for children ≥4 years of age through to adults [19].
- Base protein content claims in Canada on the proportion of quality protein (corrected by PDCAAS) found in a food relative to the DV for protein (50 g).
- Protein claims in Canada are currently based on RDIs. Rather than RDIs, it is suggested that protein content claims be based on reference amounts, which corresponds to RACCs in the USA. This permits protein quality and the corresponding claim to be directly applicable to how a food is consumed relative to the DV for protein. This also permits consumers to better quantify the contribution of protein in a food to daily protein requirements and aligns with the basis for other nutrient content claims, as well as how the DV is calculated for other Nutrition Facts Table.
- Permit the utilization of in vitro methodologies to determine the total protein digestibility of foods with unestablished PDCAAS values. In vitro determination of total protein digestibility values will expedite innovation and reformulation of food products. To permit the use of such methods, it is reasonable that Health Canada identify one or more acceptable methods for generating digestibility values in vitro as this would help limit variability and permit comparisons across foods.
- Adopt a three-tier claim framework for protein. The proposed thresholds are summarized in Table 3 (Option 2) relative to a 50 g DV for protein (see criteria 3). Under the current Canadian framework, “source of” and “good source of” protein claims are considered equivalent [16]. However, proposed Option 2 outlined in Table 3 aligns with requirements for nutrient content claims for dietary fibre, vitamins, and minerals where “source,” “good source,” and “excellent source” claims are of increasing magnitude [52]. Furthermore, a three-tier system creates more opportunities for the protein content in plant-based protein sources to be promoted as a nutritional attribute of the food. Under Option 2, minimum levels of corrected protein per reference amount for each tier of protein content claim would be 2.5 g, 5 g and 10 g protein, respectively. A threshold of ≥5 g protein/reference amount for a “good source” of claim and ≥10 g protein/reference amount for an “excellent source” claim aligns with the USA framework when a 50 g DV for protein is used to support protein claims for children ≥4 years of age through to adulthood [19]. A 2.5 g protein/reference amount limit was derived from the current Canadian framework where a 5% DV for vitamins, minerals, and fibre represents the threshold for “source of” claims [52].
- When two or more distinct foods are traditionally consumed together, it is proposed that the sum of their corrected protein levels can be used to calculate the %DV as support for a protein content claim. This consideration is already in use in the Canadian Food and Drug Regulations where the protein rating for breakfast cereal can be combined with the protein rating for 125 mL milk [16]. Again, Table 4 provides an example where the chickpea-based breakfast cereal from Table 1 would qualify for a “good source” of protein content claim under the proposed PDCAAS system outlined herein. Conversely, this same cereal would not qualify for the same claim within the current regulatory system using the PER and protein rating system.
- It is understood that protein requirements (mg/g protein) for a reference population used to calculate the PDCAAS of a food can change as data corresponding to specific age-sex groups continue to evolve. Therefore, the reference population and associated indispensable amino acid requirements (mg amino acid/g protein) should be incorporated by reference. This would permit Health Canada to make expedited changes to the regulatory framework for PDCAAS-based assessments of protein quality for foods.
5. Discussion
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Millward, D.J.; Layman, D.K.; Tome, D.; Schaafsma, G. Protein quality assessment: Impact of expanding understanding of protein and amino acid needs for optimal health. Am. J. Clin. Nutr. 2008, 87, 1576S–1581S. [Google Scholar] [PubMed]
- Kromhout, D.; Spaaij, C.J.; de Goede, J.; Weggemans, R.M. The 2015 Dutch food-based dietary guidelines. Eur. J. Clin. Nutr. 2016, 70, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Nordic Council of Ministries. Nordic Nutrition Recommendations 2012: Inegrating Nutrition and Physical Activity; Nordic Council of Ministries: Copenhagen, Denmark, 2012. [Google Scholar]
- US Department of Agriculture; US Department of Health and Human Services. Scientific Report of the 2015 Dietary Guidelines Advisory Committee; US Department of Agriculture and US Department of Health and Human Services: Washington, DC, USA, 2015.
- French Agency for Food Environmental and Occupational Health & Safety. Opinion of the French Agency for Food, Environmental and Occupational Health & Safety on the “Updating of the PNNS Guidelines: Revision of the Food-Based Dietary Guidelines”; Maison-Alfort: Paris, France, 2016. [Google Scholar]
- Aiking, H. Protein production: Planet, profit, plus people? Am. J. Clin. Nutr. 2014, 100, 483S–489S. [Google Scholar] [CrossRef] [PubMed]
- Soret, S.; Mejia, A.; Batech, M.; Jaceldo-Siegl, K.; Harwatt, H.; Sabate, J. Climate change mitigation and health effects of varied dietary patterns in real-life settings throughout North America. Am. J. Clin. Nutr. 2014, 100, 490S–495S. [Google Scholar] [CrossRef] [PubMed]
- Sabate, J.; Soret, S. Sustainability of plant-based diets: Back to the future. Am. J. Clin. Nutr. 2014, 100, 476S–482S. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations; World Health Organization. Protein Quality Evaluation: Report of the Joint FAO/WHO Expert Consultation, FAO Food and Nutrition: Paper 51; Food and Agriculture Organization of the United Nations; The World Health Organization: Rome, Italy, 1991. [Google Scholar]
- European Commission. Regulation (EC) No. 1924/2006 of the European Parliament and of the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods; No. 1924/2006; European Parliament and the Council of the European Union: Rome, Italy, 2006. [Google Scholar]
- Food Standards Australia New Zealand. Food Standards Code: 1.2.7 Nutrition, Health and Related Claims. In Standard 1.2.7, FSANZ, ed.; Federal Register of Legislative Instruments: Canberra, Australia, 2015. [Google Scholar]
- Ministry of Health(卫生部). National Standards of People’s Republic of China: National food safety standard, Standard for Nutrition Labeling of Prepackaged Foods (预包装食品营养标签通则). Available from National Health and Family Planning Commission (国家卫生与计划生育委员会). Available online: http://www.chinapop.gov.cn/sps/s7891/201111/d0c3c555e6c14aa7a3fa6306002223ba.shtml (accessed on 24 April 2017).
- South Korean Ministry of Food and Drug Safety (식품의약품안전처). Labeling Standards for Foods (식품등의 표시기준). Ministry of Food and Drug Safety (식품의약품안전처). Available from the National Law Information Center (국가법령정보센터). Available online: http://www.law.go.kr/admRulSc.do?menuId=1&p1=&subMenu=1&nwYn=1§ion=&tabNo=&query=#liBgcolor0 (accessed on 19 April 2017).
- Health Canada. Official Methods FO-1: Determination of Protein Quality; Health Protection Branch: Ottawa, ON, Canada, 1981.
- Marinangeli, C.P.F.; House, J.D. Potential impact of the digestible indispensable amino acid score as a measure of protein quality on dietary regulations and health. Nutr. Rev. 2017, in press. [Google Scholar] [CrossRef]
- Government of Canada. Food and Drug Regulations: Nutrient Content Claims, B.01.500 Table. Available online: http://laws-lois.justice.gc.ca/eng/regulations/c.r.c.,_c._870/page-25.html#h-45 (accessed on 15 December 2016).
- Government of Canada. Food and Drug Regulations: Schedule K—Reasonable Daily Intake for Various Foods. Available online: http://laws-lois.justice.gc.ca/eng/regulations/c.r.c.,_c._870/page-159.html#h-351 (accessed on 15 December 2016).
- The Canadian Food Inspection Agency. Food labeling for industry: Elements within the Nutrition Facts Table—Protein Government of Canada. Available online: http://www.inspection.gc.ca/food/labelling/food-labelling-for-industry/nutrition-labelling/elements-within-the-nutrition-facts-table/eng/1389206763218/1389206811747?chap=7 (accessed on 26 July 2016).
- Food and Drug Administration. Electronic Code of Federal Regulations: Title 21 Food and Drugs Part 101 Food Labeling. Available online: http://www.ecfr.gov/cgi-bin/retrieveECFR?gp=&SID=bad23c28ebd662323b3ace1e3f5ee94f&mc=true&n=pt21.2.101&r=PART&ty=HTML#se21.2.101_154 (accessed on 17 May 2016).
- Schaafsma, G. The Protein Digestibility-Corrected Amino Acid Score (PDCAAS)—A concept for describing protein quality in foods and food ingredients: A critical review. J. AOAC Int. 2005, 88, 988–994. [Google Scholar] [PubMed]
- Sarwar, G.; McDonough, F.E. Evaluation of protein digestibility-corrected amino acid score method for assessing protein quality of foods. J. Assoc. Off. Anal. Chem. 1990, 73, 347–356. [Google Scholar] [PubMed]
- Food and Agriculture Organization of the United Nations; World Health Organization; United Nations University. Protein and Amino acid Requirements in Human Nutrition: Report of a Joint WHO/FAO/UNU Expert Consultation Series 935; Food and Agriculture Organization of the United Nations; World Health Organization; United Nations University: Geneva, Switzerland, 2007. [Google Scholar]
- Canadian Food Inspection Agency. Food Labeling for Industry: Daily Intake—Reasonable Daily Intake for Various Foods. Canadian Food Inspection Agency. Available online: http://www.inspection.gc.ca/food/labelling/food-labelling-for-industry/nutrition-labelling/information-within-the-nutrition-facts-table/eng/1389198568400/1389198597278?chap=6 (accessed on 12 August 2016).
- Government of Canada. Table of Reference Amounts for Food. Available online: http://www.healthycanadians.gc.ca/eating-nutrition/label-etiquetage/regulatory-guidance-directives-reglementaires/reference-amounts-food-quantites-reference-aliments/index-eng.php (accessed on 8 April 2017).
- Government of Canada. Canadian Nutrient File: Milk, Fluid, Partly Skimmed, 2% M.F./(Food Code 61). Government of Canada. Available online: https://food-nutrition.canada.ca/cnf-fce/serving-portion.do?id=61 (accessed on 2 August 2017).
- Dworatzek, P.D.; Arcudi, K.; Gougeon, R.; Husein, N.; Sievenpiper, J.L.; Williams, S.L. Clinical Practice Guidelines: Nutrition Therapy. Can. J. Diabetes 2013, 37, S45–S55. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.J.; Gregoire, J.; Pearson, G.J.; Barry, A.R.; Couture, P.; Dawes, M.; Francis, G.A.; Genest, J., Jr.; Grover, S.; Gupta, M.; et al. Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia for the Prevention of Cardiovascular Disease in the Adult. Can. J. Cardiol. 2016, 32, 1263–1282. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Kendall, C.W.; Augustin, L.S.; Mitchell, S.; Sahye-Pudaruth, S.; Blanco Mejia, S.; Chiavaroli, L.; Mirrahimi, A.; Ireland, C.; Bashyam, B.; et al. Effect of legumes as part of a low glycemic index diet on glycemic control and cardiovascular risk factors in type 2 diabetes mellitus: A randomized controlled trial. Arch. Intern. Med. 2012, 172, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Wong, J.M.; Kendall, C.W.; Esfahani, A.; Ng, V.W.; Leong, T.C.; Faulkner, D.A.; Vidgen, E.; Paul, G.; Mukherjea, R.; et al. Effect of a 6-month vegan low-carbohydrate (‘Eco-Atkins’) diet on cardiovascular risk factors and body weight in hyperlipidaemic adults: A randomised controlled trial. BMJ Open 2014, 4, e003505. [Google Scholar] [CrossRef] [PubMed]
- Halton, T.L.; Willett, W.C.; Liu, S.; Manson, J.E.; Albert, C.M.; Rexrode, K.; Hu, F.B. Low-carbohydrate-diet score and the risk of coronary heart disease in women. N. Engl. J. Med. 2006, 355, 1991–2002. [Google Scholar] [CrossRef] [PubMed]
- Halton, T.L.; Liu, S.; Manson, J.E.; Hu, F.B. Low-carbohydrate-diet score and risk of type 2 diabetes in women. Am. J. Clin. Nutr. 2008, 87, 339–346. [Google Scholar] [PubMed]
- Appleby, P.N.; Key, T.J. The long-term health of vegetarians and vegans. Proc. Nutr. Soc. 2016, 75, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, M.A.; Salas-Salvado, J.; Estruch, R.; Corella, D.; Fito, M.; Ros, E.; Predimed, I. Benefits of the Mediterranean Diet: Insights From the PREDIMED Study. Prog. Cardiovasc. Dis. 2015, 58, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.A.; Wallentin, L.; Benatar, J.; Danchin, N.; Hagstrom, E.; Held, C.; Husted, S.; Lonn, E.; Stebbins, A.; Chiswell, K.; et al. Dietary patterns and the risk of major adverse cardiovascular events in a global study of high-risk patients with stable coronary heart disease. Eur. Heart J. 2016, 37, 1993–2001. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services; U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans, 8th ed. Available online: http://health.gov/dietaryguidelines/2015/guidelines/ (accessed on 4 February 2016).
- Mintel Group Ltd. The Protein Report: Meat Alternatives-US-January 2017. Mintel Group Ltd.: Chicago, IL, USA, 2017. [Google Scholar]
- Sanders, T.A. The nutritional adequacy of plant-based diets. Proc. Nutr. Soc. 1999, 58, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Clarys, P.; Deliens, T.; Huybrechts, I.; Deriemaeker, P.; Vanaelst, B.; De Keyzer, W.; Hebbelinck, M.; Mullie, P. Comparison of nutritional quality of the vegan, vegetarian, semi-vegetarian, pesco-vegetarian and omnivorous diet. Nutrients 2014, 6, 1318–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elorinne, A.L.; Alfthan, G.; Erlund, I.; Kivimaki, H.; Paju, A.; Salminen, I.; Turpeinen, U.; Voutilainen, S.; Laakso, J. Food and Nutrient Intake and Nutritional Status of Finnish Vegans and Non-Vegetarians. PLoS ONE 2016, 11, e0148235. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.A.; Rinaldi, S.; Scalbert, A.; Ferrari, P.; Achaintre, D.; Gunter, M.J.; Appleby, P.N.; Key, T.J.; Travis, R.C. Plasma concentrations and intakes of amino acids in male meat-eaters, fish-eaters, vegetarians and vegans: A cross-sectional analysis in the EPIC-Oxford cohort. Eur. J. Clin. Nutr. 2016, 70, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Marsh, K.A.; Munn, E.A.; Baines, S.K. Protein and vegetarian diets. Med. J. Aust. 2013, 199, S7–S10. [Google Scholar] [CrossRef] [PubMed]
- Codex Alimentarius Commission. Guidelines for Use of Nutrition and Health Claims: CAC/GL 23-1997. Available online: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCAC%2BGL%2B23-1997%252FCXG_023e.pdf (accessed on 9 December 2016).
- Codex Alimentarius Commission. Guidelines on Nutrition Labelling: CAC/GL 2-1985—ANNEX Adopted in 2011. Revision: 2013, 2015 and 2016; FAO; WHO: Rome, Italy, 2016. [Google Scholar]
- Health Canada. Do Canadian Adults Meet Their Nutrient Requirements through Food Intake Alone? Health Canada. Available online: http://www.hc-sc.gc.ca/fn-an/surveill/nutrition/commun/art-nutr-adult-eng.php (accessed on 3 January 2017).
- Health Canada. Do Canadian Children Meet Their Nutrient Requirements through Food Intake Alone? Health Canada. Available online: http://www.hc-sc.gc.ca/fn-an/surveill/nutrition/commun/art-nutr-child-enf-eng.php (accessed on 3 January 2017).
- Health Canada. Do Canadian Adolescents Meet Their Nutrient Requirements through Food Intake Alone? Health Canada. Available online: http://www.hc-sc.gc.ca/fn-an/surveill/nutrition/commun/art-nutr-adol-eng.php (accessed on 3 January 2017).
- Nosworthy, M.G.; House, J.D. Factors influencing the quality of dietary proteins: Implications for pulses. Cereal Chem. 2017, 94, 49–57. [Google Scholar] [CrossRef]
- Schaafsma, G. The protein digestibility-corrected amino acid score. J. Nutr 2000, 130, 1865S–1867S. [Google Scholar] [PubMed]
- Rozan, P.; Lamghari, R.; Linder, M.; Villaume, C.; Fanni, J.; Parmentier, M.; Méjean, L. In Vivo and in Vitro Digestibility of Soybean, Lupine, and Rapeseed Meal Proteins after Various Technological Processes. J. Agric. Food Chem. 1997, 45, 1762–1769. [Google Scholar] [CrossRef]
- Butts, C.A.; Monro, J.A.; Moughan, P.J. In vitro determination of dietary protein and amino acid digestibility for humans. Br. J. Nutr. 2012, 108, S282–S287. [Google Scholar] [CrossRef] [PubMed]
- Meunier, J.P.; Manzanilla, E.G.; Anguita, M.; Denis, S.; Perez, J.F.; Gasa, J.; Cardot, J.M.; Garcia, F.; Moll, X.; Alric, M. Evaluation of a dynamic in vitro model to simulate the porcine ileal digestion of diets differing in carbohydrate composition. J. Anim. Sci. 2008, 86, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Canadian Food Inspection Agency. Food Labeling for Industry: Nutrient Claims—Vitamin and Mineral Nutrient Claims. Canadian Food Inspection Agency. Available online: http://www.inspection.gc.ca/food/labelling/food-labelling-for-industry/nutrient-content/specific-claim-requirements/eng/1389907770176/1389907817577?chap=13 (accessed on 20 July 2017).
- Ministry of Health of Brazil. Dietary Guidelines for the Brazilian Population Secretariat of Health Care; Primary Health Care Department: Brasília, Brazil, 2014.
- Ruini, L.F.; Ciati, R.; Pratesi, C.A.; Marino, M.; Principato, L.; Vannuzzi, E. Working toward healthy and sustainable diets: The “Double Pyramid Model” developed by the Barilla Center for Food and Nutrition to raise awareness about the environmental and nutritional impact of foods. Front. Nutr. 2015, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- Canadian Food Inspection Agency. Food Labeling for Industry: Nutrient Claims—Protein. Canadian Food Inspection Agency. Available online: http://www.inspection.gc.ca/food/labelling/food-labelling-for-industry/nutrient-content/specific-claim-requirements/eng/1389907770176/1389907817577?chap=3 (accessed on 12 August 2016).
| Food | Description | RDI or RA | Protein (g) per RDI or RA | PER [18] | Protein Rating |
|---|---|---|---|---|---|
| Soy-based Breakfast cereal |
|
| 2.5 | 2.0 | 5.0 |
| 2.7 | 2.0 | 5.4 | |||
| 5.0 | 2.0 | 10.0 | |||
| Chickpea-based Breakfast cereal |
|
| 2.5 | 2.32 | 5.8 |
| 2.7 | 2.32 | 6.3 | |||
| 5.0 | 2.32 | 11.6 | |||
| Milk |
|
| 4.3 ¥ | 2.5 | 10.8 |
| Soy-based breakfast cereal + Milk |
|
| - | - | 16.2 (5.4 + 10.8) |
| - | - | 20.8 (10.0 + 10.8) | |||
| Chickpea-based breakfast cereal + Milk |
|
| - | - | 17.1 (6.3 + 10.8) |
| - | - | 22.4 (11.6 + 10.8) |
| Australia and New Zealand [11] | Europe [10] | Codex Alimentarius [42], China [12], and South Korea [13] |
|---|---|---|
| General Protein Claim ≥5 g protein/serving. | “Source” of Protein ≥12% of energy/serving. | “Source” of Protein * ≥10% the NRV * per 100 g (solids); or ≥5% the NRV * per 100 mL (liquids); or ≥5% the NRV * per 100 kcal (Codex and South Korea) or 420 kJ (China); or ≥10% the NRV * per serving (Codex and South Korea) |
| “Good Source” of Protein ≥10 g protein/serving. | “High Source” of Protein ≥20% energy/serving. | “High Source” of Protein At least 2x the level of protein that qualifies for a “source” claim. |
| Proposed Option 1: Removal of Protein Quality (Preferred) | Proposed Option 2: Adoption of PDCAAS (Less Preferred) | ||||
|---|---|---|---|---|---|
| Protein Content (g) per Reference Amount | Protein Content Claim | Corrected Protein Requirement per Reference Amount per DV * | Protein Content Claim | ||
| 5 g | “Good source” of protein | ≥5% DV |  | 2.5 g | “Source” of protein |
| 10 g | “Excellent source” of protein | ≥10% DV | 5 g | “Good source” of protein | |
| ≥20% DV | 10 g | “Excellent source” of protein | |||
| Example from Table 1: Chickpea-based Breakfast Cereal and Milk (2% MF) | ||
| ||
| ||
| ||
| Current Approach Using the PER † | Proposed Option 1: Removal of Protein Quality ɣ (Preferred) | Proposed Option 2: Adoption of PDCAAS in Canada ɣ (Less Preferred) |
| The food has a protein rating of 20 or more, as determined by official method FO-1, Determination of Protein Rating, October 15, 1981 ‡ (a) Per RDI; or (b) Per 30 g combined with 125 mL of milk, if the food is a breakfast cereal.
2. Protein Rating Milk 3. Total Protein Rating 4. Protein Claim Does not qualify for a protein claim |
2. Protein Claim Qualifies for a “good source” of protein claim |
2. Corrected Protein LevelMilk per RA 3. Total Corrected Protein LevelCereal + Milk 4. Protein Level Relative to the Proposed DV for Protein 5. Protein Claim Qualifies for a “good” source of protein claim |
| Food Innovation | Protein Ingredients | Current Canadian Framework * | Current USA Framework § | Proposed Framework for Canada | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein (g) per RDI † | PER ɣ | Protein Rating | Protein Claim (Y/N) | Protein (g) per RACC ‡ | PDCAAS | %DV | Protein Claim (Y/N) | Proposed Option 1: Removal of Protein Quality Ÿ (preferred) | Proposed Option 2: Adoption of PDCAAS ǂ (Less Preferred) | |||||
| Protein (g) per RA ¥ | Protein Claim (Y/N) | PDCAAS | %DV | Protein Claim (Y/N) | ||||||||||
| 1. Bread | Barley, Dry Navy Beans, cooked chickpeas, cooked lentils, yellow split peas, pinto beans, wheat gluten, soy protein, sunflower seed, whole wheat flour | 17.7 | 1.15 | 20.3 | Yes “Good Source” | 7.1 | 0.458 | 6.5 | No | 10.6 | Yes “Excellent Source” | 0.458 | 9.7 | Yes “Source” |
| 2. Breakfast Cereal (Low Density: 20 g to 42 g/250 mL) ¥ | Whole grain barley, whole grain wheat, pea protein concentrate | 5.3 | 1.68 | 9.3 | No | 7.4 | 0.671 | 9.9 | No | 5.5 | Yes “Good Source” | 0.671 | 7.4 | Yes “Source” |
| 125 mL Milk | (2% MF) | 4.3 | 2.5 | 6.8 | N/A | 4.3 | 1.00 | 8.6 | N/A | 4.3 | N/A | 1.00 | 8.6 | N/A |
| Breakfast Cereal + 125 mL Milk† | Whole grain barley, whole grain wheat, pea protein concentrate, milk (2% MF) | N/A | N/A | 16.1 | No | N/A | N/A | N/A | N/A | 9.8 | Yes “Good Source” | N/A | 16.0 | Yes “Good Source” |
| 3. Pancake Mix | Whole wheat flour, whey protein concentrate, pea protein, rice protein, soy flour, whole egg powder | 20.7 | 2.25 | 46.7 | Yes “Excellent Source” | 30.4 | 0.901 | 54.8 | Yes “Excellent Source” | 20.8 | Yes “Excellent Source” | 0.901 | 37.4 | Yes “Excellent Source” |
| 4. Tricolour Pasta | Semolina, pea protein | 12.6 | 1.37 | 17.3 | No | 8.1 | 0.549 | 8.9% | No | 8.1 | Yes “Good Source” | 0.549 | 8.9% | Yes “Source of” |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinangeli, C.P.F.; Foisy, S.; Shoveller, A.K.; Porter, C.; Musa-Veloso, K.; Sievenpiper, J.L.; Jenkins, D.J.A. An Appetite for Modernizing the Regulatory Framework for Protein Content Claims in Canada. Nutrients 2017, 9, 921. https://doi.org/10.3390/nu9090921
Marinangeli CPF, Foisy S, Shoveller AK, Porter C, Musa-Veloso K, Sievenpiper JL, Jenkins DJA. An Appetite for Modernizing the Regulatory Framework for Protein Content Claims in Canada. Nutrients. 2017; 9(9):921. https://doi.org/10.3390/nu9090921
Chicago/Turabian StyleMarinangeli, Christopher P. F., Samara Foisy, Anna K. Shoveller, Cara Porter, Kathy Musa-Veloso, John L. Sievenpiper, and David J. A. Jenkins. 2017. "An Appetite for Modernizing the Regulatory Framework for Protein Content Claims in Canada" Nutrients 9, no. 9: 921. https://doi.org/10.3390/nu9090921
APA StyleMarinangeli, C. P. F., Foisy, S., Shoveller, A. K., Porter, C., Musa-Veloso, K., Sievenpiper, J. L., & Jenkins, D. J. A. (2017). An Appetite for Modernizing the Regulatory Framework for Protein Content Claims in Canada. Nutrients, 9(9), 921. https://doi.org/10.3390/nu9090921





