Polyphenols from Root, Tubercles and Grains Cropped in Brazil: Chemical and Nutritional Characterization and Their Effects on Human Health and Diseases
Abstract
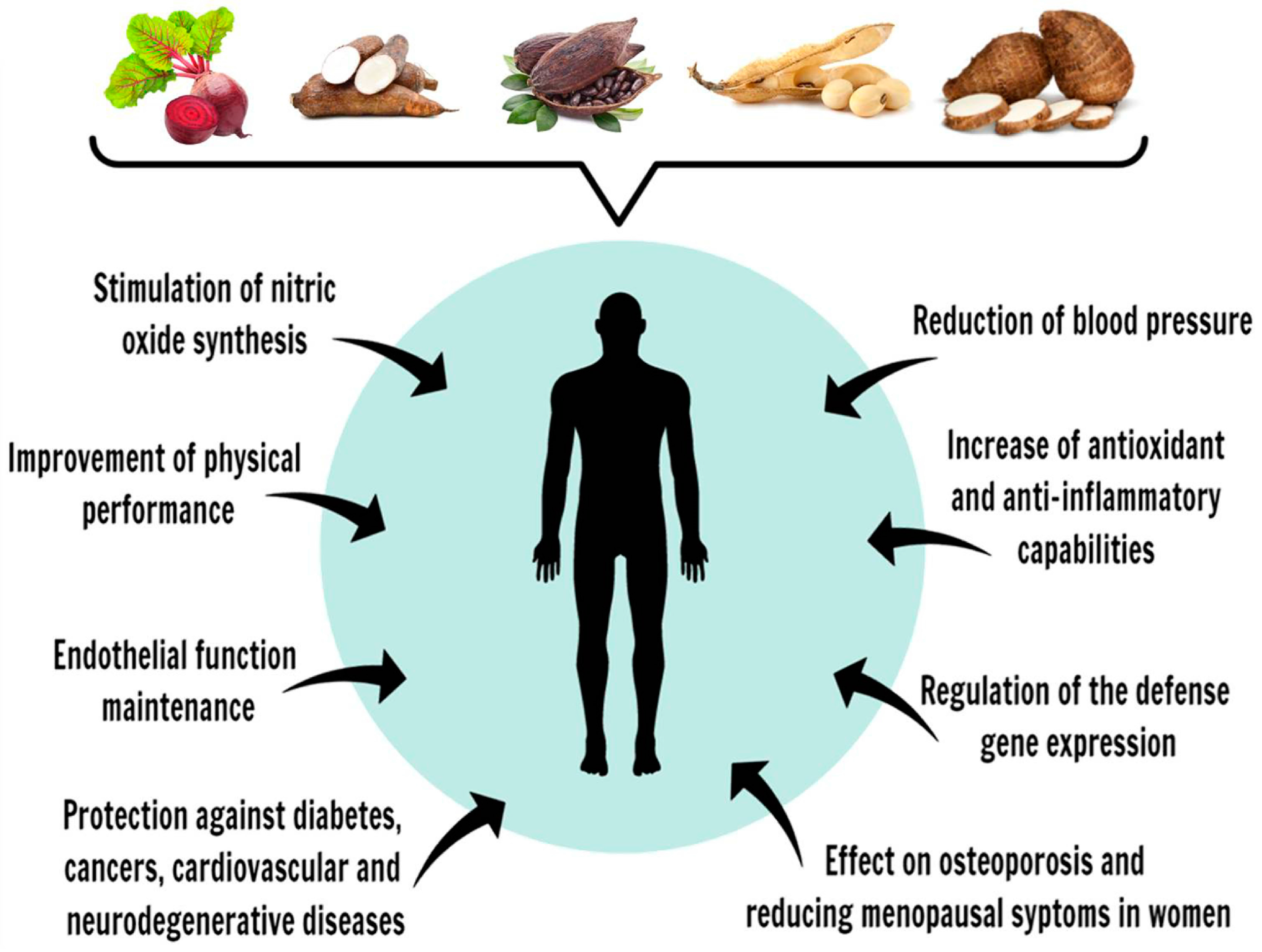
:1. Introduction
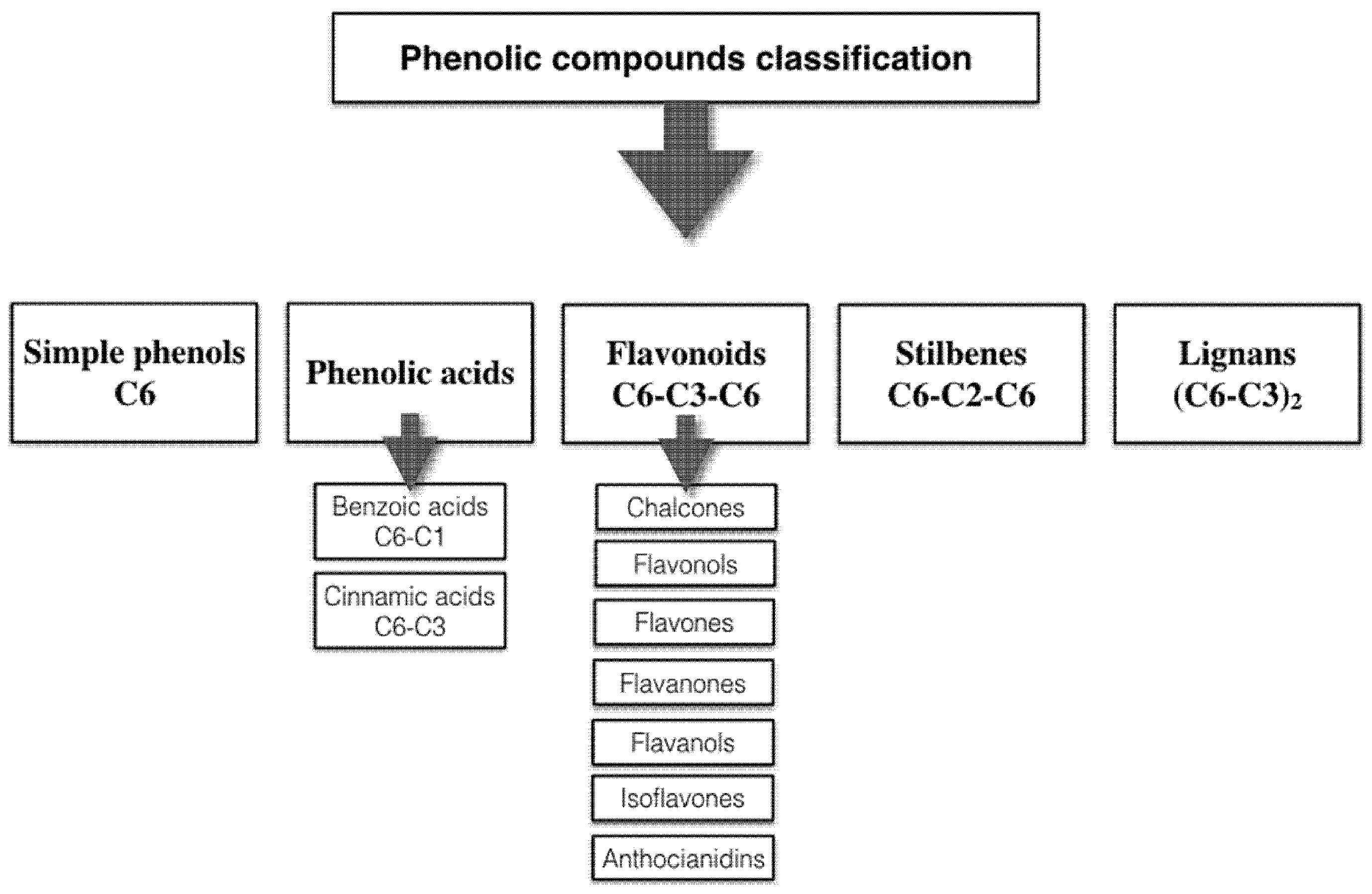
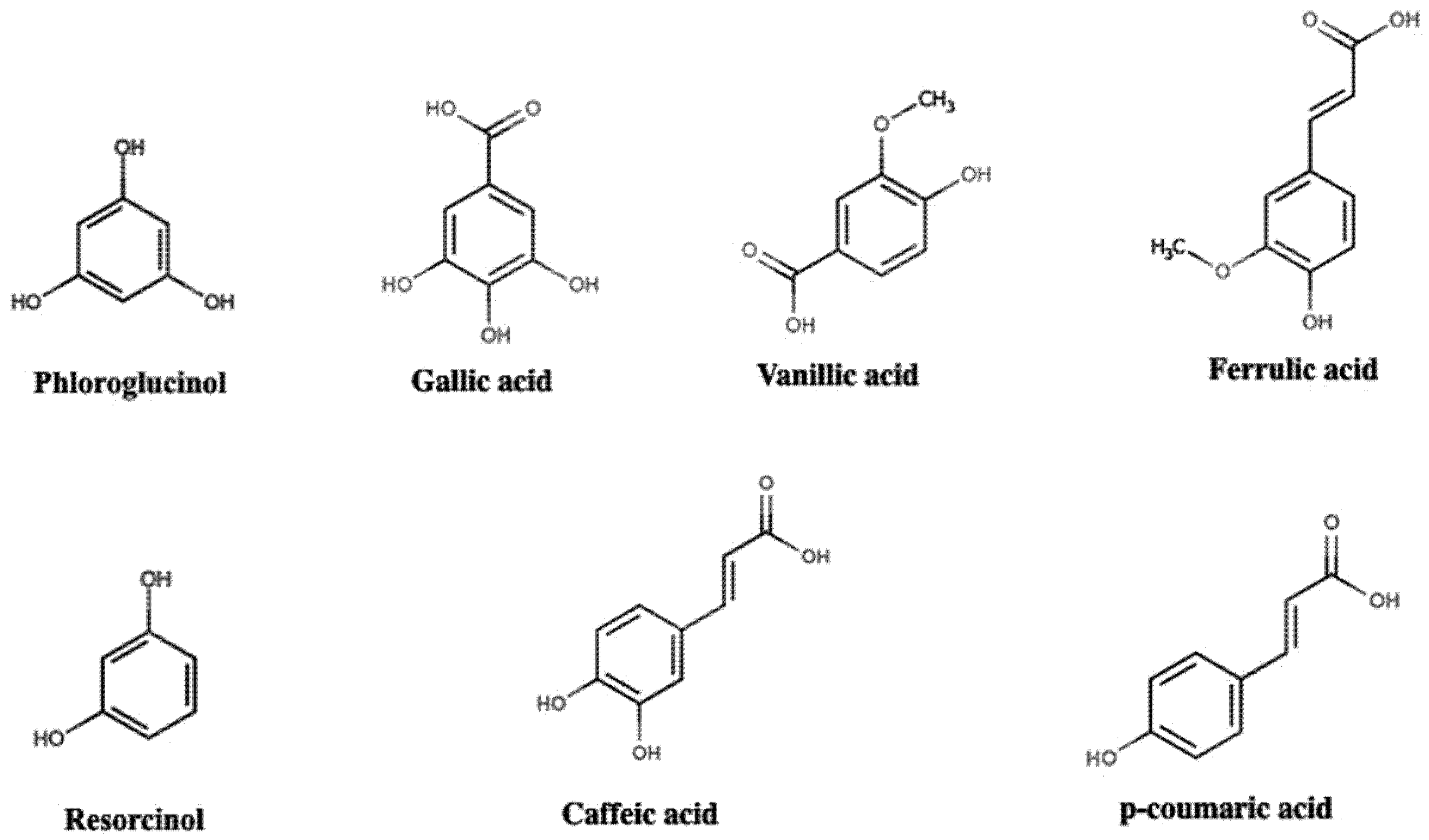
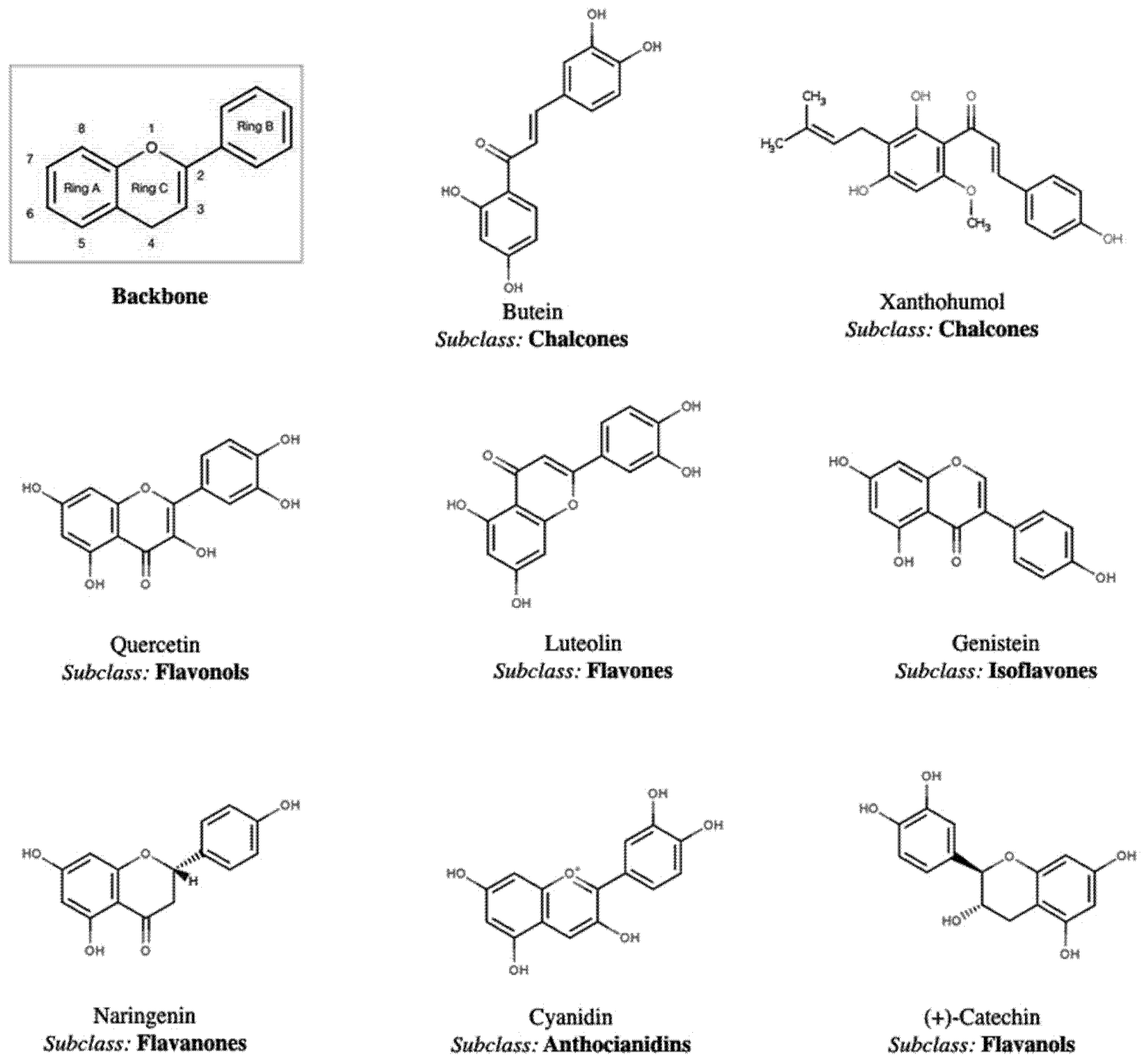
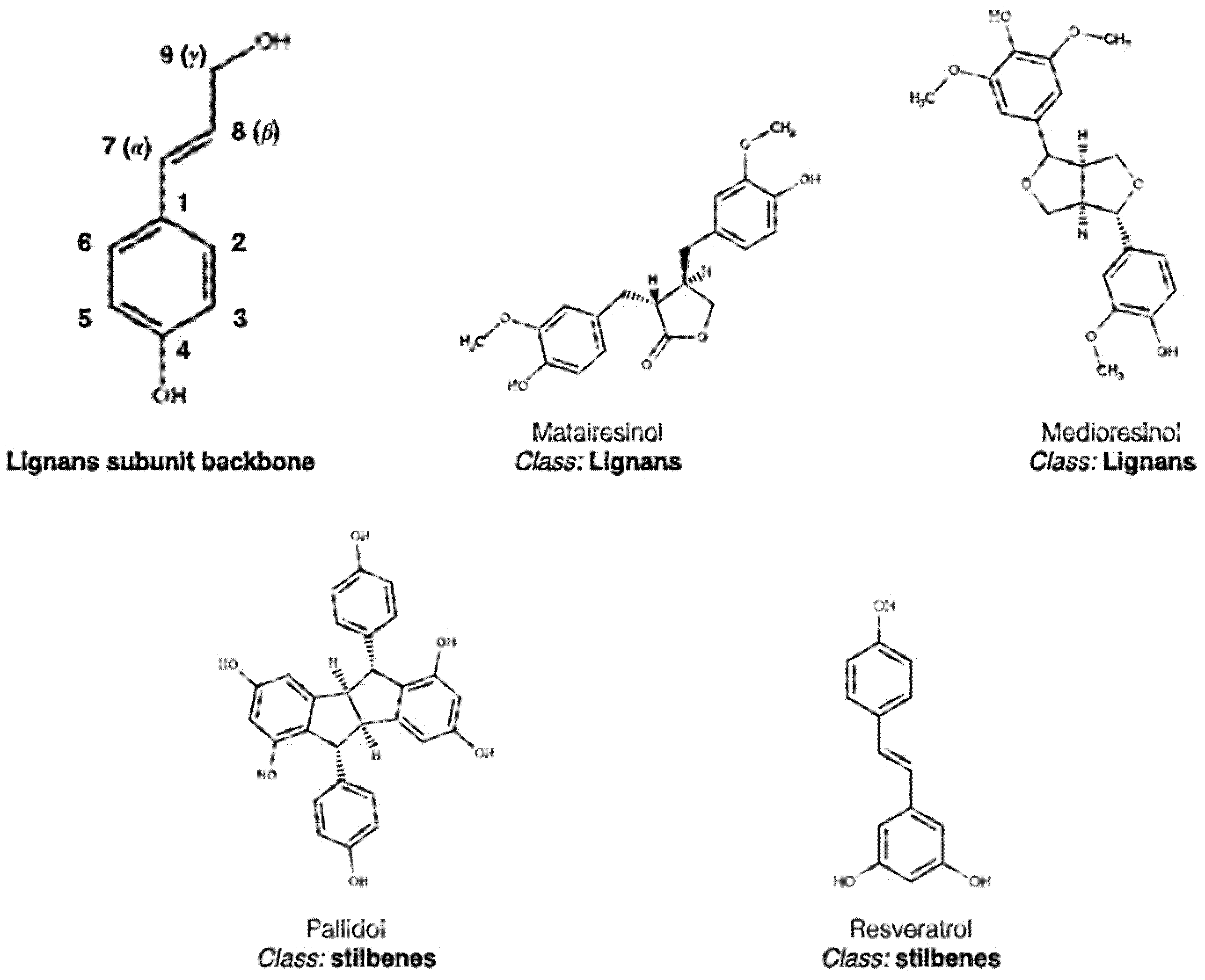
2. Polyphenol Structural Characterization and Classification
3. Bioavailability and Dietary Polyphenol Intake
4. Polyphenols and Their Cellular Effects
5. Vegetables Popularly Consumed in Brazil as Polyphenol Sources
5.1. Beetroot (Beta vulgaris sp.)
5.2. Cassava (Manihot esculenta)
5.3. Cocoa Beans (Theobroma cacao) and Cocoa-Based Products
5.4. Soybeans (Glycine max)
5.5. Taro (Colocasia esculenta)
6. Dietary Intake of Selected Plants or Their Polyphenols and Human Health in Diseases: Intracellular Targets and Molecular Mechanisms
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 richest dietary sources of polyphenols: An application of the phenol-explorer database. Eur. J. Clin. Nutr. 2010, 64, S112–S120. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Kagliwal, L.D.; Singhal, R.S. Biotransformation of polyphenols for improved bioavailability and processing stability. Adv. Food Nutr. Res. 2013, 69, 183–217. [Google Scholar] [PubMed]
- Perez-Hernandez, J.; Zaldivar-Machorro, V.J.; Villanueva-Porras, D.; Vega-Avila, E.; Chavarria, A. A potential alternative against neurodegenerative diseases: Phytodrugs. Oxid. Med. Cell Longev. 2016, 2016, 8378613. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V.; Tomas-Barberan, F.A.; Yoshida, K. Polyphenols: From Plants to a Variety of Food and Nonfood Uses. J. Agric. Food Chem. 2015, 63, 7589–7594. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Micek, A.; Godos, J.; Pajak, A.; Sciacca, S.; Galvano, F.; Giovannucci, E.L. Dietary Flavonoid and Lignan Intake and Mortality in Prospective Cohort Studies: Systematic Review and Dose-Response Meta-Analysis. Am. J. Epidemiol. 2017, 3, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Menezes, R.; Rodriguez-Mateos, A.; Kaltsatou, A.; González-Sarrías, A.; Greyling, A.; Giannaki, C.; Andres-Lacueva, C.; Milenkovic, D.; Gibney, E.R.; Dumont, J.; et al. Impact of Flavonols on Cardiometabolic Biomarkers: A Meta-Analysis of Randomized Controlled Human Trials to Explore the Role of Inter-Individual Variability. Nutrients 2017, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ouyang, Y.Y.; Liu, J.; Zhao, G. Flavonoid intake and risk of CVD: A systematic review and meta-analysis of prospective cohort studies. Br. J. Nutr. 2014, 111, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Zhan, J.; Liu, X.L.; Wang, Y.; Ji, J.; He, Q.Q. Dietary flavonoids intake and riskof type 2 diabetes: A meta-analysis of prospective cohort studies. Clin. Nutr. 2014, 33, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Vermerris, W.; Nicholson, R. Families of phenolic compounds and means of classification. In Phenolic Compound Biochemistry; Springer: Dordrecht, The Netherlands, 2006; pp. 1–34. [Google Scholar]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouysegu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. Engl. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Giada, M. Food phenolic compounds: Main classes, sources and their antioxidant power. In Oxidative Stress and Chronic Degenerative Diseases—A Role for Antioxidants, 1st ed.; Morales-Gonzále, J.A., Ed.; InTech: Rijeka, Croatia, 2013; pp. 87–112. [Google Scholar]
- Weichselbaum, E.; Buttriss, J. Polyphenols in the diet. Nutr. Bull. 2010, 35, 157–164. [Google Scholar] [CrossRef]
- Charrouf, Z.; Guillaume, D. Phenols and polyphenols from Argania spinosa. Am. J. Food Technol. 2007, 2, 679–683. [Google Scholar]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Neveu, V.; Perez-Jimenez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D. Phenol-explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.M.; Valentão, P.; Pereira, J.A.; Andrade, P.B. Phenolics: From chemistry to biology. Molecules 2009, 14, 2202–2211. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [PubMed]
- Başkan, K.S.; Tütem, E.; Akyüz, E.; Apak, R. Assessment of the contributions of anthocyanins to the total antioxidant capacities of plant foods. Eur. Food Res. Technol. 2015, 241, 529–541. [Google Scholar] [CrossRef]
- Araújo, M.M.; Fanaro, G.B.; Villavicencio, A.L.C.H. Soybean and isoflavones—From farm to fork. In Soybean-Bio-Active Compounds; El-Shemy, H.A., Ed.; InTech: Rijeka, Croatia, 2013; pp. 2–21. [Google Scholar]
- D’Archivio, D.M.; Filesi, C.; Di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, dietary sources and bioavailability. Ann. Ist. Super. Sanita 2007, 43, 348–361. [Google Scholar] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18 Pt B, 820–897. [Google Scholar] [CrossRef]
- Wallace, T.C.; Giusti, M.M. Anthocyanins. Adv. Nutr. 2015, 6, 620–622. [Google Scholar] [CrossRef] [PubMed]
- Ferrazzano, G.F.; Amato, I.; Ingenito, A.; Zarrelli, A.; Pinto, G.; Pollio, A. Plant polyphenols and their anti-cariogenic properties: A review. Molecules 2011, 16, 1486–1507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajila, C.; Brar, S.; Verma, M.; Tyagi, R.; Godbout, S.; Valero, J. Extraction and analysis of polyphenols: Recent trends. Crit. Rev. Biotechnol. 2011, 31, 227–249. [Google Scholar] [CrossRef] [PubMed]
- Tomas-Barberan, F.A.; Andres-Lacueva, C. Polyphenols and health: Current stateand progress. J. Agric. Food Chem. 2012, 60, 8773–8775. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Davalos, A. Polyphenols and cardiovascular disease: A critical summary of the evidence. Mini Rev. Med. Chem. 2011, 11, 1186–1190. [Google Scholar] [PubMed]
- Miranda, A.M.; Steluti, J.; Fisberg, R.M.; Marchoni, D.M. Dietary intake and food contributors of polyphenols in adults and elderly adults of Sao Paulo: A population-based study. Br. J. Nutr. 2016, 115, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Nascimento-Souza, M.A.; de Paiva, P.G.; Pérez-Jiménez, J.; Franceschini, S.D.C.C.; Ribeiro, A.Q. Estimated dietary intake and major food sources of polyphenols in elderly of Viçosa, Brazil: A population-based study. Eur. J. Nutr. 2016, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Marventano, S.; Mistretta, A.; Galvano, F.; Grosso, G. Dietary sources of polyphenols in the Mediterranean healthy Eating, Aging and Lifestyle (MEAL) study cohort. Int. J. Food Sci. Nutr. 2017, 68, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Vogiatzoglou, A.; Mulligan, A.A.; Lentjes, M.A.; Luben, R.J.; Spencer, J.P.; Schroeter, H.; Khaw, K.T.; Kuhnle, G.G. Flavonoid intake in European adults (18 to 64 years). PLoS ONE 2015, 10, e0128132. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.; Shin, S.; Joung, H. Estimation of dietary flavonoid intake and major food sources of Korean adults. Br. J. Nutr. 2016, 115, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Kanimozhi, S.; Bhavani, P.; Subramanian, P. Influence of the flavonoid, quercetin on antioxidant status, lipid peroxidation and histopathological changes in hyperammonemic rats. Indian J. Clin. Biochem. 2017, 32, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Peng, W. Biological activity of natural flavonoids as impacted by protein flexibility: An example flavanones. Mol. Biosyst. 2015, 11, 1119–1133. [Google Scholar] [CrossRef] [PubMed]
- Perira-Caro, G.; Borges, G.; van der Hooft, J.; Clifford, M.N.; Del Rio, D.; Lean, M.E.; Roberts, S.A.; Kellerhals, M.B.; Crozier, A. Orange juice (poly)phenols are highly bioavailable in humans. Am. J. Clin. Nutr. 2014, 100, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Maliakal, P.; Chen, L.; Meng, X.; Bondoc, F.Y.; Prabhu, S.; Lambert, G.; Mohr, S.; Yang, C.S. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: Formation of different metabolites and individual variability. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1025–1032. [Google Scholar]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Mishra, N.; Rizvi, S.I. Protective role of myricetin on markers of oxidative stress in human erythrocytes subjected to oxidative stress. Nat. Prod. Commun. 2009, 4, 221–226. [Google Scholar] [PubMed]
- Yildrim, A.B.; Guner, B.; Karakas, F.P.; Turjer, A.U. Evaluation of antibacterial, antitumor, antioxidant activities and phenolic constituents of field-grown and in vitro- grown Lysimachia vulgaris. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant Activity of Polyphenols, Flavonoids, Anthocyanins and Carotenoids: Updated Review of Mechanisms and Catalyzing Metals. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kaur, M.; Silakari, O. Flavones: An important scaffold for medicinal chemistry. Eur. J. Med. Chem. 2014, 84, 206–239. [Google Scholar] [CrossRef] [PubMed]
- Taofiq, O.; González-Paramás, A.M.; Barreiro, M.F.; Ferreira, I.C. Hydroxycinnamic acids and their derivatives: Cosmeceutical significance, challenges and future perspectives, a Review. Molecules 2017, 22, 281. [Google Scholar] [CrossRef] [PubMed]
- Pluemsamran, T.; Onkoksoong, T.; Panich, U. Caffeic acid and ferulic acid inhibit UVA-induced matrixmetalloproteinase-1 through regulation of antioxidant defense system in keratinocyte HaCaT cells. Photochem. Photobiol. 2012, 88, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Belščak-Cvitanović, A.; Komes, D.; Dujmović, M.; Karlović, S.; Biškić, M.; Brnčić, M.; Ježek, D. Physical, bioactive and sensory quality parameters of reduced sugar chocolates formulated with natural sweeteners as sucrose alternatives. Food Chem. 2015, 167, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Holst, B.; Williamson, G. Nutrients and phytochemicals: From bioavailability to bioefficacy beyond antioxidants. Curr. Opin. Biotechnol. 2008, 19, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yoshioka, H.; Kim, G.S.; Jung, J.E.; Okami, N.; Sakata, H.; Maier, C.M.; Narasimhan, P.; Goeders, C.E.; Chan, P.H. Oxidative stress in ischemic brain damage: Mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid. Redox Signal. 2011, 14, 1505–1517. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.N.; Arthur, K.N.; Orlich, M.J.; James, W.; Purty, A.; Job, J.S.; Rajaram, S.; Sabaté, J. Global epidemiology of obesity, vegetarian dietary patterns, and noncommunicable disease in Asian Indians. Am. J. Clin. Nutr. 2014, 100, 359S–364S. [Google Scholar] [CrossRef] [PubMed]
- Faller, A.L.K.; Fialho, E. The antioxidant capacity and polyphenol content of organic and conventional retail vegetables after domestic cooking. Food Res. Int. 2009, 42, 210–215. [Google Scholar] [CrossRef]
- IBGE 2017—Produção Agrícola Municipal, 2017. Brazilian Agricultural Research Corporation. Ministry of Agriculture, Livestock, and Food Supply. Available online: http://www.ibge.gov.br/home/estatistica/indicadores/agropecuaria/ (accessed on 16 September 2017).
- Kujala, T.S.; Vienola, M.S.; Klika, K.D.; Loponen, J.M.; Pihlaja, K. Betalain and phenolic compositions of four beetroot (Beta vulgaris) cultivars. Eur. Food Res. Technol. 2002, 214, 505–510. [Google Scholar] [CrossRef]
- Wruss, J.; Waldenberger, G.; Huemer, S.; Uygun, P.; Lanzerstorfer, P.; Müller, U.; Höglinger, O.; Weghuber, J. Compositional characteristics of commercial beetroot products and beetroot juice prepared from seven beetroot varieties grown in Upper Austria. J. Food Compos. Anal. 2015, 42, 46–55. [Google Scholar] [CrossRef]
- Uarrota, V.G.; Maraschin, M. Metabolomic, enzymatic, and histochemical analyzes of cassava roots during postharvest physiological deterioration. BMC Res. Notes 2015, 5, 648–663. [Google Scholar] [CrossRef] [PubMed]
- Gogbeu, S.J.; Dogbo, D.O.; Gonnety, J.T.; N’zue, B.; Zohouri, G.P.; Boka, A. Study of some characteristics of soluble polyphenol oxidases from six cultivars callus of cassava (Manihot esculenta Crantz). J. Anim. Plant Sci. 2011, 9, 1169–1179. [Google Scholar]
- Kubo, I.; Masuoka, N.; Nihei, K.; Brigitta, B. Maniçoba, a quercetin-rich Amazonian dish. J. Food Compos. Anal. 2006, 19, 579–588. [Google Scholar] [CrossRef]
- Bayoumi, A.L.; Rowan, M.G.; Beeching, J.R.; Blagbrough, I.S. Investigation of biosynthetic pathways to hydroxycoumarins during post-harvest physiological deterioration in cassava roots by using stable isotope labelling soad. ChemBioChem 2008, 9, 3013–3022. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, H.; Rodriguez, M.X.; Tohme, J.; Beeching, J.R. Accumulation of hydroxycoumarins during post-harvest deterioration of tuberous roots of cassava (Manihot esculenta Crantz). Ann. Bot. 2000, 86, 1153–1160. [Google Scholar] [CrossRef]
- Uarrota, V.G.; Nunes, E.C.; Peruch, L.A.; Neubert Ede, O.; Coelho, B.; Moresco, R.; Domínguez, M.G.; Sánchez, T.; Meléndez, J.L.; Dufour, D.; et al. Toward better understanding of postharvest deterioration: Biochemical changes in stored cassava (Manihot esculenta Crantz) roots. Food Sci. Nutr. 2015, 4, 409–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, B.; Hu, L.; Mei, W.; Zhou, K.; Wang, H.; Luo, Y.; Wei, X.; Dai, H. Antioxidant phenolic compounds of cassava (Manihot esculenta) from Hainan. Molecules 2011, 16, 10157–10167. [Google Scholar] [CrossRef] [PubMed]
- Cheeke, P.R.; Piacente, S.; Oleszek, W. Anti-inflammatory and anti-arthritic effects of Yucca schidigera: A review. J. Inflamm. 2006, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Efraim, P.; Alves, A.B.; Jardim, D.C.P. Polyphenols in cocoa and derivatives: Factors of variation and health effects. Braz. J. Food Technol. 2011, 14, 181–201. [Google Scholar] [CrossRef]
- Khan, N.; Khymenets, O.; Urpí-Sardà, M.; Tulipani, S.; Garcia-Aloy, M.; Monagas, M.; Mora-Cubillos, X.; Llorach, R.; Andres-Lacueva, C. Cocoa polyphenols and inflammatory markers of cardiovascular disease. Nutrients 2014, 6, 844–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega, N.; Romero, M.P.; Macià, A.; Reguant, J.; Anglès, N.; Morelló, J.R.; Motilva, M.J. Obtention and characterization of phenolic extracts from different cocoa sources. J. Agric. Food Chem. 2008, 56, 9621–9627. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Xu, B. A critical review on polyphenols and health benefits of black soybeans. Nutrients 2017, 9, 455. [Google Scholar] [CrossRef] [PubMed]
- Riedl, K.M.; Lee, J.H.; Renita, M.; St Martin, S.K.; Schwartz, S.J.; Vodovotz, Y. Isoflavone profiles, phenol content, and antioxidant activity of soybean seeds as influenced by cultivar and growing location in Ohio. J. Sci. Food Agric. 2007, 87, 1197–1206. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, B.W.; Kim, B.; Kim, H.T.; Ko, J.M.; Baek, I.Y.; Seo, T.W.; Kang, M.Y.; Cho, K.M. Changes in phenolic compounds (isoflavones and phenolic acids) and antioxidant properties in high-protein soybean (Glycine max L., cv. Saedanbaek) for different roasting conditions. J. Korean Soc. Appl. Biol. Chem. 2013, 56, 605–612. [Google Scholar] [CrossRef]
- United States Department of Agriculture (USDA). USDA Database for the Flavonoid Content of Selected Foods. 2013. Available online: https://www.ars.usda.gov/ARSUserFiles/80400525/Data/Flav/Flav3-1.pdf (accessed on 5 May 2017).
- Baião, D.S.; Conte-Junior, C.A.; Paschoalin, V.M.F.; Alvares, T.S. Quantitative and comparative contents of nitrate and nitrite in Beta vulgaris L. by reversed-phase high-performance liquid chromatography-fluorescence. Food Anal. Methods 2016, 9, 1002–1008. [Google Scholar] [CrossRef]
- Van Velzen, A.G.; Sips, A.J.; Schothorst, R.C.; Lambers, A.C.; Meulenbelt, J. The oral bioavailability of nitrate from nitrate-rich vegetables in humans. Toxicol. Lett. 2008, 181, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Váli, L.; Stefanovits-Bányai, E.; Szentmihályi, K.; Fébel, K.; Sárdi, E.; Lugasi, A.; Kocsis, I.; Blázovics, A. Liver-protecting effects of table beet (Beta vulgaris var. rubra) during ischemia-reperfusion. Nutrition 2007, 23, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Kanner, J.; Harel, S.; Granit, R. Betalains—A new class of dietary cationized antioxidants. J. Agric. Food Chem. 2001, 49, 5178–5185. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [PubMed]
- Asami, D.K.; Hong, Y.J.; Barrett, D.M.; Mitchell, A.E. Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried marionberry, strawberry, and corn grown using conventional, organic, and sustainable agricultural practices. J. Agric. Food Chem. 2003, 51, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Čanadanović-Brunet, J.M.; Savatović, S.S.; Ćetković, G.S.; Vulić, J.J.; Djilas, S.M.; Markov, S.L.; Cvetković, D.D. Antioxidant and antimicrobial activities of beet root pomace extracts. Czech J. Food Sci. 2011, 6, 575–585. [Google Scholar]
- Kavalcová, P.; Bystrická, J.; Tomáš, J.; Karoičová, J.; Kovarovič, J.; Lenková, M. The content of total polyphenols and antioxidante activity in red beetroot. Potravinarstvo 2015, 9, 77–83. [Google Scholar]
- Wootton-Beard, P.C.; Ryan, L. A beetroot juice shot is a significant and convenient source of bioaccessible antioxidants. J. Funct. Foods 2011, 3, 329–334. [Google Scholar] [CrossRef]
- Baião, D.S.; Conte-Junior, C.A.; Paschoalin, V.M.F.; Alvares, T.S. Beetroot juice increase nitric oxide metabolites in both men and women regardless of body mass. Int. J. Food Sci. Nutr. 2016, 67, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Vasconcellos, J.; Paschoalin, V.M.F.; Conte-Junior, C.A.; Alvares, T.S. Comparison of total antioxidant potential, and total phenolic, nitrate, sugar, and organic acid contents in beetroot juice, chips, powder, and cooked beetroot. Food Sci. Biotechnol. (Seoul) 2016, 25, 79–84. [Google Scholar] [CrossRef]
- Da Silva, D.V.T.; Silva, F.O.; Moreira, D.P.; Pierucci, A.P.R.T.; Conte-Junior, C.A.; Silveira, T.A.; Del Aguila, E.M.; Paschoalin, V.M.F. Physicochemical, nutritional and sensory analyses of a nitrate-enriched beetroot gel and its effects on plasmatic nitric oxide and blood pressure. Food Nutr. Res. 2016, 60, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Baião, D.S.; da Silva, D.V.; Del Aguila, E.M.; Paschoalin, V.M.F. Nutritional, bioactive and physicochemical characteristics of different beetroot formulations. In Food Additives; InTech: Rijeka, Croatia, 2017; pp. 1–24. [Google Scholar]
- Montagnac, J.A.; Davis, C.R.; Tanumihardjo, S.A. Nutritional value of cassava for use as a staple food and recent advances for improvement. Compr. Rev. Food Sci. Food Saf. 2009, 8, 181–194. [Google Scholar] [CrossRef]
- Pandey, A.; Soccol, C.R.; Nigam, P.; Soccol, V.T. Biotechnological potential of agro-industrial residues. II: Cassava bagasse. Bioresour. Technol. 2000, 74, 81–87. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). A Review of Cassava in Latin America and the Caribbean with Country Case Studies on Brazil and Colombia; FAO: Rome, Italy, 2004; Volume 4. [Google Scholar]
- Alvarado, P.M.; Grosmaire, L.; Dufour, D.; Toro, A.G.; Sánchez, T.; Calle, F.; Santander, M.A.M.; Ceballos, H. Combined effect of fermentation, sun-drying and genotype on bread making ability of sour cassava starch. Carbohydr. Polym. 2013, 98, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Rebouças, K.H.; Gomes, L.P.; Leite, A.M.O.; Uekane, T.M.; Rezende, C.M.; Tavares, M.I.B.; Almeida, E.L.; Del Aguila, E.M.; Paschoalin, V.M.F. Evaluating physicochemical and rheological characteristics and microbial community dynamics during the natural fermentation of cassava starch. J. Food Process. Technol. 2016, 7, 4–11. [Google Scholar] [CrossRef]
- Oladunmoye, O.O.; Aworh, O.C.; Maziya-Dixon, B.; Erukainure, O.L.; Elemo, G.N. Chemical and functional properties of cassava starch, durum wheat semolina flour, and their blends. Food Sci. Nutr. 2014, 2, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.C.; Dudey, R.; Richa, T.; Meeta, M.; Advani, U. Medicinal bioactives as antimicrobial agents: An overview. Int. J. Pharm. Dev. 2011, 3, 24–30. [Google Scholar]
- Ferreres, F.; Gonçalves, R.F.; Gil-Izquierdo, A.; Valentão, P.; Silva, A.M.; Silva, J.B.; Santos, D.; Andrade, P.B. Further knowledge on the phenolic profile of Colocasia esculenta (L.) Shott. J. Agric. Food Chem. 2012, 60, 7005–7015. [Google Scholar] [CrossRef] [PubMed]
- Bahekar, S.; Kale, R. Phytopharmacological aspects of Manihot esculenta Crantz (cassava)—A Review. Mintage J. Pharm. Med. Sci. 2013, 2, 4–5. [Google Scholar]
- Adam, S.I.Y.; Ahmed, W.A.A.; Esra, O.M.; Nazik, E.Y.; Eiman, F.A.; Shaimaa, A.A.; Abdelgadir, W.S. Antimcrobial activity of Manihot esculenta root methanolic and aqueous extracts. Eur. J. Biomed. Pharm. Sci. 2014, 1, 390–401. [Google Scholar]
- Sakai, T.; Nakagawa, Y. Diterpenic stress metabolites from cassava roots. Phytochemistry 1988, 27, 3769–3779. [Google Scholar] [CrossRef]
- Uarrota, V.G.; Moresco, R.; Coelho, B.; da Nunes, E.C.; Peruch, L.A.; Neubert, O.; Rocha, M.; Maraschin, M. Metabolomics combined with chemometric tools (PCA, HLA, PLS-DA and SVM) for screening cassava (Manihot esculenta, Crantz) roots during postharvest physiological deterioration. Food Chem. 2014, 161, 67–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rickard, J.E. Biochemical changes involved in the postharvest deterioration of cassava roots. Trop. Sci. 1981, 23, 235–237. [Google Scholar]
- Uarrota, V.G.; Nunes, E.C.; Peruch, L.A.M.; Neubert, E.O.; Coelho, B.; Moresco, R.; Ceballos, H. Toward better understanding of postharvest deterioration: Biochemical changes in stored cassava (Manihot esculenta Crantz) roots. Food Sci. Nutr. 2016, 4, 409–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oleszek, W.; Sitek, M.; Stochmal, A.; Piacente, S.; Pizza, C.; Cheeke, P. Resveratrol and other phenolics from the bark of Yucca schidigera Roezl. J. Agric. Food Chem. 2001, 49, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Piacente, S.; Montoro, P.; Oleszek, W.; Pizza, C. Yucca schidigera bark: Phenolic constituents and antioxidant activity. J. Nat. Prod. 2004, 67, 882–885. [Google Scholar] [CrossRef] [PubMed]
- Fasuyi, A.O. Nutrient composition and processing effects on cassava leaf (Manihot esculenta, Crantz) antinutrients. Pak. J. Nutr. 2005, 4, 37–42. [Google Scholar]
- Okeke, C.U.; Iweala, E. Antioxidant profile of Dioscorea rotundata, Manihot esculenta, Ipoemea batatas, Vernonia amygdalina and Aloe vera. Med. Res. Technol. 2007, 4, 4–10. [Google Scholar]
- Bradbury, J.H.; Holloway, W.D. Chemistry of Tropical Root Crops: Significance for Nutrition and Agriculture in Pacific; ACIAR: Canberra, Australia, 1988. [Google Scholar]
- Barthet, V.J. Polyphenol Oxidases from Cassava (Manihot esculenta C.) Root: Extraction, Purification and Characterization. Ph.D. Thesis, University McGill (Macdonald Campus), Montreal, QC, Canada, 1997; p. 179. [Google Scholar]
- Chandrika, G.U.; Svanberg, U.; Jansz, R. In vitro accessibility of -carotene from cooked Sri Lankan green leafy vegetables and their estimated contribution to vitamin A requirement. J. Sci. Food Agric. 2006, 86, 54–61. [Google Scholar] [CrossRef]
- Wobeto, C.; Corrêa, A.D.; Abreu, C.M.P.; Santos, C.D.; Abreu, J.R. Nutrients in the cassava (Manihot esculenta Crantz) leaf powder at three ages of the plant. Food Sci. Technol. 2006, 26, 865–869. [Google Scholar]
- Reed, J.D.; McDowell, R.E.; Van Soest, P.J.; Horvath, P.J. Condensed tannins: A factor limiting the use of cassava forage. J. Sci. Food. Agric. 1982, 33, 213–220. [Google Scholar] [CrossRef]
- Simão, A.A.; Santos, M.A.; Fraguas, R.M.; Braga, M.A.; Marques, T.R.; Duarte, M.H.; Santos, C.M.; Freire, J.M.; Correa, A.D. Antioxidants and chlorophyll in cassava leases at three plant ages. Afr. J. Agric. Res. 2013, 8, 3724–3730. [Google Scholar]
- Almeida, S.E.M.; Arruda, S.F.; Vargas, R.M.; Souza, E.M.T. β-Carotene from cassava (Manihot esculenta Crantz) leaves improves vitamin A status in mice. Compos. Biochem. Phys. C 2007, 146, 235–240. [Google Scholar]
- Buschmann, H.; Reilly, K.; Rodriguez, M.X.; Tohme, J.; Beeching, J.R. Hydrogen peroxide and flavan-3-ols in storage roots of cassava (Manihot esculenta Crantz) during postharvest deterioration. J. Agric. Food Chem. 2000, 48, 5522–5529. [Google Scholar] [CrossRef] [PubMed]
- Simão, A.A.; Lage, F.F.; Chaga, P.M.B.; Fraguas, R.M.; Frei, J.M.; Marques, T.R.; Corrêa, A.D. Antioxidants from medicinal plants used in the treatment of obesity. Eur. J. Med. Plants 2013, 3, 429–443. [Google Scholar] [CrossRef]
- Suresh, R.; Saravanakumar, M.; Suganyadevi, P. Anthocyanins from Indian cassava (Manihot esculenta Crantz) and its antioxidant properties. Int. J. Pharm. Sci. Res. 2011, 37, 1819–1828. [Google Scholar]
- Tao, H.-T.; Qiu, B.; Du, F.-L.; Xu, T.-C.; Liu, L.-N.; Lu, F.; Li, K.-M.; Liu, W. The protective effects of cassava (Manihot esculenta Crantz) leaf flavonoid extracts on liver damage of carbon tetrachloride injured mice. Afr. J. Tradit. Complement. Altern. Med. 2015, 12, 52–56. [Google Scholar] [CrossRef]
- Bokanisereme, U.F.Y.; Okechukwu, P.N. Anti-inflammatory, analgesic and anti-pyretic activity of cassava leaves extract. Asian J. Pharm. Clin. Res. 2013, 6, 89–92. [Google Scholar]
- Montoro, P.; Skhirtladze, A.; Bassarello, C.; Perrone, A.; Kemertelidze, E.; Pizza, C.; Piacente, S. Determination of phenolic compounds in Yucca gloriosa bark and root by LC-MS/MS. J. Pharm. Biomed. Anal. 2008, 47, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Nair, K.P. The Agronomy and Economy of Important Tree Crops of the Developing World; Elsevier Inc.: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Jahurul, M.; Zaidul, I.; Norulaini, N.; Sahena, F.; Jinap, S.; Azmir, J.; Sharif, K.; Omar, A.M. Cocoa butter fats and possibilities of substitution in food products concerning cocoa varieties, alternative sources, extraction methods, composition, and characteristics. J. Food Eng. 2013, 117, 467–476. [Google Scholar] [CrossRef]
- Do Carmo Brito, B.D.N.; Chisté, R.C.; da Silva Pena, R.; Gloria, M.B.A.; Lopes, A.S. Bioactive amines and phenolic compounds in cocoa beans are affected by fermentation. Food Chem. 2017, 228, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Minifie, B. Chocolate, Cocoa and Confectionery: Science and Technology; Springer Science & Business Media: New York, NY, USA, 2012; pp. 3–135. [Google Scholar]
- Bordiga, M.; Locatelli, M.; Travaglia, F.; Coïsson, J.D.; Mazza, G.; Arlorio, M. Evaluation of the effect of processing on cocoa polyphenols: Antiradical activity, anthocyanins and procyanidins profiling from raw beans to chocolate. Int. J. Food Sci. Technol. 2015, 50, 840–848. [Google Scholar] [CrossRef]
- Batista, N.N.; de Andrade, D.P.; Ramos, C.L.; Dias, D.R.; Schwan, R.F. Antioxidant capacity of cocoa beans and chocolate assessed by FTIR. Food Res. Int. 2016, 90, 313–319. [Google Scholar] [CrossRef]
- D’Souza, R.N.; Grimbs, S.; Behrends, B.; Bernaert, H.; Ullrich, M.S.; Kuhnert, N. Origin-based polyphenolic fingerprinting of Theobroma cacao in unfermented and fermented beans. Food Res. Int. 2017, 1, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Camu, N.; De Winter, T.; Addo, S.K.; Takrama, J.S.; Bernaert, H.; De Vuyst, L. Fermentation of cocoa beans: Influence of microbial activities and polyphenol concentrations on the flavour of chocolate. J. Sci. Food Agric. 2008, 88, 2288–2297. [Google Scholar] [CrossRef]
- Paoletti, R.; Poli, A.; Conti, A.; Visioli, F. Chocolate and Health; Springer: Milan, Italy, 2012. [Google Scholar]
- Ellam, S.; Williamson, G. Cocoa and human health. Ann. Rev. Nutr. 2013, 33, 105–128. [Google Scholar] [CrossRef] [PubMed]
- Bastos, V.S. Sucessão Microbiana e Dinâmica de Substratos e Metabólitos Durante a Fermentação Espontânea de Grãos de Cacau (Theobroma cacao L.), Variedade Clonal 565, Cultivado no sul da Bahia. Ph.D. Thesis, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil, 2016. [Google Scholar]
- De Vuyst, L.; Weckx, S. The cocoa bean fermentation process: From ecosystem analysis to starter culture development. J. Appl. Microbiol. 2016, 121, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Utami, R.R.; Armunanto, R.; Supriyanto, S.R.A. Effects of cocoa bean (Theobroma cacao L.) fermentation on phenolic content, antioxidant activity and functional group of cocoa bean shell. Pak. J. Nutr. 2016, 15, 948–953. [Google Scholar]
- Nazaruddin, R.; Seng, L.; Hassan, O.; Said, M. Effect of pulp preconditioning on the content of polyphenols in cocoa beans (Theobroma cacao) during fermentation. Ind. Crops Prod. 2006, 24, 87–94. [Google Scholar] [CrossRef]
- Sánchez-Rabaneda, F.; Jáuregui, O.; Casals, I.; Andrés-Lacueva, C.; Izquierdo-Pulido, M.; Lamuela-Raventós, R.M. Liquid chromatographic/electrospray ionization tandem mass spectrometric study of the phenolic composition of cocoa (Theobroma cacao). J. Mass Spectrom. 2003, 38, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Murga, L.; Tarín, J.; García-Perez, M.; Cano, A. The impact of chocolate on cardiovascular health. Maturitas 2011, 69, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Kay, C.; Abdelhamid, A.; Kroon, P.A.; Cohn, J.S.; Rimm, E.B.; Cassidy, A. Effects of chocolate, cocoa, and flav-3-ols on cardiovascular health: A systematic review and meta-analysis of randomized trials. Am. J. Clin. Nutr. 2012, 95, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Kuebler, U.; Arpagaus, A.; Meister, R.E.; von Känel, R.; Huber, S.; Ehlert, U.; Wirtz, P.H. Dark chocolate attenuates intracellular pro-inflammatory reactivity to acute psychosocial stress in men: A randomized controlled trial. Brain Behav. Immun. 2016, 57, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Almoosawi, S.; Fyfe, L.; Ho, C.; Al-Dujaili, E. The effect of polyphenol-rich dark chocolate on fasting capillary whole blood glucose, total cholesterol, blood pressure and glucocorticoids in healthy overweight and obese subjects. Br. J. Nutr. 2010, 103, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Desideri, G.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Ghiadoni, L.; Mastroiacovo, D.; Raffaele, A.; Ferri, L.; Bocale, R.; Lechiara, M.C. Benefits in cognitive function, blood pressure, and insulin resistance through cocoa flavanol consumption in elderly subjects with mild cognitive impairment novelty and significance. Hypertension 2012, 60, 794–801. [Google Scholar] [CrossRef] [PubMed]
- FAO (Food and Agriculture Organization of the United Nations). 2017. Available online: http://www.fao.org/home/en/ (accessed on 19 July 2017).
- Malenčić, D.; Maksimović, Z.; Popović, M.; Miladinović, J. Polyphenol contents and antioxidant activity of soybean seed extracts. Bioresour. Technol. 2008, 99, 6688–6691. [Google Scholar] [CrossRef] [PubMed]
- Kuligowski, M.; Pawłowska, K.; Jasińska-Kuligowska, I.; Nowak, J. Isoflavone composition, polyphenols content and antioxidative activity of soybean seeds during tempeh fermentation. CyTA-J. Food 2017, 15, 27–33. [Google Scholar] [CrossRef]
- Snyder, H.E.; Wilson, L.A. Em Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Trugo, L.C., Finglas, P.M., Eds.; Academic Press: Oxford, UK, 2003; Volume 9, pp. 5383–5389. [Google Scholar]
- Aguiar, C.L.; Haddad, R.; Eberlin, M.N.; Carrao-Panizzi, M.C.; Mui, T.S.; Park, Y.K. Thermal behavior of malonylglucoside isoflavones in soybean flour analyzed by RPHPLC/DAD and eletrospray ionization mass spectrometry. LWT Food Sci. Technol. 2012, 48, 114–119. [Google Scholar] [CrossRef]
- Tepavčević, V.; Atanacković, M.; Miladinović, J.; Malenčić, D.; Popović, J.; Cvejić, J. Isoflavone composition, total polyphenolic content, and antioxidant activity in soybeans of different origin. J. Med. Food. 2010, 13, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Barnes, S.; Kim, H.; Darley-Usmar, V.; Patel, R.; Xu, J.; Boersma, B.; Luo, M. Beyond ERα and ERβ: Estrogen receptor binding is only part of the isoflavone story. J. Nutr. 2000, 130, 656S–657S. [Google Scholar] [PubMed]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.; Ferreira, I.C. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, C.M.; Sá, M.F.; Toloi, M.R. Effects of phytoestrogens derived from soy bean on expression of adhesion molecules on HUVEC. Climacteric 2012, 15, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Zakir, M.M.; Freitas, I.R. Benefícios à saúde humana do consumo de isoflavonas presentes em produtos derivados da soja. J. Bioenergy Food Sci. 2015, 2, 107–116. [Google Scholar] [CrossRef]
- Marini, H.; Minutoli, L.; Polito, F.; Bitto, A.; Altavilla, D.; Atteritano, M.; Gaudio, A.; Mazzaferro, S.; Frisina, A.; Frisina, N.; et al. Effects of the phytoestrogen genistein on bone metabolism in osteopenic postmenopausal women: A randomized trial. Ann. Intern. Med. 2007, 146, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.J.; Ismail, R.; Taylor-Swanson, L.; Cray, L.; Schnall, J.G.; Mitchell, E.S.; Woods, N.F. Effects of isoflavones and amino acid therapies for hot flashes and co-occurring symptoms during the menopausal transition and early postmenopause: A systematic review. Maturitas 2014, 78, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Uehara, M. Isoflavone metabolism and bone-sparing effects of daidzein-metabolites. J. Clin. Biochem. Nutr. 2013, 52, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Koh, K.; Youn, J.E.; Kim, H.S. Identification of anthocyanins in black soybean (Glycine max (L.) Merr.) varieties. Food Sci. Technol. 2014, 51, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.M.; Ha, T.J.; Lee, Y.B.; Seo, W.D.; Kim, J.Y.; Ryu, H.W.; Lee, J.H. Soluble phenolics and antioxidant properties of soybean (Glycine max L.) cultivars with varying seed coat colours. J. Funct. Foods 2013, 5, 1065–1076. [Google Scholar] [CrossRef]
- Prajapati, R.; Kalariya, M.; Umbarkar, R.; Parmar, S.; Sheth, N. Colocasia esculenta: A potent indigenous plant. Int. J. Nutr. Pharmacol. Neurol. Dis. 2011, 1, 90–96. [Google Scholar] [CrossRef]
- Zárate, N.A.H.; Vieira, M.C.; Hiane, P.A. Produção e composição nutritiva de taro em função do propágulo, em solo hidromórfico do Pantanal Sul-Mato-Grossense. Semin. Cien. Agrar. 2006, 27, 361–366. [Google Scholar] [CrossRef]
- Zárate, N.A.H.; Vieira, M.C.; Rego, N.H. Produtividade de clones de taro em função da população de plantas na época seca do pantanal sul-mato-grossense. Pesqui. Agropecu. Trop. 2007, 36, 141–143. [Google Scholar]
- Oscarsson, K.V.; Savage, G.P. Composition and availability of soluble and insoluble oxalates in raw and cooked taro (Colocasia esculenta var. Schott) leaves. Food Chem. 2007, 101, 559–562. [Google Scholar] [CrossRef]
- Owusu-Darko, P.G.; Paterson, A.; Omenyo, E.L. Cocoyam (corms and cormels)—An underexploited food and feed resource. J. Agric. Chem. Environ. 2014, 3, 22–29. [Google Scholar] [CrossRef]
- Adegunwa, M.O.; Alamu, E.O.; Omitogun, L.A. Effect of processing on the nutritional contents of yam and cocoyam tubers. J. Appl. Biosci. 2011, 6, 3086–3092. [Google Scholar]
- Olajide, R.; Akinsoyinu, A.O.; Babayemi, O.J.; Omojola, A.B.; Abu, A.O.; Afolabi, K.D. Effect of processing on energy values, nutrient and anti-nutrient components of wild cocoyam (Colocasia esculenta (L.) Schott) corm. Pak. J. Nutr. 2011, 10, 29–34. [Google Scholar] [CrossRef]
- McDougall, G.J. Phenolic-enriched foods: Sources and processing for enhanced health benefits. Proc. Nutr. Soc. 2017, 76, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.F.; Silva, A.M.; Silva, A.M.; Valentão, P.; Ferreres, F.; Gil-Izquierdo, A.; Silva, J.B.; Santos, D.; Andrade, P.B. Influence of taro (Colocasia esculenta L. Shott) growth conditions on the phenolic composition and biological properties. Food Chem. 2013, 141, 3480–3485. [Google Scholar] [CrossRef] [PubMed]
- Agyare, C.; Boakye, Y.D.; Apenteng, J.A.; Dapaah, S.O.; Appiah, T. Antimicrobial and anti-inflammatory properties of Anchomanes difformis (Bl.) Engl. and Colocasia esculenta (L.) Schott. Biochem. Pharmacol. 2016, 5, 201. [Google Scholar]
- Simsek, S.; El, S.N. In vitro starch digestibility, estimated glycemic index and antioxidant potential of taro (Colocasia esculenta L. Schott) corm. Food Chem. 2015, 168, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Takebayashi, J.; Oki, T.; Watanabe, J.; Yamasaki, K.; Chen, J.; Sato-Furukawa, M.; Tsubota-Utsugi, M.; Taku, K.; Kazuhisa, G. Hydrophilic antioxidant capacities of vegetables and fruits commonly consumed in Japan and estimated average daily intake of hydrophilic antioxidants from these foods. J. Food Compos. Anal. 2013, 29, 25–31. [Google Scholar] [CrossRef]
- Awa, E.; Eleazu, C. Bioactive constituents and antioxidant activities of raw and processed cocoyam (Colocasia esculenta). Nutrafoods 2015, 14, 133–140. [Google Scholar] [CrossRef]
- Terasawa, N.; Saotome, A.; Tachimura, Y.; Mochizuki, A.; Ono, H.; Takenaka, M.; Murata, M. Identification and some properties of anthocyanin isolated from Zuiki, stalk of Colocasia esculenta. J. Agric. Food Chem. 2007, 55, 4154–4159. [Google Scholar] [CrossRef] [PubMed]
- Alcantara, M.; Hurtada, A.; Dizon, I. The nutritional value and phytochemical components of taro Colocasia esculenta (L.) Schott powder and its selected processed foods. J. Nutr. Food Sci. 2013, 3, 3–10. [Google Scholar] [CrossRef]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P.E. Polyphenols and Human Health: Prevention of Disease and Mechanisms of Action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81, 243S–255S. [Google Scholar] [PubMed]
- Rimbach, G.; Melchin, M.; Moehring, J.; Wagner, A.E. Polyphenols from Cocoa and Vascular Health—A Critical Review. Int. J. Mol. Sci. 2009, 10, 4290–4309. [Google Scholar] [CrossRef] [PubMed]
- Potter, S.M.; Baum, J.A.; Teng, H.; Stillman, R.J.; Shay, N.F.; Erdman, J.W., Jr. Soy protein and isoflavones: Their effects on blood lipids and bone density in postmenopausal women. Am. J. Clin. Nutr. 1998, 68, 1375S–1379S. [Google Scholar] [PubMed]
- Morabito, N.; Crisafulli, A.; Vergara, C.; Gaudio, A.; Lasco, A.; Frisina, N.; D’Anna, R.; Corrado, F.; Pizzoleo, M.A.; Cincotta, M.; et al. Effects of genistein and hormone-replacement therapy on bone loss in early postmenopausal women: A randomized double-blind placebo-controlled study. J. Bone Miner. Res. 2002, 17, 1904–1912. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, H.; O’Reilly, J.D.; Adlercreutz, H.; Mallet, A.I.; Bowey, E.A.; Rowland, I.R.; Sanders, T.A. Isoflavone phytoestrogens consumed in soy decrease F(2)-isoprostane concentrations and increase resistance of low-density lipoprotein to oxidation in humans. Am. J. Clin. Nutr. 2000, 72, 395–400. [Google Scholar] [PubMed]
- Mathur, S.; Devaraj, S.; Grundy, S.M.; Jialal, I. Cocoa products decrease low density lipoprotein oxidative susceptibility but do not affect biomarkers of inflammation in humans. J. Nutr. 2002, 132, 3663–3667. [Google Scholar] [PubMed]
- Fisher, N.D.; Hughes, M.; Gerhard-Herman, M.; Hollenberg, N.K. Flavanol-rich cocoa induces nitric-oxide-dependent vasodilation in healthy humans. J. Hypertens. 2003, 21, 2281–2286. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.; Dejam, A.; Kleinbongard, P.; Schewe, T.; Sies, H.; Kelm, M. Vascular effects of cocoa rich in flavan-3-ols. JAMA 2003, 290, 1030–1031. [Google Scholar] [CrossRef] [PubMed]
- Flammer, A.J.; Hermann, F.; Sudano, I.; Spieker, L.; Hermann, M.; Cooper, K.A.; Serafini, M.; Luscher, T.F.; Ruschitzka, F.; Noll, G.; et al. Dark chocolate improves coronary vasomotion and reduces platelet reactivity. Circulation 2007, 116, 2376–2382. [Google Scholar] [CrossRef] [PubMed]
- Balzer, J.; Rassaf, T.; Heiss, C.; Kleinbongard, P.; Lauer, T.; Merx, M.; Heussen, N.; Gross, H.B.; Keen, C.L.; Schroeter, H.; et al. Sustained benefits in vascular function through flavanol-containing cocoa in medicated diabetic patients a double-masked, randomized, controlled trial. J. Am. Coll. Cardiol. 2008, 51, 2141–2149. [Google Scholar] [CrossRef] [PubMed]
- Clifford, T.; Howatson, G.; West, D.J.; Stevenson, E.J. The potential benefits of red beetroot supplementation in health and disease. Nutrients 2015, 7, 2801–2822. [Google Scholar] [CrossRef] [PubMed]
- Luqman, S.; Rizvi, S.I. Protection of lipid peroxidation and carbonyl formation in proteins by capsaicin in human erythrocytes subjected to oxidative stress. Phytother. Res. 2006, 20, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Andújar, I.; Recio, M.C.; Giner, R.M.; Rios, J.L. Cocoa polyphenols and their potential benefits for human health. Oxid. Med. Cell. Longev. 2012, 2012, 906252. [Google Scholar] [CrossRef] [PubMed]
- García-Lafuente, A.; Guillamón, E.; Villares, A.; Rostagno, M.A.; Martínez, J.A. Flavonoids as antiinflammatory agents: Implications in cancer and cardiovascular disease. Inflamm. Res. 2009, 58, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Balestrieri, C.; Felice, F.; Piacente, S.; Pizza, C.; Montoro, P.; Oleszek, W.; Visciano, V.; Balestrieri, M.L. Relative effects of phenolic constituents from Yucca schidigera Roezl. bark on Kaposi’s sarcoma cell proliferation, migration, and PAF synthesis. Biochem. Pharmacol. 2006, 71, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Yamori, Y.; Moriguchi, E.H.; Teramoto, T.; Miura, A.; Fukui, Y.; Honda, K.; Fukui, M.; Nara, Y.; Taira, K.; Moriguchi, Y. Soybean isoflavones reduce postmenopausal bone resorption in female Japanese immigrants in Brazil: A ten-week study. J. Am. Coll. Nutr. 2002, 21, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Landau, J.M.; Huang, M.T.; Newmark, H.L. Inhibition of carcinogenesis by dietary polyphenolic compounds. Ann. Rev. Nutr. 2001, 21, 381–406. [Google Scholar] [CrossRef] [PubMed]
- Johnson, I.T.; Williamson, G.; Musk, S.R.R. Anticarcinogenic factors in plant foods: A new class of nutrients? Nutr. Res. Rev. 1994, 7, 175–204. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Kendall, C.W.; Garsetti, M.; Rosenberg-Zand, R.S.; Jackson, C.J.; Agarwal, S.; Rao, A.V.; Diamandis, E.P.; Parker, T.; Faulkner, D.; et al. Effect of soy protein foods on low-density lipoprotein oxidation and ex vivo sex hormone receptor activity—A controlled crossover trial. Metabolism 2000, 49, 537–543. [Google Scholar] [CrossRef]
- Russo, J.; Russo, I.H. The role of estrogen in the initiation of breast cancer. J. Steroid. Biochem. Mol. Biol. 2006, 102, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Nah, J.; Chun, S.; Park, H.; Yang, S.E.; Min, W.K. In vivo antioxidant effect of green tea. Eur. J. Clin. Nutr. 2000, 54, 527–529. [Google Scholar] [CrossRef] [PubMed]
- Young, J.F.; Dragstedt, L.O.; Haraldsdottir, J.; Daneshvar, B.; Kall, M.A.; Loft, S.; Nilsson, L.; Nielsen, S.E.; Mayer, B.; Skibsted, L.H.; et al. Green tea extract only affects markers of oxidative status postprandially: Lasting antioxidant effect of flavonoid-free diet. Br. J. Nutr. 2002, 87, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Buijsse, B.; Feskens, E.J.; Kok, F.J.; Kromhout, D. Cocoa intake, blood pressure, and cardiovascular mortality: The Zutphen Elderly Study. Arch. Intern. Med. 2006, 166, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Keen, C.L. Evidence that the antioxidant flavonoids in tea and cocoa are beneficial for cardiovascular health. Curr. Opin. Lipidol. 2002, 13, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Kroon, P.A.; Rimm, E.B.; Cohn, J.S.; Harvey, I.; le Cornu, K.A.; Ryder, J.J.; Hall, W.L.; Cassidy, A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2008, 88, 38–50. [Google Scholar] [PubMed]
- Jia, L.; Liu, X.; Bai, Y.Y.; Li, S.H.; Sun, K.; He, C.; Hui, R. Short-term effect of cocoa product consumption on lipid profile: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2010, 92, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Ludovici, V.; Barthelmes, J.; Nägele, M.P.; Enseleit, F.; Ferri, C.; Flammer, A.J.; Ruschitzka, F.; Sudano, I. Cocoa, blood pressure, and vascular function. Front. Nutr. 2017, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.; Natsume, M.; Yasuda, A.; Nakamura, Y.; Tamura, T.; Osakabe, N.; Kanegae, M.; Kondo, K. Plasma LDL and HDL cholesterol and oxidized LDL concentrations are altered in normo- and hypercholesterolemic humans after intake of different levels of cocoa powder. J. Nutr. 2007, 137, 1436–1441. [Google Scholar] [PubMed]
- Di Renzo, L.; Rizzo, M.; Sarlo, F.; Colica, C.; Iacopino, L.; Domino, E.; Sergi, D.; De Lorenzo, A. Effects of dark chocolate in a population of normal weight obese women: A pilot study. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2257–2266. [Google Scholar] [PubMed]
- Monahan, K.D. Effect of cocoa/chocolate ingestion on brachial artery flow-mediated dilation and its relevance to cardiovascular health and disease in humans. Arch. Biochem. Biophys. 2012, 527, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Schewe, T.; Heiss, C.; Kelm, M. Cocoa polyphenols and inflammatory mediators. Am. J. Clin. Nutr. 2005, 81, 304S–312S. [Google Scholar] [PubMed]
| Plant Source | Polyphenols | Class | Compound | Ref. |
|---|---|---|---|---|
| Beetroot (B. vulgaris) | flavonoids | flavanone | betagarin | [50] |
| flavone | cochliophilin a | [50] | ||
| flavonol | dihydroisorhamnetin | [50] | ||
| isoflavone | betavulgarin | [50] | ||
| non-flavonoids | hydrobenzoic acids | n-trans-ferruloylhomovanillylamine, n-trans-ferruloyltyramine | [50] | |
| hydroxycinnamic acids | caffeic acid, ferulic acid, gallic acid, p-cumaric, p-hydroxybenzoic, syringic acid, vanillic acid | [51] | ||
| Cassava (M. esculanta) | flavonoids | anthocyanidins | cyanidin, delphinidin | [52] |
| flavan-3-ols | catechin, gallocatechin | [53] | ||
| flavonols | kaempferol, quercetin, rutin | [54,55,56,57] | ||
| non-flavonoids | coumarins | scopoletin | [58] | |
| hydrobenzoic acids | coniferaldehyde, gallic acid, isovanillin, syringsledehyde, resveratrol | [53,58] | ||
| hydroxycinnamic acids | chlorogenic acid, p-coumaric acid | [53,58] | ||
| lignans | balanophonin, pinoresinol | [58] | ||
| stilbene | trans-3,3′,5,5′-tetrahydroxy-4′-methoxystilbene | [59] | ||
| Cocoa nibs (T. cacao) | flavonoids | anthocyanidins | arabinosidil, cyaniding, galactosidyl | [60,61] |
| flavan-3-ols | cathechin, epicatechin, hyperoside, isovitexin, procyanidin b1, procyanidin b2, vitexin | [62] | ||
| flavonols | quercetin, quercetin 3-O-arabinoside | [62] | ||
| flavonones | apigenin, luteolin, luteolin-7-O-glucoside | [60] | ||
| tannins | procyanidins | [60] | ||
| Soybean (G. max) | flavonoids | anthocyanidins | cyanidin, delphinidin, pelargonidin, petunidin | [63] |
| hydrobenzoic acids | gallic acid, gentistic, protocatechuic acid | [64,65] | ||
| non-flavonoids | hydroxycinnamic acids | caffeic acid, chlorogenic acid, ferulic acid, sinapic acid, p-coumaric acid, t-cinnamic acid | [65] | |
| isoflavonoids | β-glucosides: daidzin, genistin, glycitin malonyl-β-glucosides: malonyldaidzin, malonylgenistin, malonylglycitin acetyl-β-glucosides: acetyldaidzin, acetylgenistin, acetylglycitin aglycones: daidzein, glycitein | [64,65] | ||
| Taro (C. esculenta) (corms/leaves) | flavonoids | anthocyanidins | cyanidin, delphinidin | [66] |
| flavonols | isorhamnetin, kaempferol, myricetin, quercetin | [66] | ||
| non-flavonoids | hydroxycinnamic acids | chlorogenic acid, p-coumaric acid | [67] |
| Polyphenols Source | Polyphenol Content(s) | Experimental Population | Number of Volunteers | Duration (Days) | Effect(s) |
|---|---|---|---|---|---|
| Isolated soy protein containing moderate and high isoflavones concentration | Two dietary groups: −56 mg of isoflavones −90 mg of isoflavones | Hypercholesterolemic postmenopausal women | 66 | 168 | Increases HDL cholesterol, mononuclear cell LDL receptor mRNA, both bone mineral content and density in the lumbar spine after ingestion of two dietary groups decreases in non-HDL cholesterol after ingestion of the two dietary groups (56 and 90 mg of isoflavones) [164]. |
| Genistein (soy phytoestrogen) | 54 mg | Healthy and postmenopausal women (range 47–57 years) | 90 | 364 | Decreased excretion of pyridinium and deoxypyridinoline (PYR: −54 ± 10%; DPYR: −55 ± 13%) after 6 and 12 (PYR: −42 ± 12%; DPYR: −44 ± 16%) months of genistein administration. Increases in serum bone-specific ALP (B-ALP) and osteocalcin (bone Gla protein [BGP]) after 6 (B-ALP: 23 ± 4%; BGP: 29 ± 11%) and 12 (B-ALP: 25 ± 7%; BGP: 37 ± 16%) months of genistein administration. Furthermore, significantly increases in femur (femoral neck: 3.6 ± 3% and lumbar spine (3 ± 2%) bone mineral density (BMD) were observed [165]. |
| Textured soy protein high in isoflavones (HI); Textured soy protein low in isoflavones (LI) | HI 21.2 mg of daidzein; 34.8 mg of genistein; LI 0.9 mg of daidzein 1.0 mg of genistein | Healthy men and women (range 19–40 years) | 24 | 14 | Decreased plasma 8-epi-PGF2α after high-isoflavone dietary treatment (326 ± 32 ng L−1) when compared to the low-isoflavone dietary treatment (405 ± 50 ng L−1). The lag time for copper-ion-induced LDL oxidation was longer after high-isoflavone dietary treatment (48 ± 2.4 min) than low-isoflavone dietary treatment (44 ± 1.9 min). No changes in plasma malondialdehyde, LDL α-tocopherol, polyunsaturated fatty acids, and isoflavonoids after dietary treatments [166]. |
| Cocoa supplementation (dark chocolate bar and cocoa powder drink) | 651 mg of procyanidins | Healthy men and women (range 20 to 60 years) | 25 | 42 | Decreased LDL oxidizability (evidenced by a longer lag time, 101.0 ± 20.7 min) after cocoa supplementation compared with baseline (91.3 ± 18.0 min) and washout (96.4 ± 7.5 min). No changes in urinary F(2) isoprostane concentration and markers of inflammation including the whole-blood cytokines, interleukin-1 beta, interleukin-6 and tumor necrosis factor-alpha, high sensitivity C-reactive protein and P-selectin [167]. |
| Cocoa drink | 821 mg of total flavonoids | Healthy men and women (range 18–72 years) | 27 | 35 | Increased peripheral vasodilation after four days of cocoa drink ingestion. After five days of cocoa drink consumption, pulse wave amplitude exhibited a large additional acute response [168]. |
| Cocoa drink | 176 mg of flavan-3-ols (70 mg of epicatechin plus catechin and 106 mg of procyanidins) | Outpatients with at least 1 cardiovascular risk factor (means, 41 years) | 26 | 2 | Increased flow-mediated dilatation maximally at 2 h from 3.4% to 6.3% after cocoa drink ingestion. Increases nitrosylated and nitrosated species from 22 to 36 nmol L−1 after ingestion of cocoa rich in flavan-3-ols [169]. |
| Dark chocolate | 15.6 mg of epicatechin equivalents per gram | Heart transplant recipients volunteers (range 35–70 years) | 22 | Acute | Increased coronary artery diameter from 2.36 ± 0.51 to 2.51 ± 0.59 mm after ingestion of flavonoid-rich dark chocolate. Decreased platelet adhesion from 4.9 ± 1.1% to 3.8 ± 0.8% after ingestion of flavonoid-rich dark chocolate [170]. |
| Powder cocoa drink | 963 mg of flavonoids | Diabetes mellitus II men and women (for at least 5 years, range 50 to 80 years) | 41 | 28 | Increased flow-mediated dilatation (FMD) by 30% after ingestion of flavanol-containing cocoa. Treatment was well tolerated, without evidence of tachyphylaxia. No changes in endothelium-independent responses, blood pressure, heart rate, and glycemic control after ingestion of the cocoa drink [171]. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baião, D.D.S.; De Freitas, C.S.; Gomes, L.P.; Da Silva, D.; Correa, A.C.N.T.F.; Pereira, P.R.; Aguila, E.M.D.; Paschoalin, V.M.F. Polyphenols from Root, Tubercles and Grains Cropped in Brazil: Chemical and Nutritional Characterization and Their Effects on Human Health and Diseases. Nutrients 2017, 9, 1044. https://doi.org/10.3390/nu9091044
Baião DDS, De Freitas CS, Gomes LP, Da Silva D, Correa ACNTF, Pereira PR, Aguila EMD, Paschoalin VMF. Polyphenols from Root, Tubercles and Grains Cropped in Brazil: Chemical and Nutritional Characterization and Their Effects on Human Health and Diseases. Nutrients. 2017; 9(9):1044. https://doi.org/10.3390/nu9091044
Chicago/Turabian StyleBaião, Diego Dos Santos, Cyntia Silva De Freitas, Laidson Paes Gomes, Davi Da Silva, Anna Carolina N. T. F. Correa, Patricia Ribeiro Pereira, Eduardo Mere Del Aguila, and Vania Margaret Flosi Paschoalin. 2017. "Polyphenols from Root, Tubercles and Grains Cropped in Brazil: Chemical and Nutritional Characterization and Their Effects on Human Health and Diseases" Nutrients 9, no. 9: 1044. https://doi.org/10.3390/nu9091044
APA StyleBaião, D. D. S., De Freitas, C. S., Gomes, L. P., Da Silva, D., Correa, A. C. N. T. F., Pereira, P. R., Aguila, E. M. D., & Paschoalin, V. M. F. (2017). Polyphenols from Root, Tubercles and Grains Cropped in Brazil: Chemical and Nutritional Characterization and Their Effects on Human Health and Diseases. Nutrients, 9(9), 1044. https://doi.org/10.3390/nu9091044








