High Consumption of Iron Exacerbates Hyperlipidemia, Atherosclerosis, and Female Sterility in Zebrafish via Acceleration of Glycation and Degradation of Serum Lipoproteins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Purification of Human Lipoproteins and ApoA-I
2.3. Treatment of Lipoproteins with Ferrous Ion
2.4. Acceleration of LDL Oxidation
2.5. Western Blotting
2.6. LDL Phagocytosis Assay
2.7. Cellular Senescence Assay
2.8. Zebrafish
2.9. Supplementation of Ferrous Ion to Zebrafish
2.10. Histologic Analysis
2.11. Statistical Analysis
3. Results
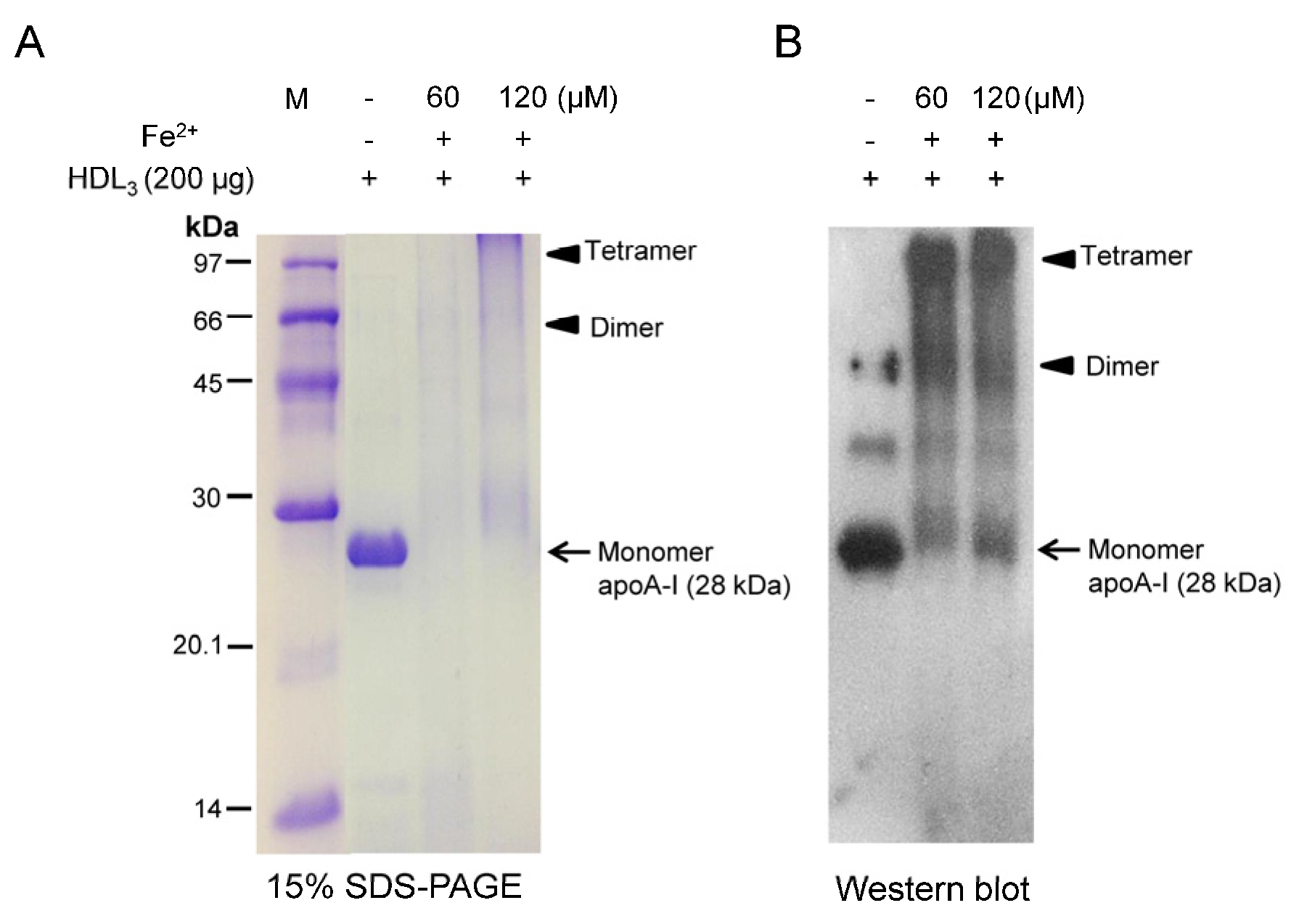
3.1. Lipoprotein Modification by the FeSO4
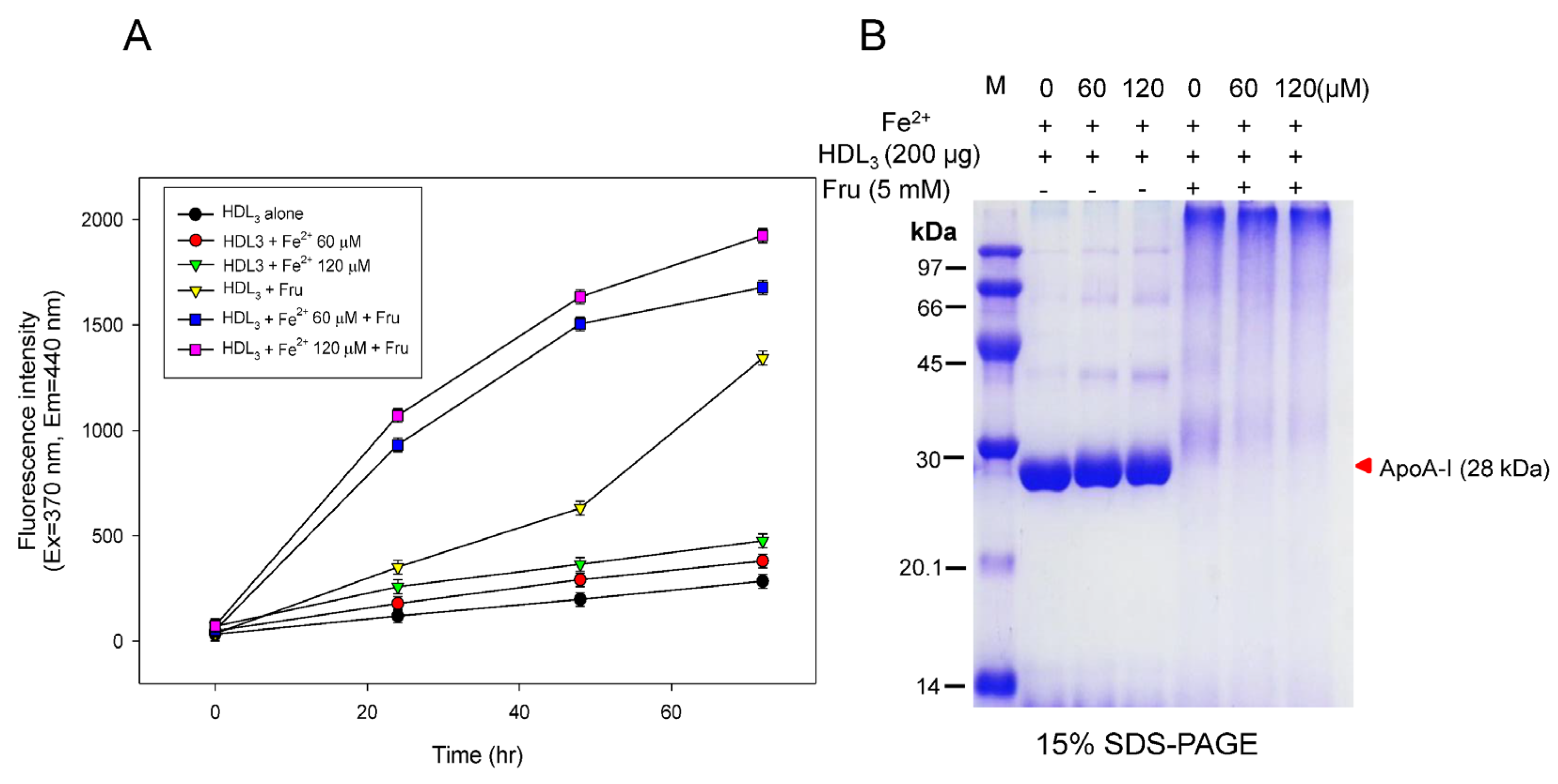
3.2. Acceleration of HDL Glycation by FeSO4
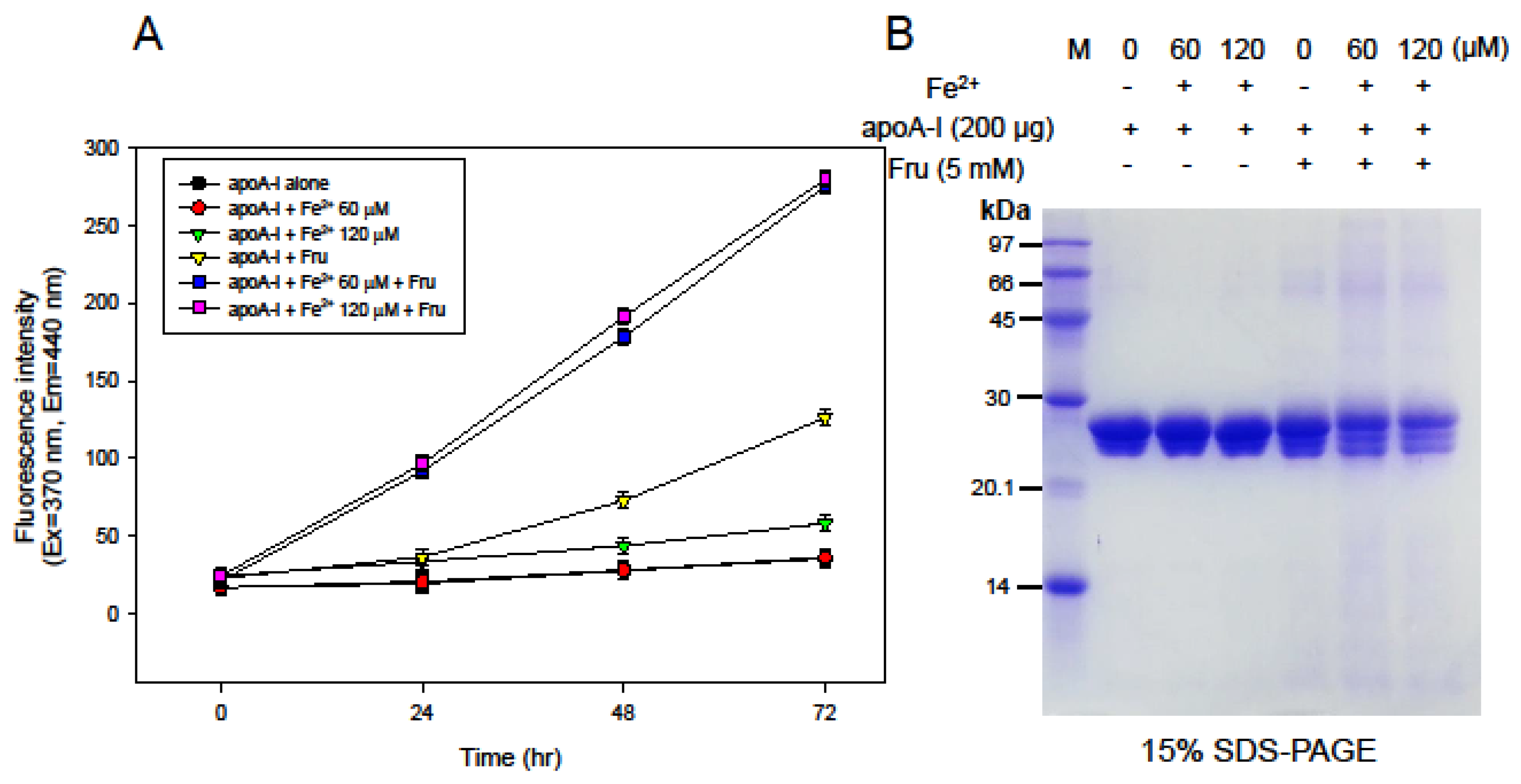
3.3. Synergistic Effect of ApoA-I Glycation by FeSO4 and Fructose
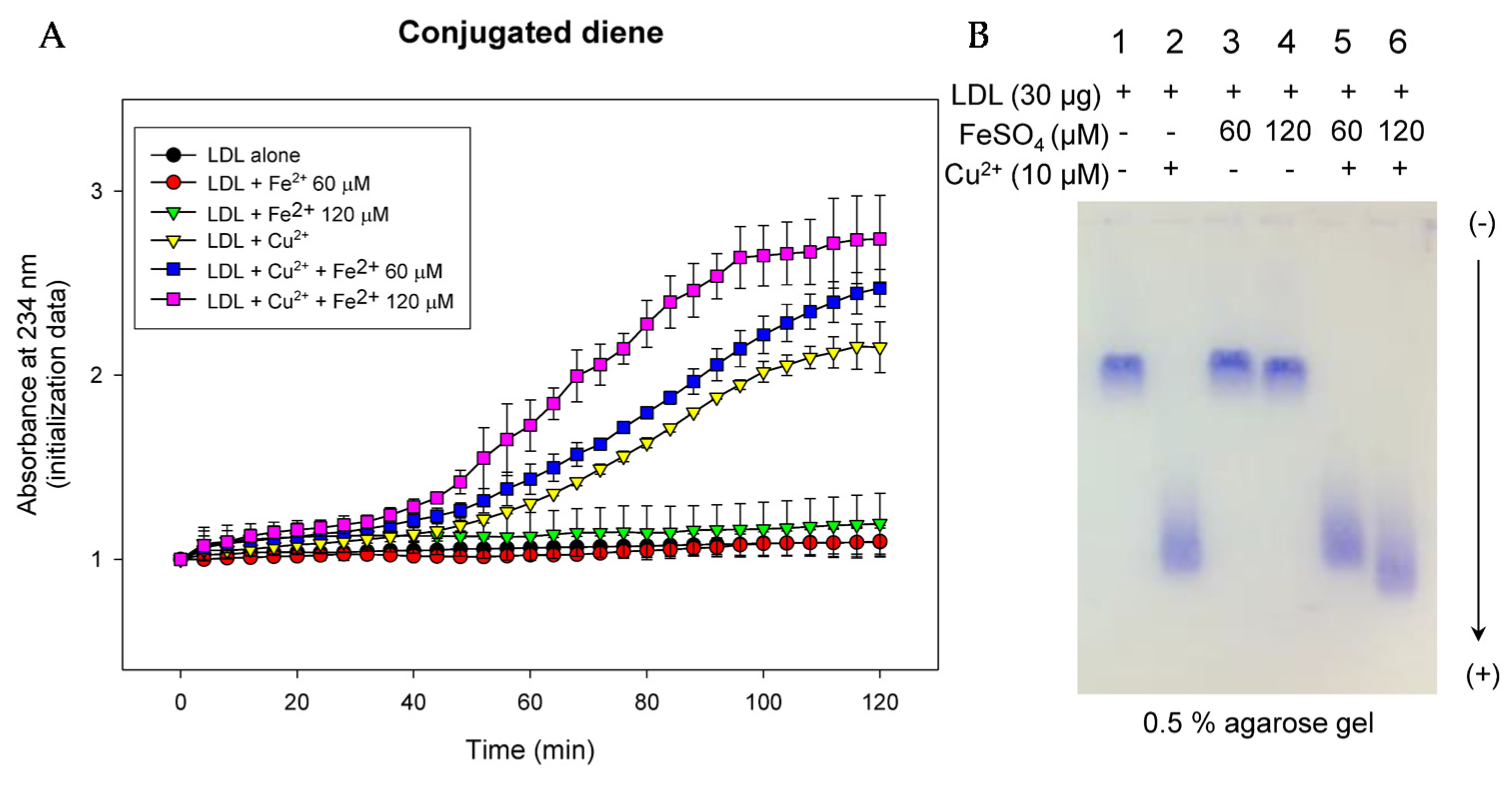
3.4. LDL Oxidation and Phagocytosis into Macrophage Was More Facilitated by FeSO4
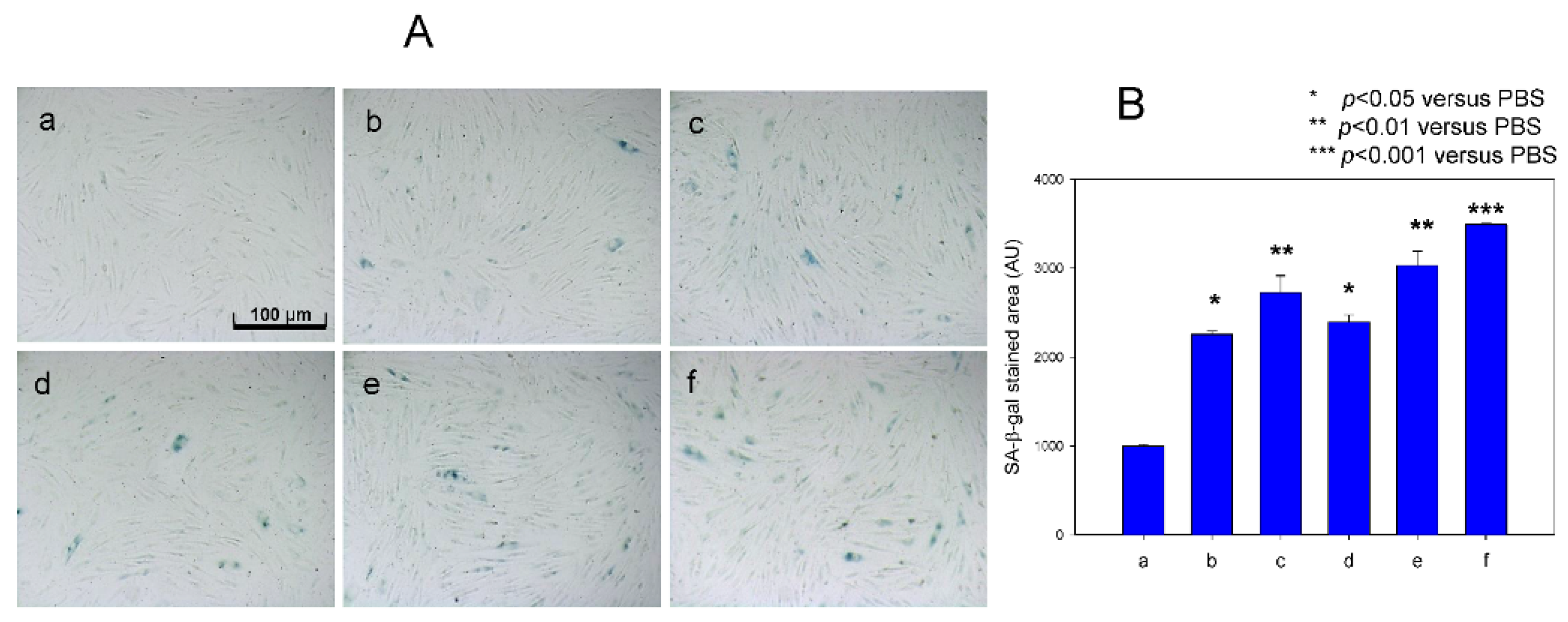
3.5. Cellular Senescence Was More Accelerated by FeSO4
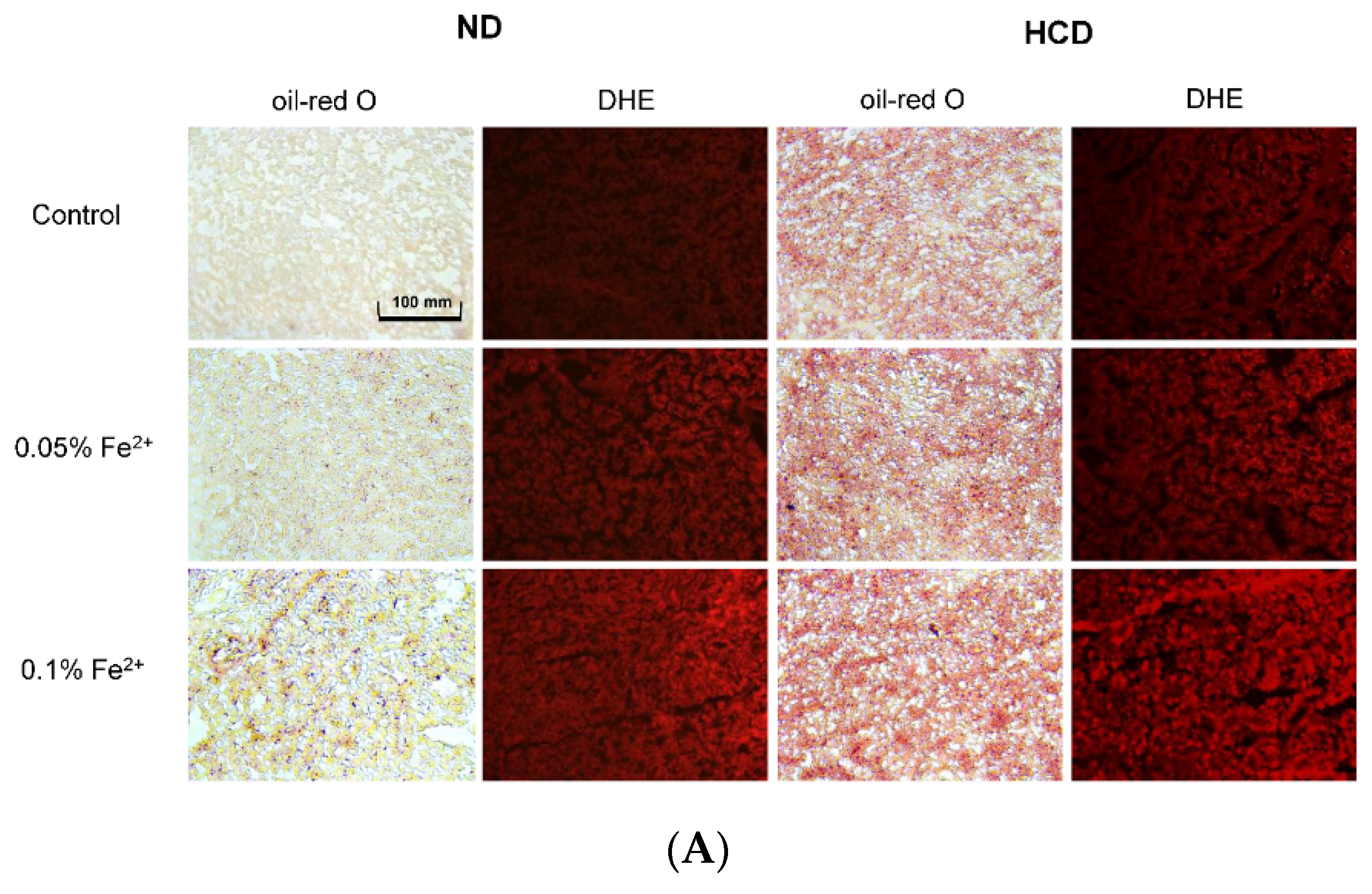
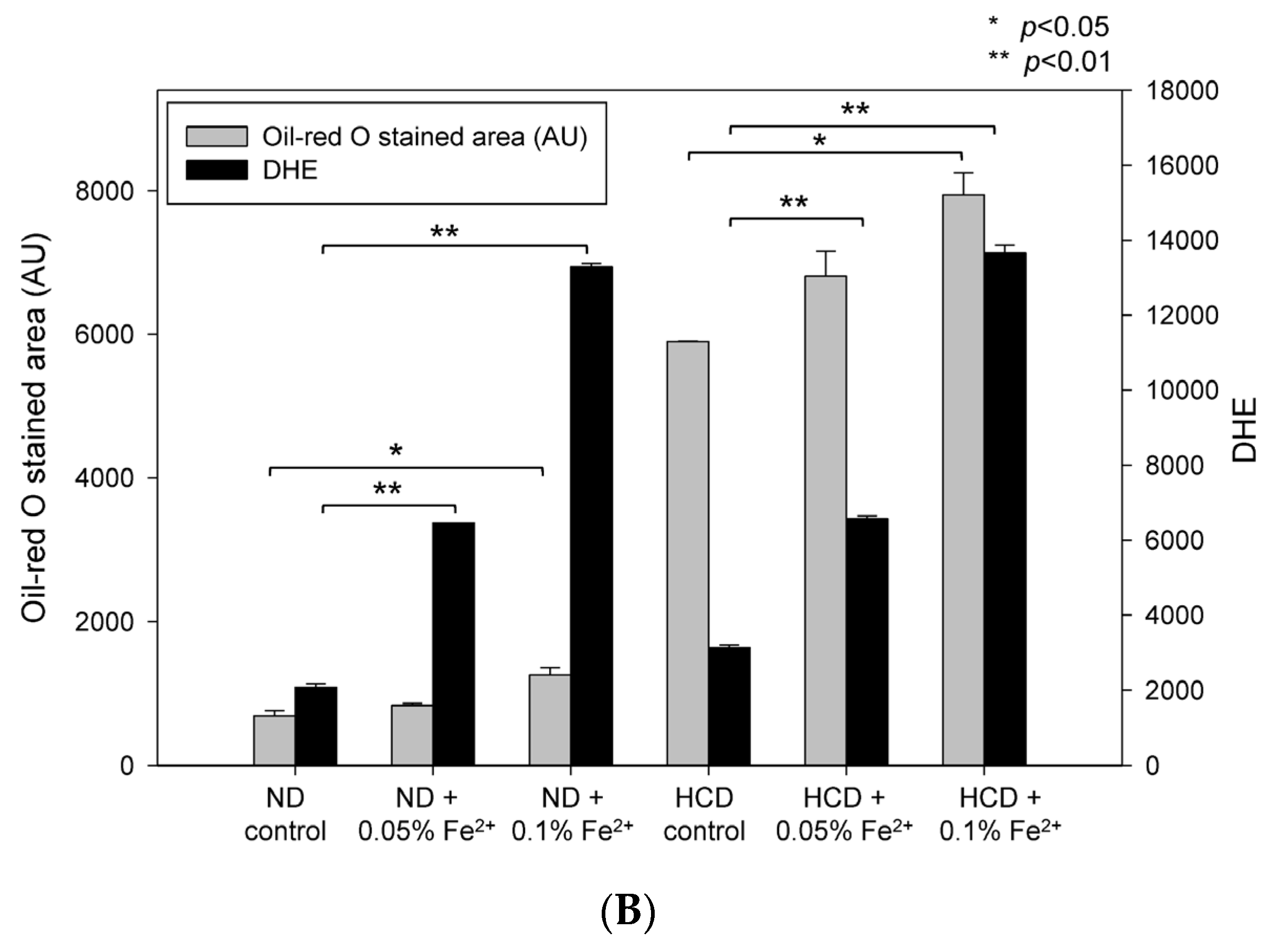
3.6. High Iron Diet Causes Hyperlipidemia in Zebrafish
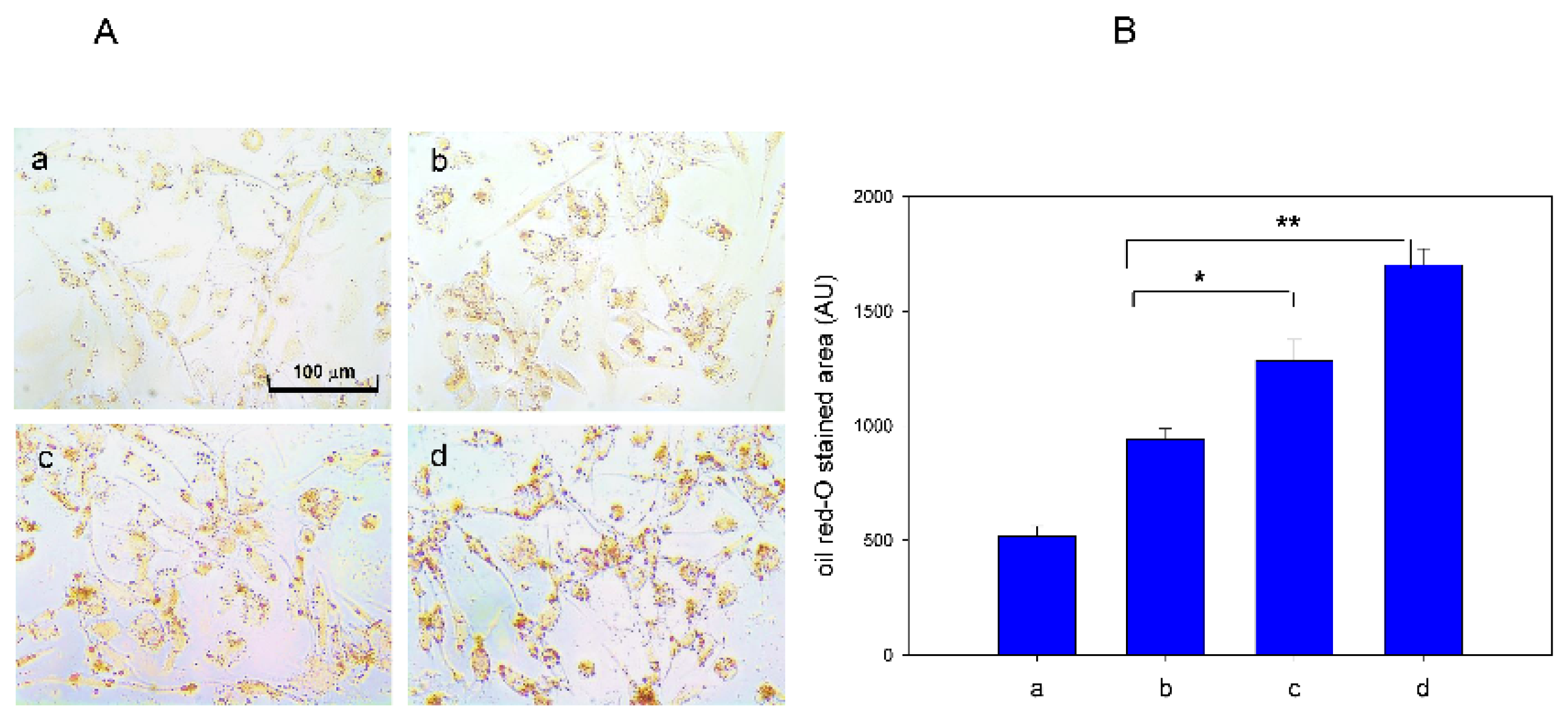
3.7. Fatty Liver Induction upon Iron Supplementation
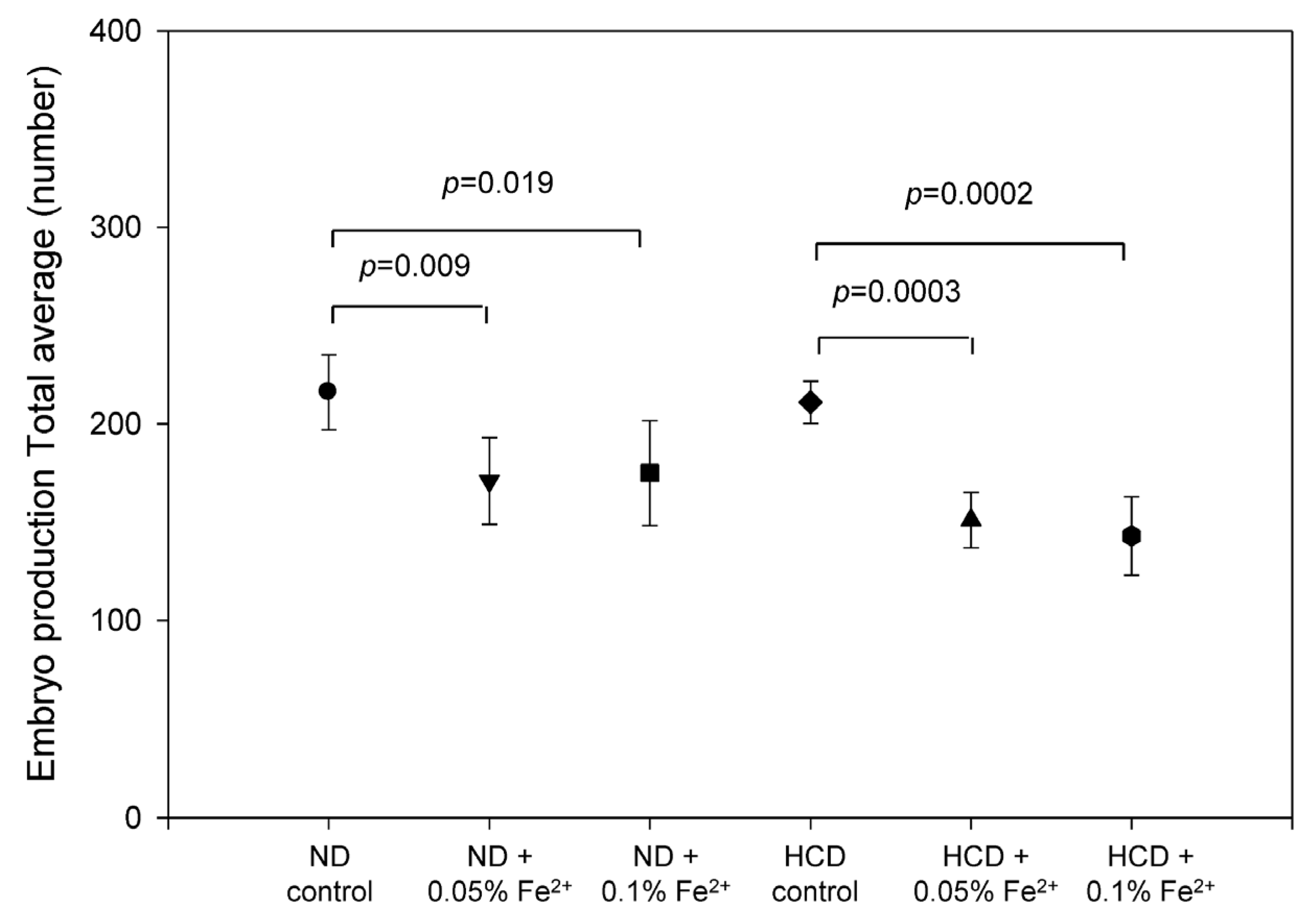
3.8. FeSO4 Consumption Reduces Embryo Production
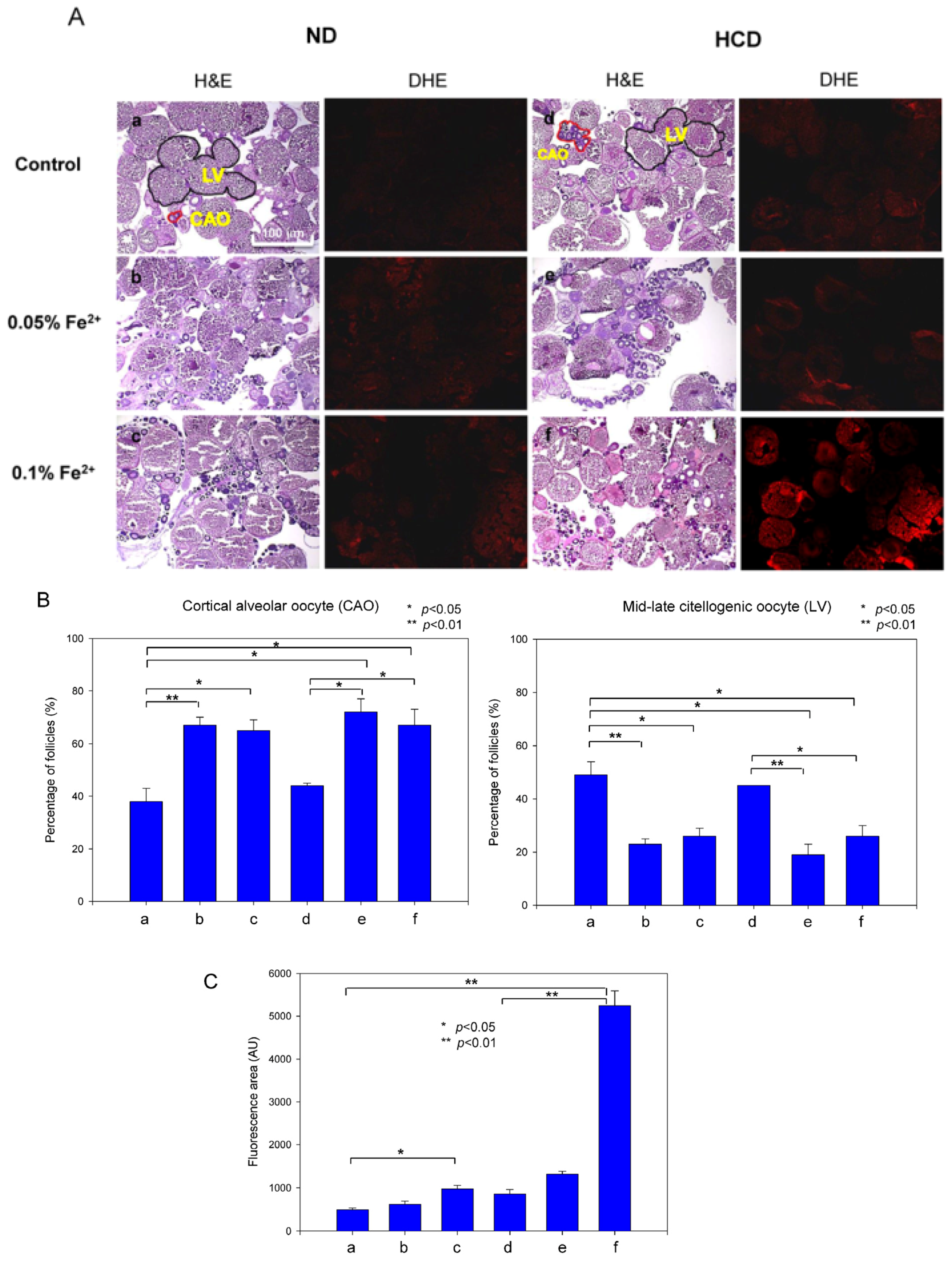
3.9. Ovarian Tissue Was Damaged by Iron Consumption
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gao, W.; Li, X.; Gao, Z.; Li, H. Iron increases diabetes-induced kidney injury and oxidative stress in rats. Biol. Trace Elem. Res. 2014, 160, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Rajpathak, S.N.; Crandall, J.P.; Wylie-Rosett, J.; Kabat, G.C.; Rohan, T.E.; Hu, F.B. The role of iron in type 2 diabetes in humans. Biochim. Biophys. Acta. 2009, 1790, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pantopoulos, K. Regulation of cellular iron metabolism. Biochem. J. 2011, 434, 365–381. [Google Scholar] [CrossRef]
- Radlowski, E.C.; Johnson, R.W. Perinatal iron deficiency and neurocognitive development. Front. Hum. Neurosci. 2013, 7, 585. [Google Scholar] [CrossRef]
- Altamura, S.; Muckenthaler, M.U. Iron toxicity in diseases of aging: Alzheimer’s disease, Parkinson’s disease and atherosclerosis. J. Alzheimer’s Dis. 2009, 16, 879–895. [Google Scholar] [CrossRef] [PubMed]
- Tsay, J.; Yang, Z.; Ross, F.P.; Cunningham-Rundles, S.; Lin, H.; Coleman, R.; Mayer-Kuckuk, P.; Doty, S.B.; Grady, R.W.; Giardina, P.J. Bone loss caused by iron overload in a murine model: Importance of oxidative stress. Blood 2010, 116, 2582–2589. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.O. Chronic iron overload and toxicity: Clinical chemistry perspective. Clin. Lab. Sci. 2001, 14, 209–219. [Google Scholar] [PubMed]
- Berliner, J.A.; Heinecke, J.W. The role of oxidized lipoproteins in atherogenesis. Free Radic. Biol. Med. 1996, 20, 707–727. [Google Scholar] [CrossRef]
- Nagy, L.; Tontonoz, P.; Alvarez, J.G.; Chen, H.; Evans, R.M. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARγ. Cell 1998, 93, 229–240. [Google Scholar] [CrossRef]
- Backe, M.B.; Moen, I.W.; Ellervik, C.; Hansen, J.B.; Mandrup-Poulsen, T. Iron Regulation of pancreatic beta-cell functions and oxidative stress. Ann. Rev. Nutr. 2016, 36, 241–273. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Stampfer, M.J. Body iron stores and coronary heart disease. Clin. Chem. 2002, 48, 601–603. [Google Scholar] [PubMed]
- Ziouzenkova, O.; Asatryan, L.; Akmal, M.; Tetta, C.; Wratten, M.L.; Loseto-Wich, G.; Jürgens, G.; Heinecke, J.; Sevanian, A. Oxidative cross-linking of ApoB100 and hemoglobin results in low density lipoprotein modification in blood relevance to atherogenesis caused by hemodialysis. J. Biol. Chem. 1999, 274, 18916–18924. [Google Scholar] [CrossRef] [PubMed]
- Barter, P.J.; Nicholls, S.; Rye, K.-A.; Anantharamaiah, G.; Navab, M.; Fogelman, A.M. Antiinflammatory properties of HDL. Circ. Res. 2004, 95, 764–772. [Google Scholar] [CrossRef]
- Cho, K.-H. Biomedicinal implications of high-density lipoprotein: Its composition, structure, functions, and clinical applications. BMB Rep. 2009, 42, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Li, X.-A. Dysfunctional high-density lipoprotein. Curr. Opin. Endocrinol. Diabetes Obes. 2009, 16, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Havel, R.J.; Eder, H.A.; Bragdon, J.H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Investig. 1955, 34, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Brewer, H.B.; Ronan, R.; Meng, M.; Bishop, C. Isolation and characterization of apolipoproteins AI, A-II, and A-IV. Methods Enzymol. 1986, 128, 223–246. [Google Scholar]
- Markwell, M.A.K.; Haas, S.M.; Bieber, L.; Tolbert, N. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 1978, 87, 206–210. [Google Scholar] [CrossRef]
- Noble, R.P. Electrophoretic separation of plasma lipoproteins in agarose gel. J. Lipid Res. 1968, 9, 693–700. [Google Scholar] [PubMed]
- Park, K.-H.; Jang, W.; Kim, K.-Y.; Kim, J.-R.; Cho, K.-H. Fructated apolipoprotein AI showed severe structural modification and loss of beneficial functions in lipid-free and lipid-bound state with acceleration of atherosclerosis and senescence. Biochem. Biophys. Res. Commun. 2010, 392, 295–300. [Google Scholar] [CrossRef]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef] [PubMed]
- Nusslein-Volhard, C.; Dahm, R. Zebrafish: A practical Approach; Oxford University Press: New York, NY, USA, 2002; Volume 4. [Google Scholar]
- Piñero, D.J.; Jones, B.C.; Beard, J.L. Variations in dietary iron alter behavior in developing rats. J. Nutr. 2001, 131, 311–318. [Google Scholar]
- Krijt, J.; Frýdlová, J.; Kukacková, L.; Fujikura, Y.; Prikryl, P.; Vokurka, M.; Necas, E. Effect of iron overload and iron deficiency on liver hemojuvelin protein. PLoS ONE 2012, 7, e37391. [Google Scholar] [CrossRef] [PubMed]
- Stoletov, K.; Fang, L.; Choi, S.-H.; Hartvigsen, K.; Hansen, L.F.; Hall, C.; Pattison, J.; Juliano, J.; Miller, E.R.; Almazan, F. Vascular lipid accumulation, lipoprotein oxidation, and macrophage lipid uptake in hypercholesterolemic zebrafish. Circ. Res. 2009, 104, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Sabaliauskas, N.A.; Foutz, C.A.; Mest, J.R.; Budgeon, L.R.; Sidor, A.T.; Gershenson, J.A.; Joshi, S.B.; Cheng, K.C. High-throughput zebrafish histology. Methods 2006, 39, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Koc, N.D.; Teksöz, N.; Ural, M.; Akbulut, C. Histological structure of zebrafish (Danio rerio, Hamilton, 1822) testicles. Elix. Aquac. 2012, 46, 8117–8120. [Google Scholar]
- Selman, K.; Wallace, R.A.; Barr, V. Oogenesis in fundulus heteroclitus. V. The relationship of yolk vesicles and cortical alveoli. J. Exp. Zool. Part A 1988, 246, 42–56. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.F. Iron metabolism and the polycystic ovary syndrome. Trends Endocrinol. Metab. 2012, 23, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; DesMarais, T.L.; Tong, Z.; Yao, Y.; Costa, M. Oxidative stress alters global histone modification and DNA methylation. Free Radic. Biol. Med. 2015, 82, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.H.; Kim, K.E.; Lee, Y.-J.; Nam, J.-H.; Hong, Y.M.; Shin, H.-J. Associations of matrix metalloproteinase (MMP)-8, MMP-9, and their inhibitor, tissue inhibitor of metalloproteinase-1, with obesity-related biomarkers in apparently healthy adolescent boys. Korean J. Pediatr. 2014, 57, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Habib, A.; Finn, A.V. The role of iron metabolism as a mediator of macrophage inflammation and lipid handling in atherosclerosis. Front. Pharmacol. 2014, 5, 195. [Google Scholar] [CrossRef] [PubMed]
- Vinchi, F.; Muckenthaler, M.U.; Da Silva, M.C.; Balla, G.; Balla, J.; Jeney, V. Atherogenesis and iron: From epidemiology to cellular level. Front. Pharmacol. 2014, 5, 94. [Google Scholar] [CrossRef]
- Holvoet, P.; Collen, D. Oxidized lipoproteins in atherosclerosis and thrombosis. FASEB J. 1994, 8, 1279–1284. [Google Scholar] [PubMed]
- Pietrangelo, A. Iron and the liver. Liver Int. 2016, 36, 116–123. [Google Scholar] [CrossRef]
- Eid, R.; Arab, N.T.; Greenwood, M.T. Iron mediated toxicity and programmed cell death: A review and a re-examination of existing paradigms. Biochim. Biophys. Acta 2017, 1864, 399–430. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, E.D. Iron toxicity: New conditions continue to emerge. Oxid. Med. Cell. Longev. 2009, 2, 107–109. [Google Scholar] [CrossRef] [PubMed]
| ND 1 | HCD 2 | |||||
|---|---|---|---|---|---|---|
| Control (n = 100) | 0.05% Fe2+ (n = 100) | 0.1% Fe2+ (n = 100) | Control (n = 100) | 0.05% Fe2+ (n = 100) | 0.1% Fe2+ (n = 100) | |
| Weight (mg) | 521 ± 36 a,3 | 465 ± 38 b | 465 ± 36 b | 578 ± 39 a | 435 ± 37 b | 470 ± 38 b |
| Weight (mg)/height (mm) | 14.5 ± 2.2 a | 12.2 ± 1.3 b | 12.7 ± 1.1 b | 14.7 ± 1.8 a | 11.7 ± 2.7 b | 12.2 ± 1.9 b |
| Total cholesterol (mg/dL) | 160 ± 12 a | 590 ± 19 b | 340 ±1 2 c | 623 ± 17 a | 893 ± 24 b | 847 ± 10 b |
| Triacylglyceride (mg/dL) | 347 ± 12 a | 500 ± 11 b | 604 ± 26 b | 397 ± 12 a | 382 ±16 b | 405 ±12 b |
| Glucose (mg/dL) | 188 ± 11 a | 455 ± 24 b | 369 ± 35 b | 206 ± 15 a | 253 ± 12 b | 278 ± 21 b |
| AST (Karmen/mL) | 264 ± 14 a | 390 ± 16 b | 539 ± 21 c | 351 ± 24 a | 441 ± 57 b | 551 ± 49 c |
| ALT (Karmen/mL) | 26 ± 4 a | 33 ± 9 a,b | 36 ± 12 b | 35 ± 3 a | 34 ± 10 a | 50 ± 2 b |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-H.; Yadav, D.; Kim, S.-J.; Kim, J.-R.; Cho, K. High Consumption of Iron Exacerbates Hyperlipidemia, Atherosclerosis, and Female Sterility in Zebrafish via Acceleration of Glycation and Degradation of Serum Lipoproteins. Nutrients 2017, 9, 690. https://doi.org/10.3390/nu9070690
Kim S-H, Yadav D, Kim S-J, Kim J-R, Cho K. High Consumption of Iron Exacerbates Hyperlipidemia, Atherosclerosis, and Female Sterility in Zebrafish via Acceleration of Glycation and Degradation of Serum Lipoproteins. Nutrients. 2017; 9(7):690. https://doi.org/10.3390/nu9070690
Chicago/Turabian StyleKim, So-Hee, Dhananjay Yadav, Suk-Jeong Kim, Jae-Ryong Kim, and Kyung‐Hyun Cho. 2017. "High Consumption of Iron Exacerbates Hyperlipidemia, Atherosclerosis, and Female Sterility in Zebrafish via Acceleration of Glycation and Degradation of Serum Lipoproteins" Nutrients 9, no. 7: 690. https://doi.org/10.3390/nu9070690
APA StyleKim, S.-H., Yadav, D., Kim, S.-J., Kim, J.-R., & Cho, K. (2017). High Consumption of Iron Exacerbates Hyperlipidemia, Atherosclerosis, and Female Sterility in Zebrafish via Acceleration of Glycation and Degradation of Serum Lipoproteins. Nutrients, 9(7), 690. https://doi.org/10.3390/nu9070690







