Toxoplasma Gondii Moderates the Association between Multiple Folate-Cycle Factors and Cognitive Function in U.S. Adults
Abstract
:1. Introduction
2. Materials and Methods
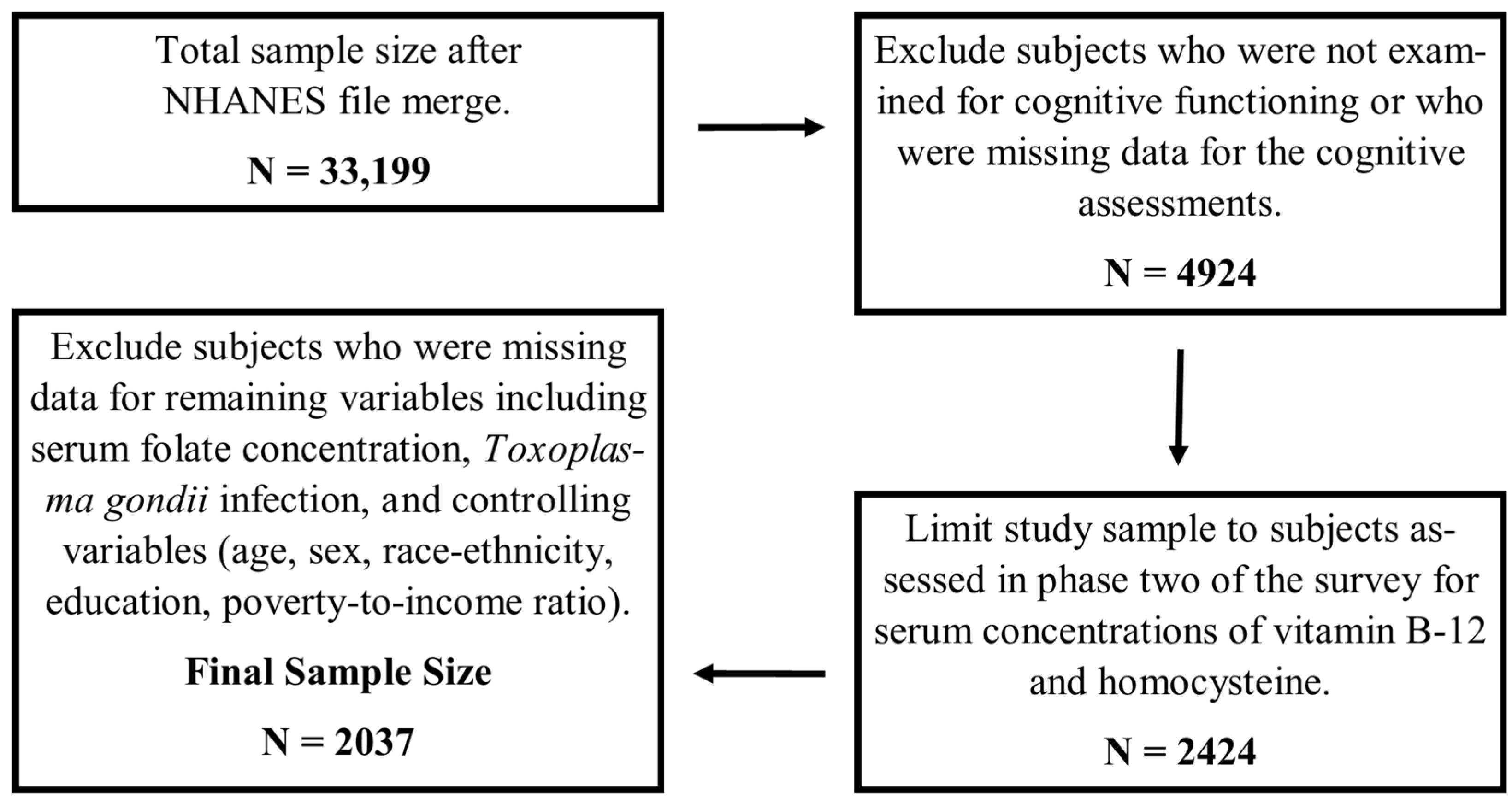
2.1. Study Sample
2.2. Toxoplasma Gondii
2.3. B-Vitamin Biomarkers
2.4. Cognitive Functioning
2.5. Covariates
2.6. Statistical Analysis
3. Results
4. Discussion and Conclusions
Author Contributions
Conflicts of Interest
References
- Jones, J.L.; Kruszon-Moran, D.; Rivera, H.N.; Price, C.; Wilkins, P.P. Toxoplasma gondii seroprevalence in the United States 2009–2010 and comparison with the past two decades. Am. J. Trop. Med. Hyg. 2014, 90, 1135–1139. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S.A.; Jones, J.L.; Conrad, P.A.; Patton, S.; Lindsay, D.S.; Dubey, J.P. Toxoplasma gondii: Epidemiology, feline clinical aspects, and prevention. Trends Parasitol. 2010, 26, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, V.B.; Suzuki, Y. Effects of Toxoplasma gondii infection on the brain. Schizophr. Bull. 2007, 33, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Martin, H.L.; Alsaady, I.; Howell, G.; Prandovszky, E.; Peers, C.; Robinson, P.; McConkey, G.A. Effect of parasitic infection on dopamine biosynthesis in dopaminergic cells. Neuroscience 2015, 306, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Prandovszky, E.; Gaskell, E.; Martin, H.; Dubey, J.P.; Webster, J.P.; McConkey, G.A. The neurotropic parasite Toxoplasma gondii increases dopamine metabolism. PLoS ONE 2011, 6, e23866. [Google Scholar] [CrossRef] [PubMed]
- Massimine, K.M.; Doan, L.T.; Atreya, C.A.; Stedman, T.T.; Anderson, K.S.; Joiner, K.A.; Coppens, I. Toxoplasma gondii is capable of exogenous folate transport. A likely expansion of the BT1 family of transmembrane proteins. Mol. Biochem. Parasitol. 2005, 144, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Folate in Health and Disease; Marcel Dekker, Inc.: New York, NY, USA, 1995; ISBN 0824792807.
- Kruman, I.I.; Mouton, P.R.; Emokpae, R., Jr.; Cutler, R.G.; Mattson, M.P. Folate deficiency inhibits proliferation of adult hippocampal progenitors. Neuropharmacol. Neurotoxicol. 2005, 16, 1055–1059. [Google Scholar] [CrossRef]
- Temudo, T.; Rios, M.; Prior, C.; Carrilho, I.; Santos, M.; Maciel, P.; Sequeiros, J.; Fonseca, M.; Monteiro, J.; Cabral, P.; et al. Evaluation of CSF neurotransmitters and folate in 25 patients with Rett disorder and effects of treatment. Brain Dev. 2009, 31, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Pu, D.; Shen, Y.; Wu, J. Association between MTHFR gene polymorphisms and the risk of autism spectrum disorders: A meta-analysis. Autism Res. 2013, 6, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Hultberg, B.; Isaksson, A.; Nilsson, K.; Gustafson, L. Markers for the functional availability of cobalamin/folate and their association with neuropsychiatric symptoms in the elderly. Int. J. Geriatr. Psychiatry 2001, 16, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Shea, T.B. Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci. 2003, 26, 137–146. [Google Scholar] [CrossRef]
- Michelakos, T.; Kousoulis, A.A.; Katsiardanis, K.; Dessypris, N.; Anastasiou, A.; Katsiardani, K.P.; Kanavidis, P.; Stefanadis, C.; Papadopoulos, F.C.; Petridou, E.T. Serum folate and B12 levels in association with cognitive impairment among seniors: Results from the Velestino study in Greece and meta-analysis. J. Aging Health 2013, 25, 589–616. [Google Scholar] [CrossRef] [PubMed]
- McGarel, C.; Pentieva, K.; Strain, J.J.; McNulty, H. Emerging roles for folate and related B-vitamins in brain health across the lifecycle. Proc. Nutr. Soc. 2015, 74, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Rai, V. Methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism and Alzheimer disease risk: A meta-analysis. Mol. Neurobiol. 2016, 54, 1173–1186. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Zhao, H.; Kong, Y.; Ye, M. Association between the MTHFR gene and Alzheimer’s disease: A meta-analysis. Int. J. Neurosci. 2011, 121, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Gunter, E.W.; Lewis, B.G.; Koncikowski, S.M. Laboratory Procedures Used for the Third National Health and Nutrition Examination Survey (NHANES III), 1988–1994; U.S. Department of Health and Human Services: Atlanta, GA, USA, 1996.
- Wyman, C.P.; Gale, S.D.; Hedges-Muncy, A.; Erickson, L.D.; Wilson, E.; Hedges, D.W. Association between Toxoplasma gondii seropositivity and memory function in nondemented older adults. Neurobiol. Aging 2017, 53, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Konishi, E. Annual change in immunoglobulin G and M antibody levels to Toxoplasma gondii in human sera. Microbiol. Immunol. 1989, 33, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Havlíček, J.; Gašová, Z.; Smith, A.P.; Zvára, K.; Flegr, J. Decrease of psychomotor performance in subjects with latent ‘asymptomatic’ toxoplasmosis. Parasitology 2001, 122, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Laboratory Procedure Manual. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2003-2004/labmethods/l06_c_met_folates-b12.pdf (accessed on 2 June 2017).
- Kessler, D.A.; Shalala, D.E. Food Standards: Amendment of Standards of Identity for Enriched Grain Products to Require Addition of Folic Acid; Department of Health and Human Services: Food and Drug Administration; Federal Register: Washington, DC, USA, 1996; Volume 61.
- Edward, F.; Krieg, J.; Chrislip, D.W.; Letz, R.E.; Otto, D.A.; Crespo, C.J.; Brightwell, W.S.; Ehrenberg, R.L. Neurobehavioral test performance in the third national health and nutrition examination survey. Neurotoxicol. Teratol. 2001, 23, 569–589. [Google Scholar] [CrossRef]
- StataCorp. Stata Statistical Software: Release 14, StataCorp LP: College Station, TX, USA, 2015.
- Jones, J.L.; McAuley, J.B.; Kruszon-Moran, D.; Wilson, M.; McQuillan, G.; Navin, T.; McAuley, J.B. Toxoplasma gondii infection in the United States: Seroprevalence and risk factors. Am. J. Epidemiol. 2001, 154, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Pappas, G.; Roussos, N.; Falagas, M.E. Toxoplasmosis snapshots: Global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int. J. Parasitol. 2009, 39, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Wahlin, T.-B.R.; Wahlin, A.; Winblad, B.; Backman, L. The influence of serum vitamin B12 and folate status on cognitive functioning in very old age. Biol. Psychol. 2001, 56, 247–265. [Google Scholar] [CrossRef]
- Goldfarb, E.V.; Chun, M.M.; Phelps, E.A. Memory-guided attention: Independent contributions of the hippocampus and striatum. Neuron 2016, 89, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Hannula, D.E.; Ranganath, C. Medial temporal lobe activity predicts successful relational memory binding. J. Neurosci. 2008, 28, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Kruman, I.I.; Kumaravel, T.S.; Lohani, A.; Pedersen, W.A.; Cutler, R.G.; Kruman, Y.; Haughey, N.; Lee, J.; Evans, M.; Mattson, M.P. Folic acid deficiency and homocysteine impair DNA repair in hippocampal neurons and sensitize them to amyloid toxicity in experimental models of Alzheimer’s disease. J. Neurosci. 2002, 22, 1752–1762. [Google Scholar] [PubMed]
- Madsen, S.K.; Rajagopalan, P.; Joshi, S.H.; Toga, A.W.; Thompson, P.M.; the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Higher homocysteine associated with thinner cortical gray matter in 803 participants from the Alzheimer’s disease neuroimaging initiative. Neurobiol. Aging 2015, 36 (Suppl. 1), S203–S210. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, P.; Hua, X.; Toga, A.W.; Jack, C.R., Jr.; Weiner, M.W.; Thompson, P.M. Homocysteine effects on brain volumes mapped in 732 elderly individuals. Neuroreport 2011, 22, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Ramaekers, V.T.; Thony, B.; Sequeira, J.M.; Ansseau, M.; Philippe, P.; Boemer, F.; Bours, V.; Quadros, E.V. Folinic acid treatment for schizophrenia associated with folate receptor autoantibodies. Mol. Genet. Metab. 2014, 113, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, S.; Gerretsen, P.; Takeuchi, H.; Caravaggio, F.; Chow, T.; Le Foll, B.; Mulsant, B.; Pollock, B.; Graff-Guerrero, A. The potential role of dopamine D3 receptor neurotransmission in cognition. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2013, 23, 799–813. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.T.; McDonnell, A.P.; Kelly, C.B. Folic acid: Neurochemistry, metabolism and relationship to depression. Hum. Psychopharmacol. 2004, 19, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Terry, A.V., Jr.; Buccafusco, J.J.; Wilson, C. Cognitive dysfunction in neuropsychiatric disorders: Selected serotonin receptor subtypes as therapeutic targets. Behav. Brain Res. 2008, 195, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Berrett, A.N.; Gale, S.D.; Erickson, L.D.; Brown, B.L.; Hedges, D.W. Folate and inflammatory markers moderate the association between Helicobacter pylori exposure and cognitive function in us adults. Helicobacter 2016, 21, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Kountouras, J.; Gavalas, E.; Boziki, M.; Zavos, C. Helicobacter pylori may be involved in cognitive impairment and dementia development through induction of atrophic gastritis, vitamin B-12–folate deficiency, and hyperhomocysteinemia sequence. Am. J. Clin. Nutr. 2007, 86, 805–809. [Google Scholar] [PubMed]
- Cheng, D.M.; Jiang, Y.G.; Huang, C.Y.; Kong, H.Y.; Pang, W.; Yang, H.P. Polymorphism of MTHFR C677T, serum vitamin levels and cognition in subjects with hyperhomocysteinemia in China. Nutr. Neurosci. 2010, 13, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Ho, V.; Massey, T.E.; King, W.D. Effects of methionine synthase and methylenetetrahydrofolate reductase gene polymorphisms on markers of one-carbon metabolism. Genes Nutr. 2013, 8, 571–580. [Google Scholar] [CrossRef] [PubMed]
| T. Gondii Seropositive N = 417 | T. Gondii Seronegative N = 1620 | ||||
|---|---|---|---|---|---|
| Mean or Frequency | SE | Mean or Frequency | SE | p | |
| Sociodemographic Factors | |||||
| Age (years) | 41.54 | 1.01 | 36.16 | 0.63 | <0.001 |
| Poverty-to-income Ratio | 3.23 | 0.25 | 3.38 | 0.13 | 0.316 |
| Education (years) | 12.36 | 0.23 | 13.13 | 0.12 | <0.001 |
| Female | 45.63% | 2.65% | 51.55% | 1.08% | 0.050 |
| Race-ethnicity | |||||
| Non-Hispanic White | 72.73% | 3.92% | 77.98% | 1.27% | 0.159 |
| Non-Hispanic Black | 10.77% | 1.64% | 10.55% | 0.47% | 0.896 |
| Hispanic | 5.87% | 0.81% | 5.50% | 0.40% | 0.691 |
| Other | 10.62% | 3.25% | 5.97% | 1.14% | 0.097 |
| Biochemistry | |||||
| Folate (ng/mL) | 7.08 | 0.51 | 6.89 | 0.24 | 0.807 |
| Vitamin B-12 (pg/mL) | 521.83 | 44.25 | 474.01 | 7.08 | 0.623 |
| Homocysteine (umol/L) | 9.36 | 0.27 | 9.56 | 0.25 | 0.282 |
| Cognitive Testing | |||||
| Symbol Digit Substitution Score | 2.85 | 0.05 | 2.55 | 0.03 | <0.001 |
| Serial Digit Learning Score | 5.45 | 0.31 | 3.91 | 0.15 | <0.001 |
| Reaction Time (sec) | 240.1 | 3.03 | 234.47 | 1.71 | 0.070 |
| Symbol Digit Substitution | Serial Digit Learning | Reaction Time | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | p | β | 95% CI | p | β | 95% CI | p | |
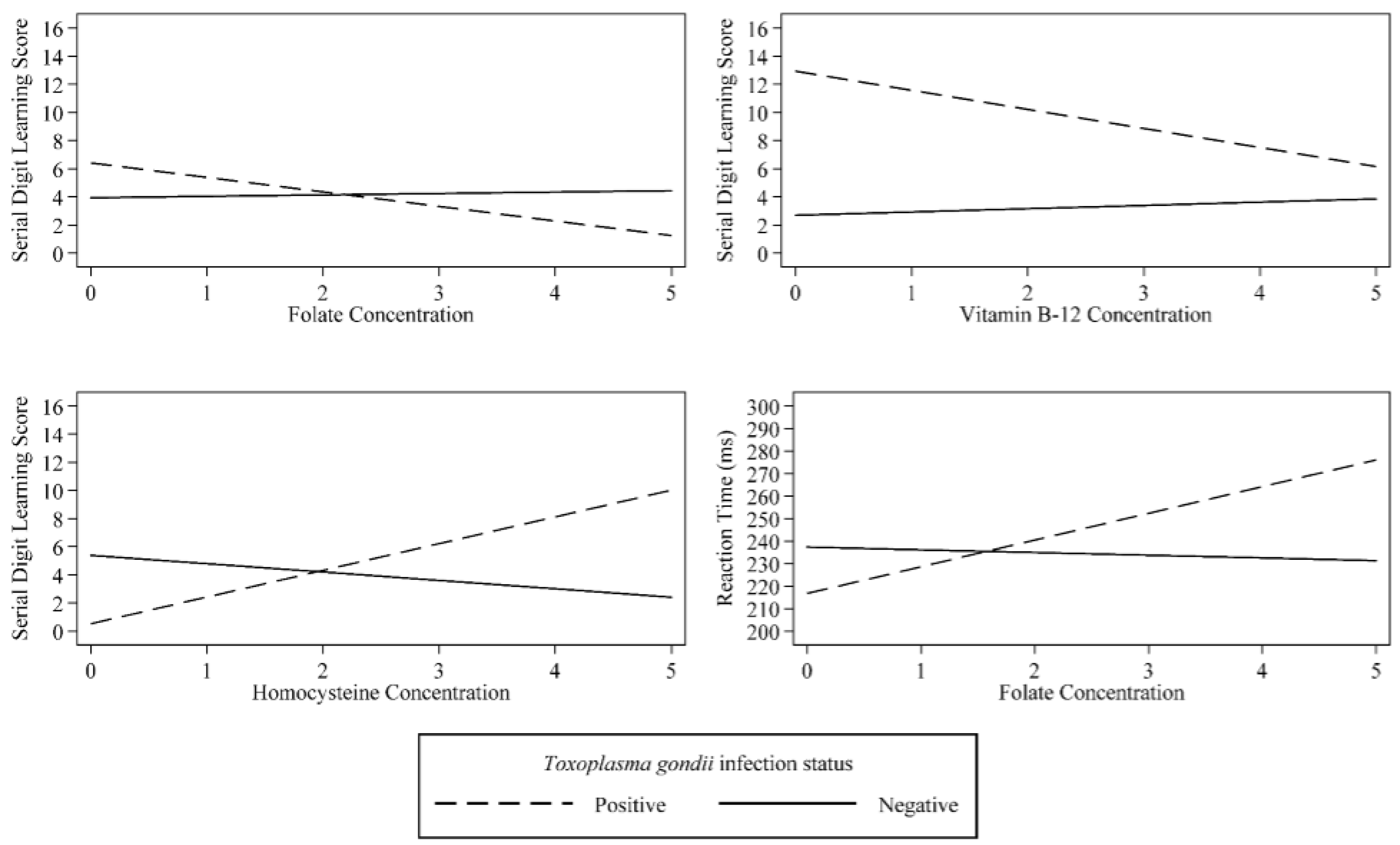
| Folate | 0.01 | (−0.04, 0.05) | 0.786 | 0.10 | (−0.33, 0.53) | 0.641 | −1.19 | (−5.55, 3.16) | 0.585 |
| T. gondii | 0.15 | (−0.08, 0.38) | 0.185 | 2.48 | (1.02, 3.94) | 0.001 | −20.60 | (−34.38, −6.83) | 0.004 |
| T. gondii × Folate Interaction | −0.07 | (−0.20, 0.07) | 0.344 | −1.13 | (−1.97, −0.29) | 0.009 | 13.07 | (4.20, 21.93) | 0.005 |
| Race-Ethnicity | |||||||||
| Non-Hispanic White (ref) | - | - | - | - | - | - | - | - | - |
| Non-Hispanic Black | 0.30 | (0.21, 0.39) | <0.001 | 1.72 | (1.15, 2.29) | <0.001 | 5.92 | (0.14, 11.70) | 0.045 |
| Mexican American | 0.27 | (0.18, 0.37) | <0.001 | 2.52 | (1.95, 3.09) | <0.001 | 1.23 | (−6.86, 9.32) | 0.761 |
| Other | 0.29 | (0.10, 0.47) | 0.003 | 1.88 | (0.69, 3.08) | 0.003 | −4.15 | (−14.36, 6.06) | 0.418 |
| Female | −0.17 | (−0.24, −0.10) | <0.001 | 0.08 | (−0.23, 0.38) | 0.611 | 8.63 | (1.60, 15.66) | 0.017 |
| Age (years) | 0.03 | (0.03, 0.03) | <0.001 | 0.09 | (0.07, 0.11) | <0.001 | 0.36 | (0.14, 0.58) | 0.002 |
| PIR | −0.03 | (−0.04, −0.02) | <0.001 | −0.25 | (−0.35, −0.14) | <0.001 | −3.13 | (−4.33, −1.92) | <0.001 |
| Education (years) | −0.10 | (−0.11, −0.08) | <0.001 | −0.46 | (−0.57, −0.35) | <0.001 | −2.93 | (−4.08, −1.79) | <0.001 |
| Constant | 2.91 | (2.70, 3.12) | <0.001 | 6.77 | (4.96, 8.57) | <0.001 | 267.68 | (250.01, 285.34) | <0.001 |
| Symbol Digit Substitution | Serial Digit Learning | Reaction Time | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | p | β | 95% CI | p | β | 95% CI | p | |
| Vitamin B-12 | 0.05 | (−0.00, 0.11) | 0.057 | 0.23 | (−0.26, 0.72) | 0.346 | −3.04 | (−8.77, 2.68) | 0.291 |
| T. gondii | 1.06 | (−0.12, 2.25) | 0.076 | 10.24 | (2.14, 18.33) | 0.014 | 5.26 | (−123.23, 133.74) | 0.935 |
| T. gondii Vitamin B-12 Interaction | −0.17 | (−0.36, 0.03) | 0.087 | −1.59 | (−2.92, −0.26) | 0.020 | −0.61 | (−21.40, 20.19) | 0.954 |
| Race-Ethnicity | |||||||||
| Non-Hispanic White (ref) | - | - | - | - | - | - | - | - | - |
| Non-Hispanic Black | 0.30 | (0.21, 0.39) | <0.001 | 1.78 | (1.19, 2.37) | <0.001 | 6.09 | (0.18, 12.01) | 0.044 |
| Mexican American | 0.27 | (0.18, 0.37) | <0.001 | 2.53 | (1.96, 3.10) | <0.001 | 1.81 | (−6.22, 9.84) | 0.653 |
| Other | 0.29 | (0.10, 0.47) | 0.003 | 1.87 | (0.67, 3.08) | 0.003 | −4.59 | (−14.98, 5.81) | 0.380 |
| Female | −0.17 | (−0.24, −0.10) | <0.001 | 0.08 | (−0.21, 0.36) | 0.583 | 8.52 | (1.64, 15.40) | 0.016 |
| Age (years) | 0.03 | (0.03, 0.03) | <0.001 | 0.09 | (0.07, 0.11) | <0.001 | 0.40 | (0.17, 0.63) | 0.001 |
| PIR | −0.03 | (−004, −0.02) | <0.001 | −0.24 | (−0.34, −0.14) | <0.001 | −3.14 | (−4.32, −1.97) | <0.001 |
| Education (years) | −0.10 | (−0.11, −0.08) | <0.001 | −0.47 | (−0.58, −0.35) | <0.001 | −2.83 | (−4.03, −1.64) | <0.001 |
| Constant | 2.61 | (2.22, 2.99) | <0.001 | 5.68 | (2.42, 8.93) | 0.001 | 281.51 | (239.25, 323.77) | <0.001 |
| Symbol Digit Substitution | Serial Digit Learning | Reaction Time | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | p | β | 95% CI | p | β | 95% CI | p | |
| Homocysteine | −0.05 | (−0.13, 0.02) | 0.166 | −0.59 | (−1.22, 0.03) | 0.060 | −2.72 | (−9.05, 3.62) | 0.393 |
| T. gondii | −0.35 | (−1.17, 0.48) | 0.403 | −4.87 | (−7.88, −1.85) | 0.002 | −10.87 | (−45.43, 23.70) | 0.531 |
| T. gondii × Homocysteine Interaction | 0.18 | (−0.20, 0.56) | 0.351 | 2.49 | (1.09, 3.89) | 0.001 | 5.67 | (−11.10, 22.43) | 0.501 |
| Race-Ethnicity | |||||||||
| Non-Hispanic White (ref) | - | - | - | - | - | - | - | - | - |
| Non-Hispanic Black | 0.31 | (0.22, 0.40) | <0.001 | 1.79 | (1.19, 2.38) | <0.001 | 5.50 | (−0.33, 11.34) | 0.064 |
| Mexican American | 0.27 | (0.17, 0.37) | <0.001 | 2.52 | (1.96, 3.08) | <0.001 | 1.41 | (−6.81, 9.62) | 0.732 |
| Other | 0.29 | (0.11, 0.48) | 0.002 | 1.98 | (0.81, 3.15) | 0.001 | −3.92 | (−13.95, 6.11) | 0.436 |
| Female | −0.18 | (−0.25, −0.10) | <0.001 | 0.02 | (−0.31, 0.34) | 0.926 | 8.13 | (1.12, 15.13) | 0.024 |
| Age (years) | 0.03 | (0.03, 0.03) | <0.001 | 0.09 | (0.07, 0.11) | <0.001 | 0.42 | (0.18, 0.66) | 0.001 |
| PIR | -0.03 | (−0.05, −0.02) | <0.001 | −0.25 | (−0.35, −0.15) | <0.001 | −3.21 | (−4.45, −1.98) | <0.001 |
| Education (years) | -0.10 | (−0.11, −0.08) | <0.001 | −0.47 | (−0.59, −0.36) | <0.001 | −2.83 | (−4.02, −1.64) | <0.001 |
| Constant | 3.04 | (2.78, 3.30) | <0.001 | 8.36 | (6.08, 10.65) | <0.001 | 268.43 | (243.08, 293.78) | <0.001 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berrett, A.N.; Gale, S.D.; Erickson, L.D.; Brown, B.L.; Hedges, D.W. Toxoplasma Gondii Moderates the Association between Multiple Folate-Cycle Factors and Cognitive Function in U.S. Adults. Nutrients 2017, 9, 564. https://doi.org/10.3390/nu9060564
Berrett AN, Gale SD, Erickson LD, Brown BL, Hedges DW. Toxoplasma Gondii Moderates the Association between Multiple Folate-Cycle Factors and Cognitive Function in U.S. Adults. Nutrients. 2017; 9(6):564. https://doi.org/10.3390/nu9060564
Chicago/Turabian StyleBerrett, Andrew N., Shawn D. Gale, Lance D. Erickson, Bruce L. Brown, and Dawson W. Hedges. 2017. "Toxoplasma Gondii Moderates the Association between Multiple Folate-Cycle Factors and Cognitive Function in U.S. Adults" Nutrients 9, no. 6: 564. https://doi.org/10.3390/nu9060564
APA StyleBerrett, A. N., Gale, S. D., Erickson, L. D., Brown, B. L., & Hedges, D. W. (2017). Toxoplasma Gondii Moderates the Association between Multiple Folate-Cycle Factors and Cognitive Function in U.S. Adults. Nutrients, 9(6), 564. https://doi.org/10.3390/nu9060564





