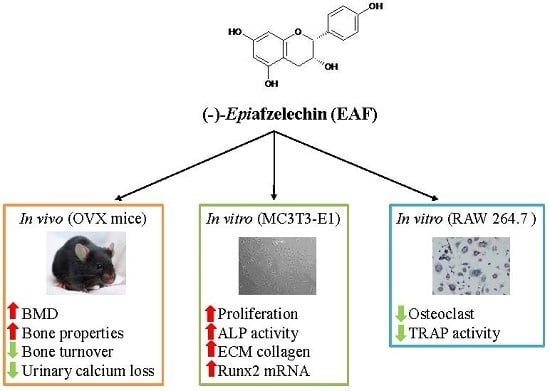
(−)-Epiafzelechin Protects against Ovariectomy-induced Bone Loss in Adult Mice and Modulate Osteoblastic and Osteoclastic Functions In Vitro
Abstract
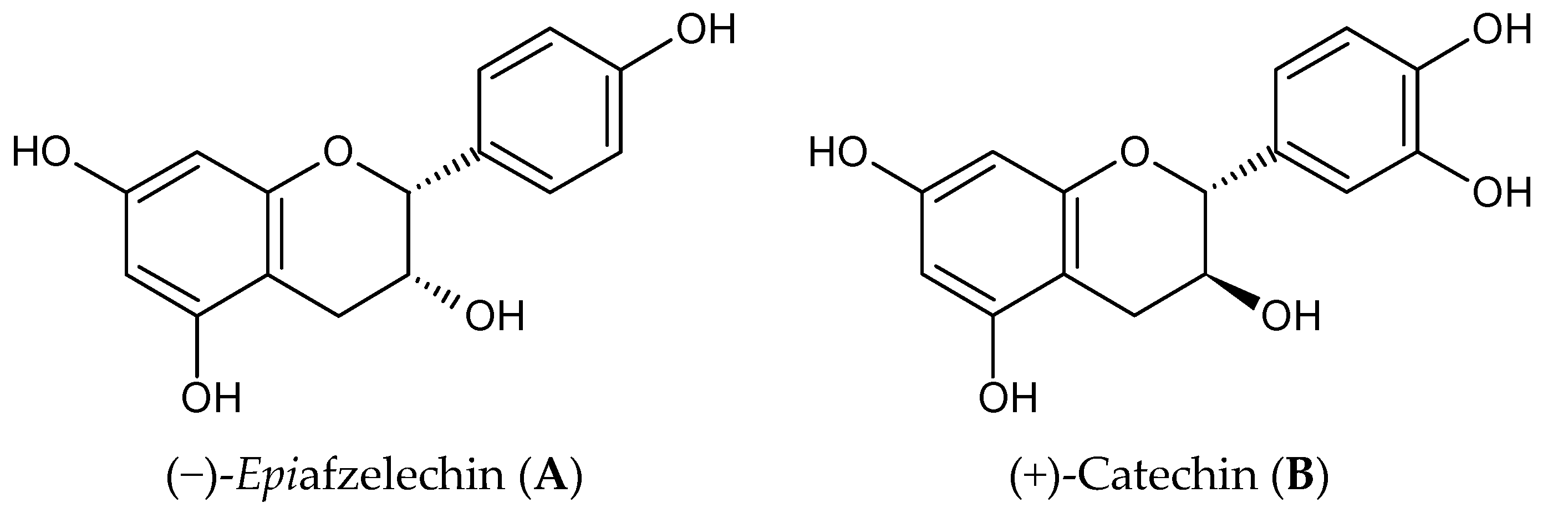
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animal Experiments
2.3. Biochemical Assays of Serum and Urine Samples
2.4. Microcomputed Tomography (μCT)
2.5. Culture of Murine Pre-osteoblastic MC3T3-E1
2.6. Gene Expression of MC3T3-E1 Cells
2.7. Culture of Murine Leukemia RAW 264.7 Cells
2.8. Statistical Analysis
3. Results
3.1. EAF Suppressed Body Weight Gain, Excessive Urinary Ca, and Bone Markers in OVX Mice
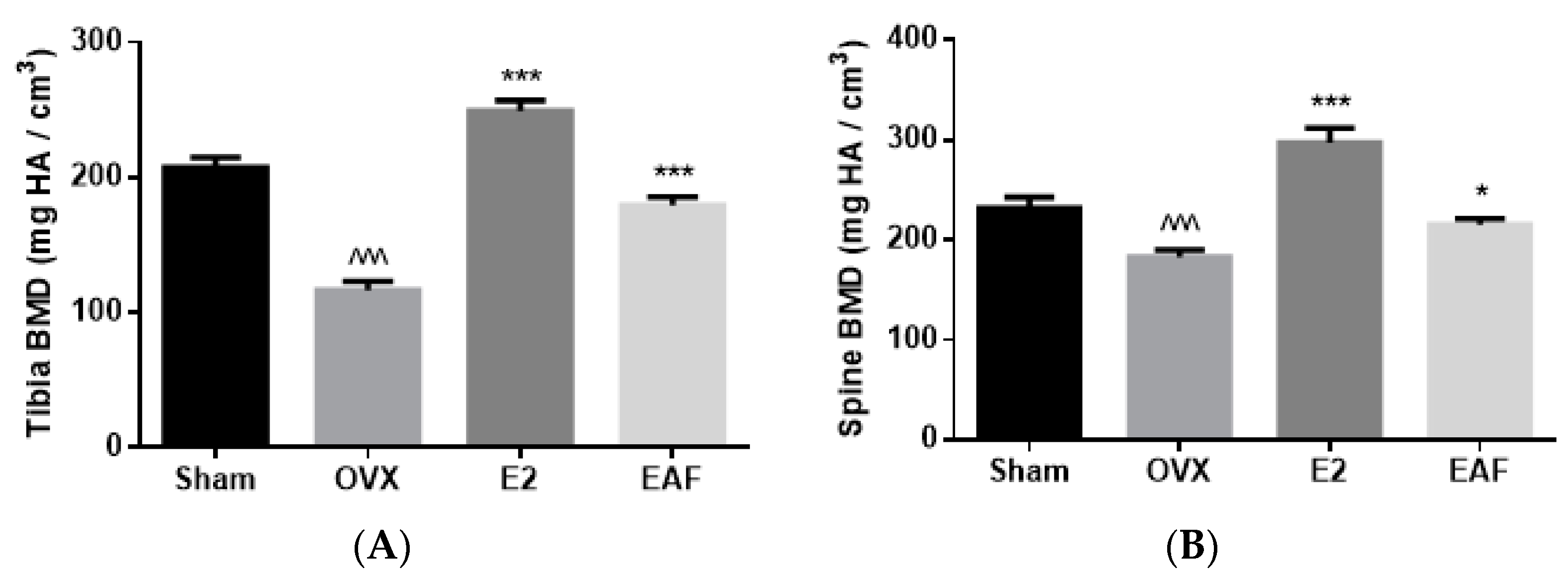
3.2. EAF Improved Bone Properties of Proximal Tibia and Lumbar Vertebra (L4) in OVX Mice
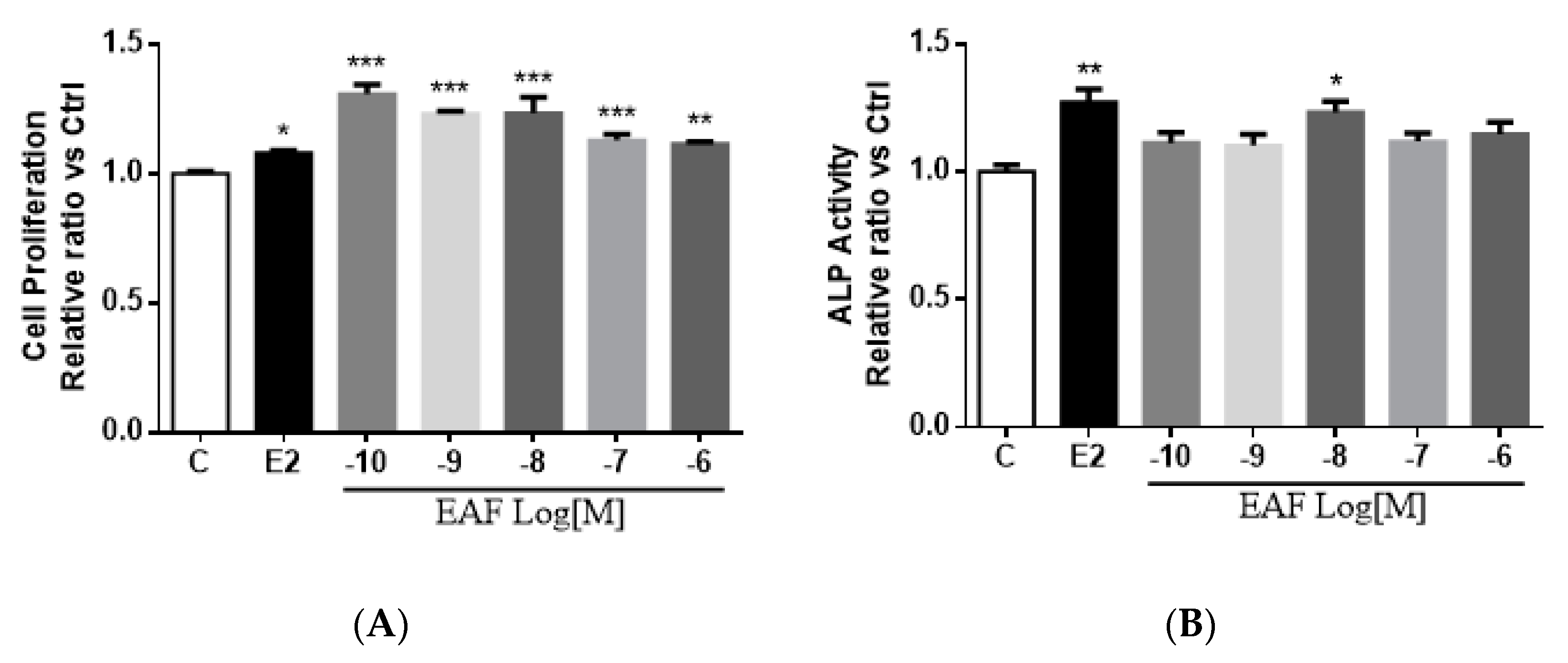
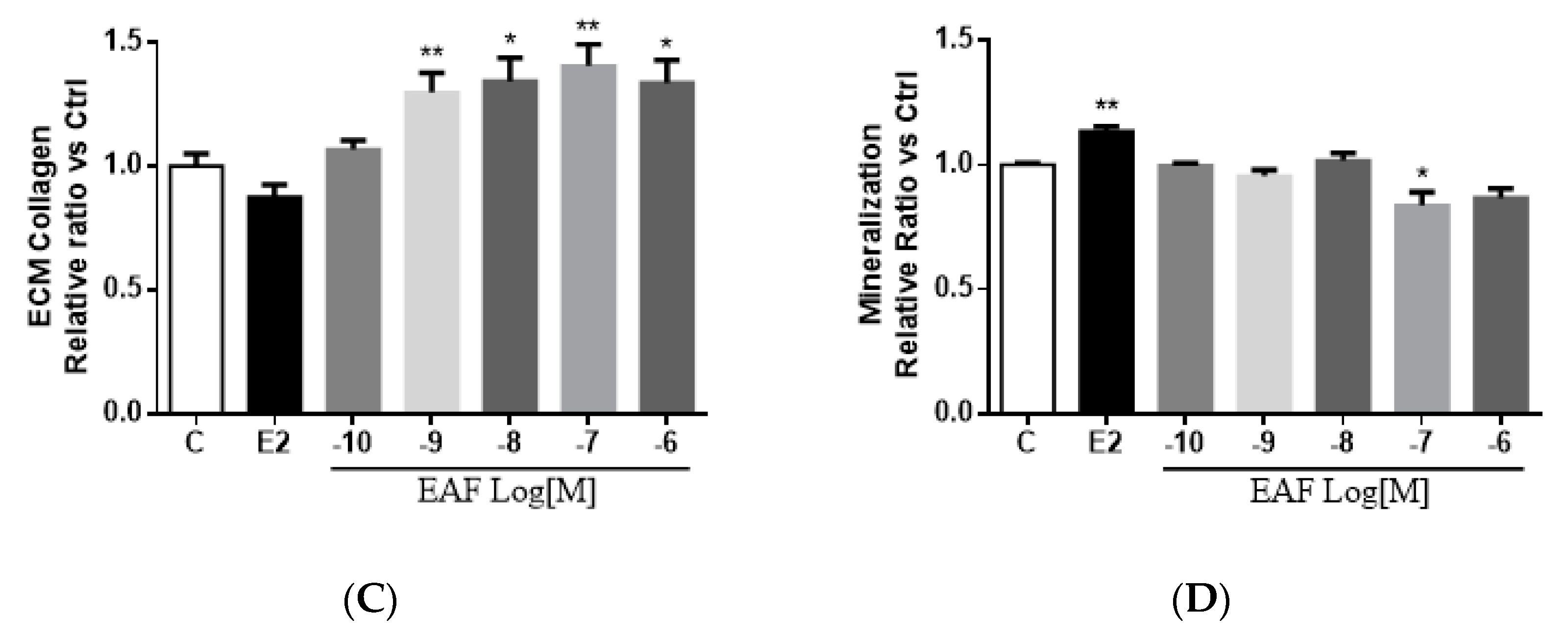
3.3. EAF Induced Osteoblastic Function in MC3T3-E1 Cells
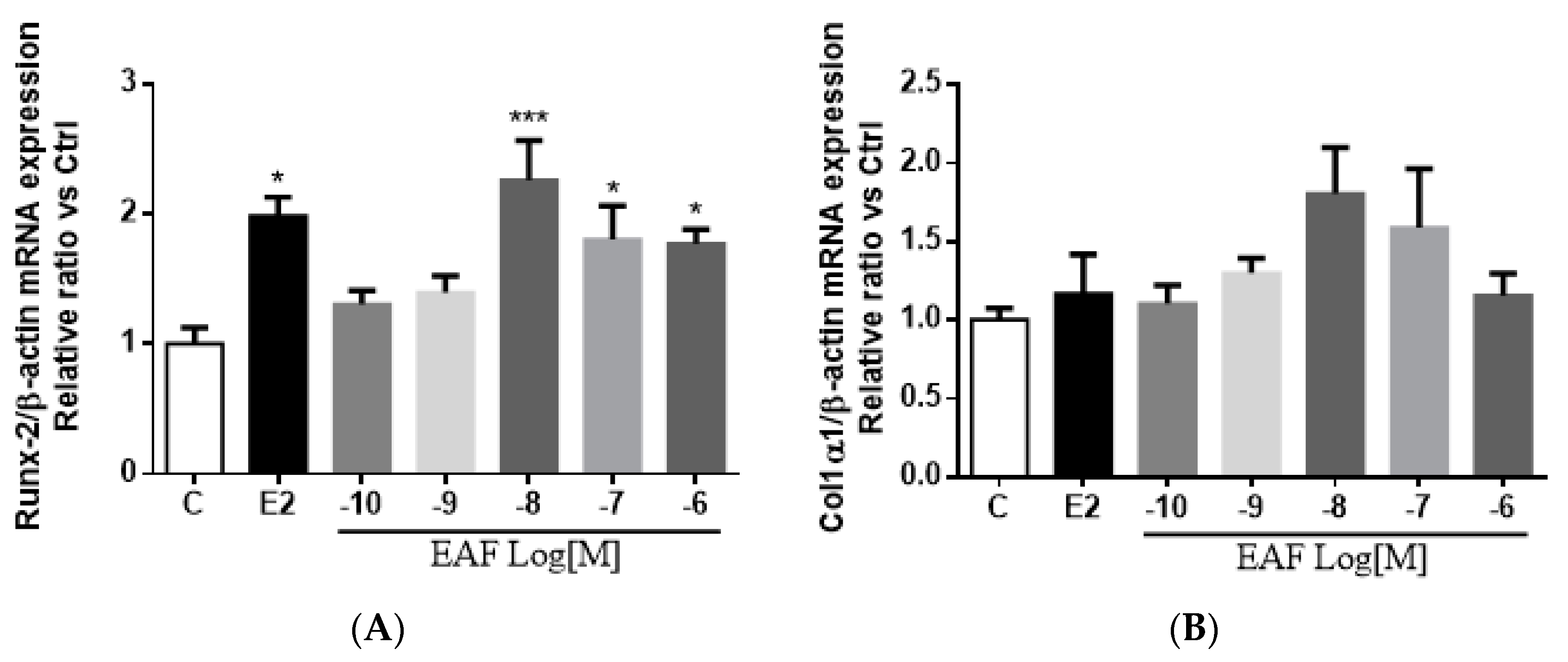
3.4. EAF Increased Osteoblast-specific mRNA Expression in MC3T3-E1 Cells
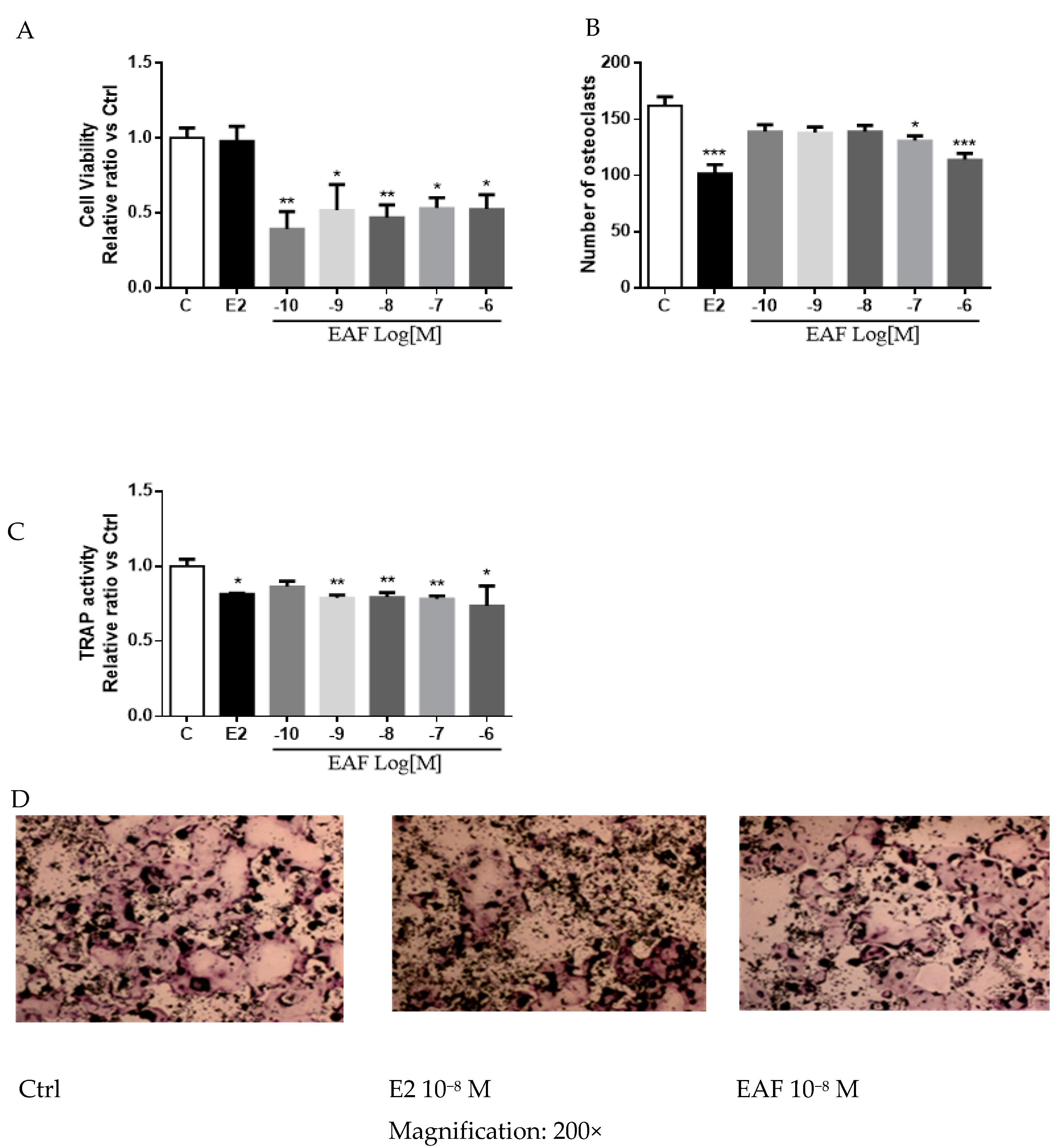
3.5. EAF Reduced Osteoclastic Activities in Receptor Activator of NF-κB Ligand (RANKL) Induced RAW 264.7 Cells
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hardcastle, A.C.; Aucott, L.; Reid, D.M.; Macdonald, H.M. Associations between dietary flavonoid intakes and bone health in a Scottish population. J. Bone Miner. Res. 2011, 26, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Welch, A.; MacGregor, A.; Jennings, A.; Fairweather-Tait, S.; Spector, T.; Cassidy, A. Habitual flavonoid intakes are positively associated with bone mineral density in women. J. Bone Miner. Res. 2012, 27, 1872–1878. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Q.; He, L.P.; Liu, Y.H.; Liu, J.; Su, Y.X.; Chen, Y.M. Association between dietary intake of flavonoid and bone mineral density in middle aged and elderly Chinese women and men. Osteoporos. Int. 2014, 25, 2417–2425. [Google Scholar] [CrossRef] [PubMed]
- Myers, G.; Prince, R.L.; Kerr, D.A.; Devine, A.; Woodman, R.J.; Lewis, J.R.; Hodgson, J.M. Tea and flavonoid intake predict osteoporotic fracture risk in elderly Australian women: A prospective study. Am. J. Clin. Nutr. 2015, 102, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.Y.; Wang, X.L.; Mok, S.K.; Lai, W.P.; Chow, H.K.; Leung, P.C.; Yao, X.S.; Wong, M.S. Naringin improves bone properties in ovariectomized mice and exerts oestrogen-like activities in rat osteoblast-like (UMR-106) cells. Br. J. Pharmacol. 2010, 159, 1693–1703. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.; Ma, Y.; Sheng, Z.; Jin, Y.; Zhang, Y.; Fang, L.; Fan, H.; Liao, E. Effects of genistein on vertebral trabecular bone microstructure, bone mineral density, microcracks, osteocyte density, and bone strength in ovariectomized rats. J. Bone Miner. Metab. 2008, 26, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Kang, L.; Lin, R.W.; Fu, Y.C.; Lin, Y.S.; Chang, J.K.; Chen, H.T.; Chen, C.H.; Lin, S.Y.; Wang, G.J.; et al. (−)-Epigallocatechin-3-gallate improves bone microarchitecture in ovariectomized rats. Menopause 2013, 20, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Mok, S.K.; Chen, W.F.; Lai, W.P.; Leung, P.C.; Wang, X.L.; Yao, X.S.; Wong, M.S. Icariin protects against bone loss induced by oestrogen deficiency and activates oestrogen receptor-dependent osteoblastic functions in UMR 106 cells. Br. J. Pharmacol. 2010, 159, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Q.; Wan, H.Y.; Helferich, W.G.; Wong, M.S. Genistein and a soy extract differentially affect three-dimensional bone parameters and bone-specific gene expression in ovariectomized mice. J. Nutr. 2009, 139, 2230–2236. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M. Isoflavone and Bone Metabolism: Its Cellular Mechanism and Preventive Role in Bone Loss. J. Health Sci. 2002, 48, 209–222. [Google Scholar] [CrossRef]
- Chen, X.W.; Garner, S.C.; Anderson, J.J. Isoflavones regulate interleukin-6 and osteoprotegerin synthesis during osteoblast cell differentiation via an estrogen-receptor-dependent pathway. Biochem. Biophys. Res. Commun. 2002, 295, 417–422. [Google Scholar] [CrossRef]
- Windahl, S.H.; Lagerquist, M.K.; Andersson, N.; Jochems, C.; Kallkof, A.; Hakansson, C.; Inzunza, J.; Gustafsson, J.A.; Saag, P.T.; Calsten, H.; et al. Identification of target cells for the genomic effects of estrogens in bone. Endocrinology 2007, 148, 5688–5695. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, T.B.; Utian, W.H.; Barnes, S.; Gold, E.B.; Basaria, S.S.; Aso, T.; Kronenberg, F.; Frankenfeld, C.L.; Cline, J.M.; Landgren, B.M.; et al. The role of soy isoflavones in menopausal health: Report of The North American Menopause Society/Wulf H. Utian Translational Science Symposium in Chicago, IL (October 2010). Menopause 2011, 18, 732–753. [Google Scholar]
- Chen, C.H.; Ho, M.L.; Chang, J.K.; Hung, S.H.; Wang, G.J. Green tea catechin enhances osteogenesis in a bone marrow mesenchymal stem cell line. Osteoporos. Int. 2005, 16, 2039–2045. [Google Scholar] [CrossRef] [PubMed]
- Vali, B.; Rao, L.G.; El-Sohemy, A. Epigallocatechin-3-gallate increases the formation of mineralized bone nodules by human osteoblast-like cells. J. Nutr. Biochem. 2007, 18, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.M.; Hwang, J.K. Effects of (+)-catechin on the function of osteoblastic cells. Biol. Pharm. Bull. 2003, 26, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Oka, Y.; Iwai, S.; Amano, H.; Irie, Y.; Yatomi, K.; Ryu, K.; Yamada, S.; Inagaki, K.; Oguchi, K. Tea polyphenols inhibit rat osteoclast formation and differentiation. J. Pharmacol. Sci. 2012, 118, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.L.; Yeh, J.K.; Cao, J.J.; Wang, J.S. Green tea and bone metabolism. Nutr. Res. 2009, 29, 437–456. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Lambert, J.D.; Yang, C.S. Bioavailability and stability issues in understanding the cancer preventive effects of tea polyphenols. J. Sci. Food Agric. 2006, 86, 2256–2265. [Google Scholar] [CrossRef]
- Min, K.R.; Hwang, B.Y.; Lim, H.S.; Kang, B.S.; Oh, G.J.; Lee, J.; Kang, S.H.; Lee, K.S.; Ro, J.S.; Kim, Y. (−)-Epiafzelechin: Cyclooxygenase-1 inhibitor and anti-inflammatory agent from aerial parts of Celastrus orbiculatus. Planta. Med. 1999, 65, 460–462. [Google Scholar] [CrossRef] [PubMed]
- Kpegba, K.; Agbonon, A.; Petrovic, A.G.; Amouzou, E.; Gbeassor, M.; Proni, G.; Nesnas, N. Epiafzelechin from the root bark of Cassia sieberiana: Detection by DART mass spectrometry, spectroscopic characterization, and antioxidant properties. J. Nat. Prod. 2011, 74, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.A.; Zhao, Y.M. Isolation and indentification of chemical compounds from Drynaria fortunei. China J. Chin. Mater. Med. 2005, 30, 443–444. [Google Scholar]
- Zeng, X.; Tian, J.; Cai, K.; Wu, X.; Wang, Y.; Zheng, Y.; Su, Y.; Cui, L. Promoting osteoblast differentiation by the flavanes from Huangshan Maofeng tea is linked to a reduction of oxidative stress. Phytomedicine 2014, 21, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Law, M.C.; Wong, K.C.; Pang, W.Y.; Wong, M.S.; Chan, T.H. Chemical synthesis and biological study of 4 beta-carboxymethyl-epiafzelechin acid, an osteoprotective compound from the rhizomes of Drynaria fortunei. Medchemcomm 2012, 3, 801–806. [Google Scholar] [CrossRef]
- Wong, K.C.; Law, M.C.; Wong, M.S.; Chan, T.H. Development of a UPLC-MS/MS bioanalytical method for the pharmacokinetic study of (−)-epiafzelechin, a flavan-3-ol with osteoprotective activity, in C57BL/6J mice. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014, 967, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.B.; Chan, T.H. Enantioselective synthesis of afzelechin and epiafzelechin. Tetrahedron 2004, 60, 8207–8211. [Google Scholar] [CrossRef]
- Wang, D.; Christensen, K.; Chawla, K.; Xiao, G.; Krebsbach, P.H.; Franceschi, R.T. Isolation and characterization of MC3T3-E1 preosteoblast subclones with distinct in vitro and in vivo differentiation/mineralization potential. J. Bone Miner. Res. 1999, 14, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Karsenty, G. Role of Cbfa1 in osteoblast differentiation and function. Semin. Cell Dev. Biol. 2000, 11, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Cuetara, B.L.; Crotti, T.N.; O’Donoghue, A.J.; McHugh, K.P. Cloning and characterization of osteoclast precursors from the RAW264.7 cell line. In Vitro Cell Dev. Biol. Anim. 2006, 42, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S. Minireview: The OPG/RANKL/RANK system. Endocrinology 2001, 142, 5050–5055. [Google Scholar] [CrossRef] [PubMed]
- Quarles, L.D.; Yohay, D.A.; Lever, L.W.; Caton, R.; Wenstrup, R.J. Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: An in vitro model of osteoblast development. J. Bone Miner. Res. 1992, 7, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Zuo, C.; Huang, Y.; Bajis, R.; Sahih, M.; Li, Y.P.; Dai, K.; Zhang, X. Osteoblastogenesis regulation signals in bone remodeling. Osteoporos. Int. 2012, 23, 1653–1663. [Google Scholar] [CrossRef] [PubMed]
- Merriman, H.L.; Vanwijnen, A.J.; Hiebert, S.; Bidwell, J.P.; Fey, E.; Lian, J.; Stein, J.; Stein, G.S. The Tissue-Specific Nuclear Matrix Protein, Nmp-2, Is a Member of the Aml/Cbf/Pebp2/Runt Domain Transcription Factor Family—Interactions with the Osteocalcin Gene Promoter. Biochemistry 1995, 34, 13125–13132. [Google Scholar] [CrossRef] [PubMed]
- Ducy, P.; Zhang, R.; Geoffroy, V.; Ridall, A.L.; Karsenty, G. Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell 1997, 89, 747–754. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Wan, H.Y.; Xiao, H.H.; Lai, W.P.; Yao, X.S.; Wong, M.S. Study of the mechanisms by which Sambucus williamsii HANCE extract exert protective effects against ovariectomy-induced osteoporosis in vivo. Osteoporos. Int. 2011, 22, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Gan, M.; Zou, J.; Zhu, X.; Shi, Q.; Zhao, H.; Luo, Z.; Zhang, W.; Li, S.; Niu, J.; et al. Effect of (−)-epigallocatechin-3-gallate in preventing bone loss in ovariectomized rats and possible mechanisms. Int. J. Clin. Exp. Med. 2014, 7, 4183–4190. [Google Scholar] [PubMed]
- Peng, Y.; Yu, B.; Liu, F. Epigallocatechin-3-gallate promotes osteoblastic activity in human osteoblast-like cells. Trop. J. Pharm. Res. 2016, 15, 313–317. [Google Scholar] [CrossRef]
- Kamon, M.; Zhao, R.; Sakamoto, K. Green tea polyphenol (−)-epigallocatechin gallate suppressed the differentiation of murine osteoblastic MC3T3-E1 cells. Cell Biol. Int. 2009, 34, 109–116. [Google Scholar] [PubMed]
- Hammes, S.R.; Levin, E.R. Extranuclear steroid receptors: Nature and actions. Endocr. Rev. 2007, 28, 726–741. [Google Scholar] [CrossRef] [PubMed]
- Kousteni, S.; Han, L.; Chen, J.R.; Almeida, M.; Plotkin, L.I.; Bellido, T.; Manolagas, S.C. Kinase-mediated regulation of common transcription factors accounts for the bone-protective effects of sex steroids. J. Clin. Invest. 2003, 111, 1651–1664. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Han, L.; O’Brien, C.A.; Kousteni, S.; Manolagas, S.C. Classical genotropic versus kinase-initiated regulation of gene transcription by the estrogen receptor alpha. Endocrinology 2006, 147, 1986–1996. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Maliakal, P.; Chen, L.; Meng, X.; Bondoc, F.Y.; Prabhu, S.; Lambert, G.; Mohr, S.; Yang, C.S. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: Formation of different metabolites and individual variability. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1025–1032. [Google Scholar]
- Chen, L.; Lee, M.J.; Li, H.; Yang, C.S. Absorption, distribution, elimination of tea polyphenols in rats. Drug Metab. Dispos. 1997, 25, 1045–1050. [Google Scholar] [PubMed]
| Body Weight (% of Change) | Uterine Index (mg/g) | Serum Ca (mg/dL) | Serum P (mg/dL) | Urinary Ca/Cr (mg/mg) | Urinary P/Cr (mg/mg) | Serum OCN (ng/mL) | Urinary DPD (nmol/mmol) | |
|---|---|---|---|---|---|---|---|---|
| Sham | 1.25 ± 0.66 | 0.32 ± 0.03 | 8.16 ± 0.23 | 7.57 ± 0.36 | 0.21 ± 0.03 | 7.26 ± 0.39 | 77.2 ± 1.4 | 9.4 ± 0.5 |
| OVX | 7.28 ± 1.29 ^^^ | 0.09 ± 0.02 ^^^ | 8.86 ± 0.22 | 7.60 ± 0.56 | 0.42 ± 0.04 ^^^ | 7.55 ± 0.63 | 86.6 ± 2.9 ^ | 14.4 ± 1.2 ^^^ |
| E2 | −6.59 ± 1.11 *** | 0.70 ± 0.08 *** | 9.21 ± 0.20 | 6.72 ± 0.64 | 0.29 ± 0.02 * | 6.46 ± 0.23 | 72.0 ± 3.1 ** | 8.2 ± 0.6 *** |
| EAF | 1.43 ± 1.01 ** | 0.13 ± 0.01 | 9.01 ± 0.14 | 7.22 ± 0.28 | 0.23 ± 0.04 ** | 6.74 ± 0.42 | 78.1 ± 2.2 * | 10.3 ± 0.7 * |
| Tibia | BV/TV (%) | Tb.N (mm−1) | Tb.Th (mm) | Tb.Sp (mm) | Conn.D (mm3) | SMI |
| Sham | 23.3 ± 1.1 | 4.51 ± 0.24 | 0.051 ± 0.001 | 0.175 ± 0.011 | 196.1 ± 15.9 | 1.52 ± 0.13 |
| OVX | 11.5 ± 1.0 ^^^ | 3.08 ± 0.37 | 0.043 ± 0.002 ^^^ | 0.312 ± 0.035 ^^^ | 105.6 ± 8.3 ^^ | 2.26 ± 0.12 ^^^ |
| E2 | 27.9 ± 1.4 *** | 5.38 ± 0.29 *** | 0.054 ± 0.001 *** | 0.126 ± 0.017 *** | 237.2 ± 27.7 *** | 1.54 ± 0.13 *** |
| EAF | 19.3 ± 0.6 *** | 3.71 ± 0.15 | 0.050 ± 0.001 *** | 0.210 ± 0.005 ** | 159.4 ± 15.3 * | 1.85 ± 0.05 * |
| Spine | BV/TV (%) | Tb.N (mm−1) | Tb.Th (mm) | Tb.Sp (mm) | Conn.D (mm3) | SMI |
| Sham | 29.4 ± 1.1 | 4.62 ± 0.12 | 0.063 ± 0.001 | 0.154 ± 0.006 | 141.1 ± 7.1 | 0.88 ± 0.08 |
| OVX | 21.1 ± 0.6 ^^^ | 3.82 ± 0.08 ^^^ | 0.058 ± 0.002 | 0.210 ± 0.005 ^^^ | 100.2 ± 4.9 ^^^ | 1.37 ± 0.04 ^^^ |
| E2 | 37.0 ± 1.7 *** | 4.89 ± 0.11 *** | 0.075 ± 0.002 *** | 0.130 ± 0.006 *** | 139.7± 4.3 *** | 0.63 ± 0.08 *** |
| EAF | 25.2 ± 0.7 ** | 4.42 ± 0.06 *** | 0.057 ± 0.001 | 0.169 ± 0.003 *** | 134.0 ± 1.7 *** | 0.82 ± 0.09 *** |
| EAF | EGCG | |
|---|---|---|
| Osteoblast cell line | ↑Proliferation (0.1 nM–1 μM), ↑ALP activity (10 nM), ↑mRNA expression of collagen, Runx2 at (10 nM–1 μM) | ↑ALP activity, ↑mineralization via ↑mRNA expression of Runx2, osterix, OC, ALP at 1–100 μM [18] |
| Osteoclast cell line | ↓Proliferation and TRAP activity at 1 nM–1 μM | ↓Proliferation and TRAP activity at 10–100 μM [18] |
| Bone protection in vivo | Prevent bone loss in 3-mo-old OVX mice after gavage of 0.5 mg/kg/day EAF for 6 weeks | Prevent bone loss in 12-week old OVX rat after i.p. of 10 mg/kg/day EGCG for 12 weeks [35] |
| Prevent bone loss in 6-month-old OVX rat after i.p. of 3.4 mg/kg/day EGCG for 12 weeks [7] | ||
| PK study | The maximum concentrations (Cmax) of (−)-epiafzelechin in blood by i.v. and i.p. injection of 10 mg/kg to C57BL/6J mice were found to be 10.6 and 6.0 μg/mL [24] | Cmax of EGCG by a single oral dose EGCG (2 mg/kg) to eight human subjects was 77.9 ± 22.2 ng/mL [42] |
| Cmax of EGCG by i.v. (10 mg/kg) and i.g. (75 mg/kg) administration of EGCG to rat were 4.7 ± 0.9 μg/mL and 19.8 ± 3.5 ng/mL, respectively [43] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, K.-C.; Cao, S.; Dong, X.; Law, M.-C.; Chan, T.-H.; Wong, M.-S. (−)-Epiafzelechin Protects against Ovariectomy-induced Bone Loss in Adult Mice and Modulate Osteoblastic and Osteoclastic Functions In Vitro. Nutrients 2017, 9, 530. https://doi.org/10.3390/nu9050530
Wong K-C, Cao S, Dong X, Law M-C, Chan T-H, Wong M-S. (−)-Epiafzelechin Protects against Ovariectomy-induced Bone Loss in Adult Mice and Modulate Osteoblastic and Osteoclastic Functions In Vitro. Nutrients. 2017; 9(5):530. https://doi.org/10.3390/nu9050530
Chicago/Turabian StyleWong, Ka-Chun, Sisi Cao, Xiaoli Dong, Man-Chun Law, Tak-Hang Chan, and Man-Sau Wong. 2017. "(−)-Epiafzelechin Protects against Ovariectomy-induced Bone Loss in Adult Mice and Modulate Osteoblastic and Osteoclastic Functions In Vitro" Nutrients 9, no. 5: 530. https://doi.org/10.3390/nu9050530
APA StyleWong, K.-C., Cao, S., Dong, X., Law, M.-C., Chan, T.-H., & Wong, M.-S. (2017). (−)-Epiafzelechin Protects against Ovariectomy-induced Bone Loss in Adult Mice and Modulate Osteoblastic and Osteoclastic Functions In Vitro. Nutrients, 9(5), 530. https://doi.org/10.3390/nu9050530