Effects of Polyphenols on Oxidative Stress-Mediated Injury in Cardiomyocytes
Abstract
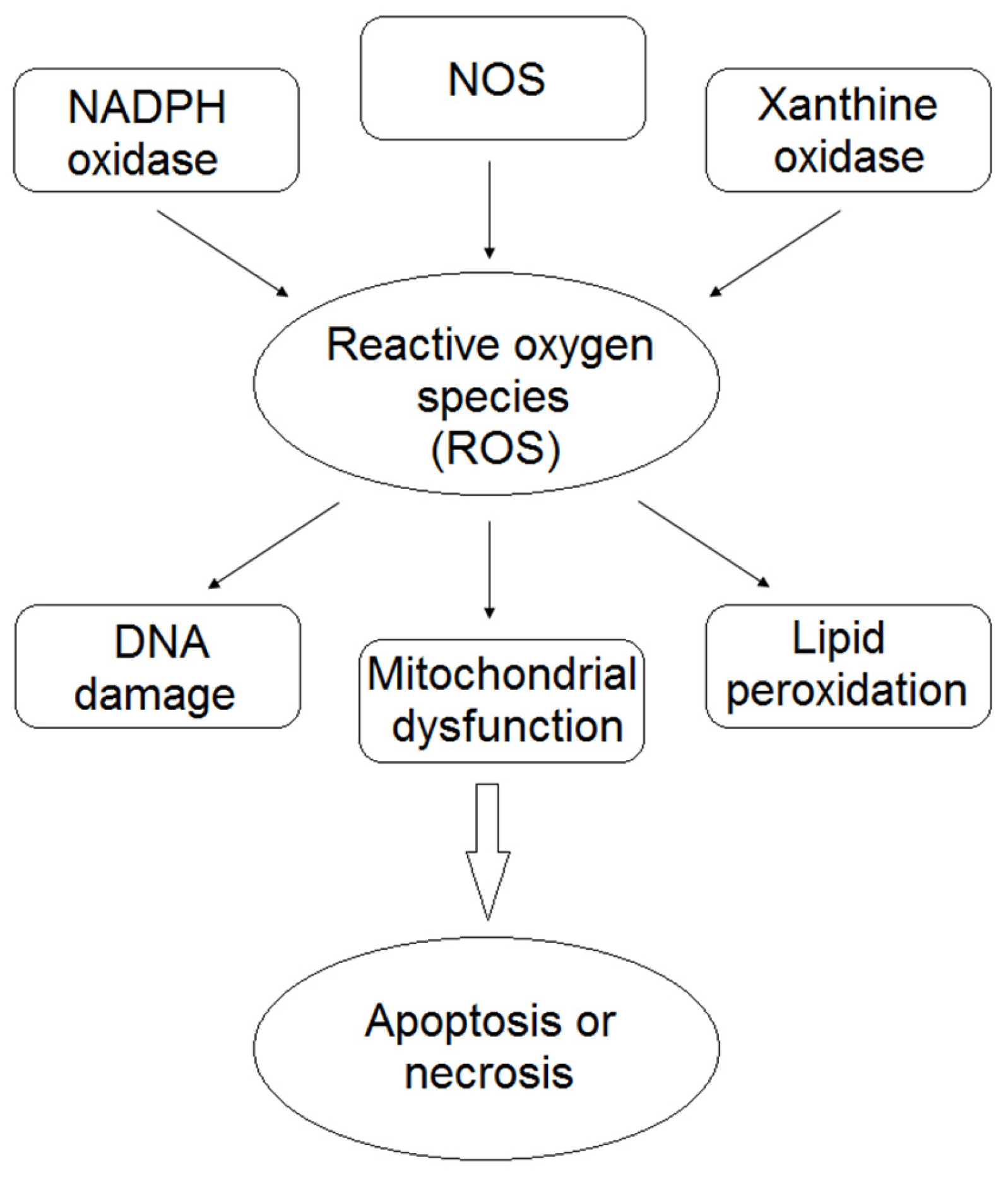
:1. Introduction
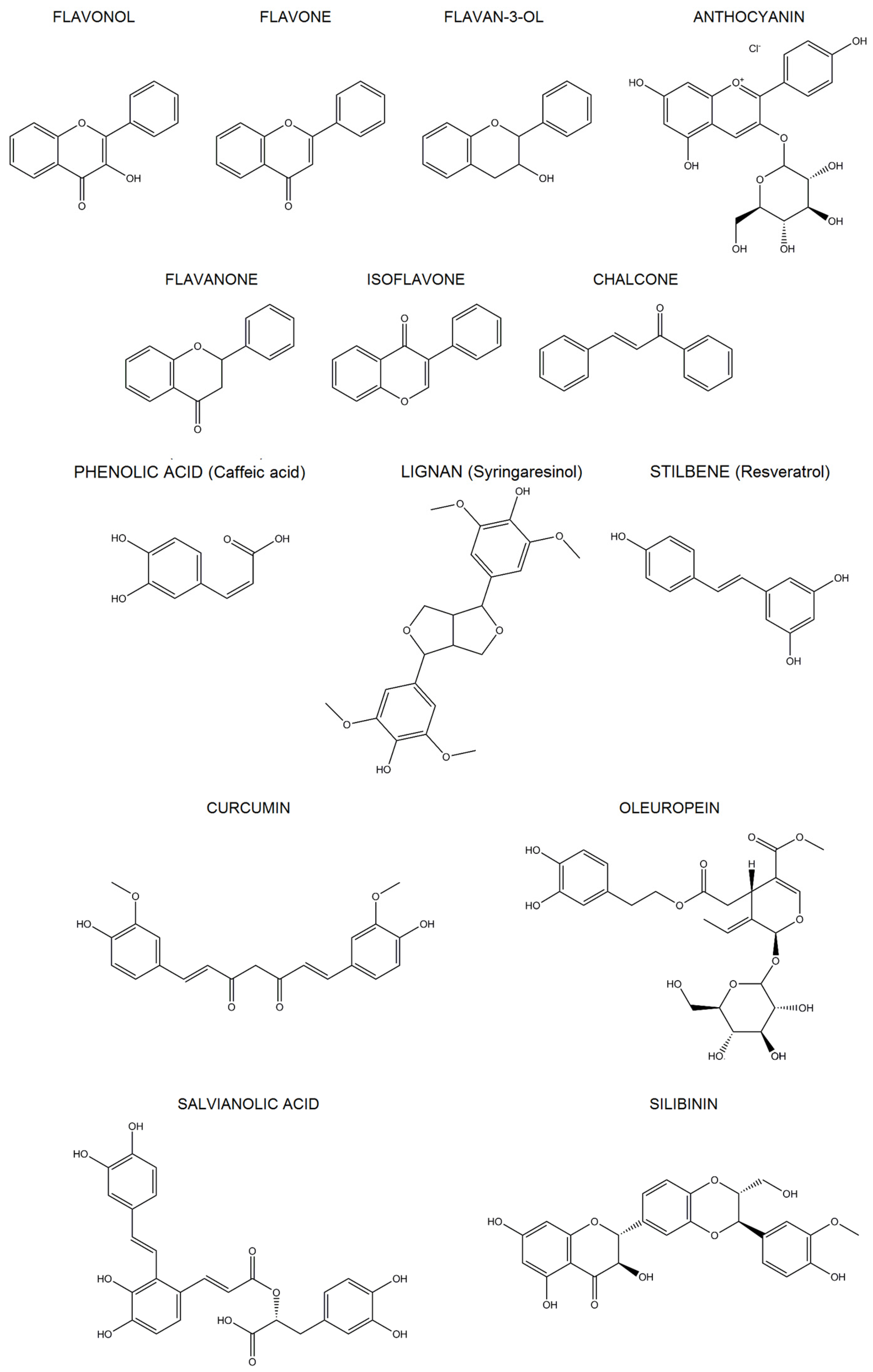
2. Polyphenols
2.1. Classification
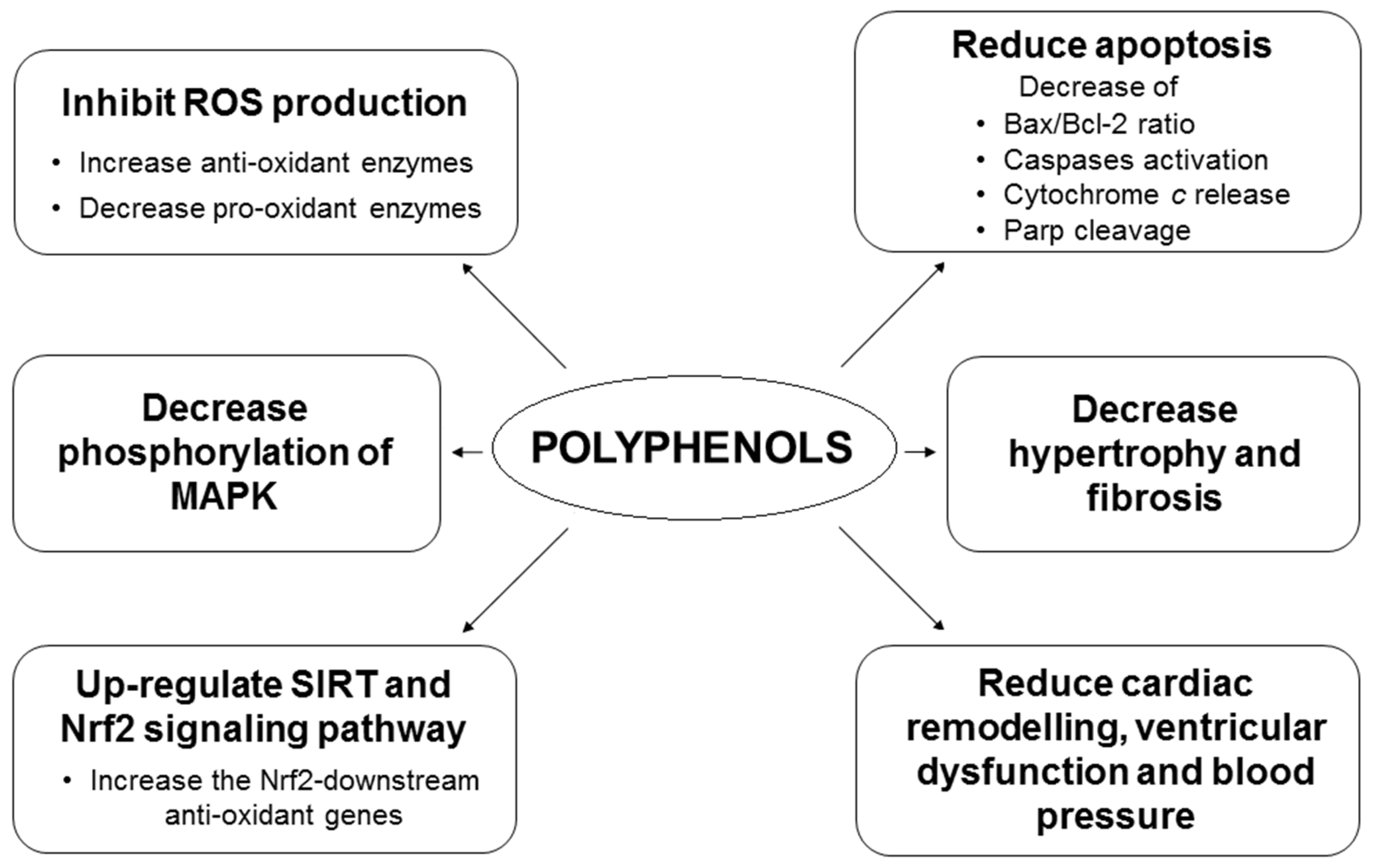
2.2. Polyphenols and Oxidative Stress and Epigenetic Regulation
3. In Vitro Effects of Polyphenols Against Oxidative Stress-Induced Cardiotoxicity
3.1. Flavonoids
3.1.1. Flavonols
3.1.2. Flavones
3.1.3. Flavan-3-Ols
3.1.4. Anthocyanins
3.1.5. Flavanones
3.1.6. Isoflavones
3.1.7. Chalcones and Dihydrochalcones
3.2. Phenolic Acids
3.3. Lignans
3.4. Stilbenes (Resveratrol)
3.5. Other Polyphenols
3.5.1. Curcumin
3.5.2. Olive Oil Polyphenols
3.5.3. Salvianolic Acid
3.5.4. Silymarin and Silibinin
3.6. Combination of Polyphenols and Comparative Studies
4. Bioavailability of Polyphenols: Is the Effective Polyphenols Dose Feasible In Vivo?
5. In Vivo Effects of Polyphenols Against Oxidative Stress-Induced Cardiotoxicity
5.1. Flavonoids
5.1.1. Flavonols
5.1.2. Flavones
5.1.3. Flavan-3-Ols
5.1.4. Anthocyanins
5.1.5. Flavanones
5.1.6. Isoflavones
5.1.7. Chalcones and Dihydrochalcones
5.2. Phenolic Acids
5.3. Lignans
5.4. Stilbenes (Resveratrol)
5.5. Other Polyphenols
5.5.1. Curcumin
5.5.2. Olive Oil Polyphenols
5.5.3. Silymarin and Silibinin
5.6. Combination of Polyphenols
6. Evidence from Human Studies
7. Limitations of the Polyphenols Studies
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Townsend, N.; Wilson, L.; Bhatnagar, P.; Wickramasinghe, K.; Rayner, M.; Nichols, M. Cardiovascular disease in Europe: Epidemiological update 2016. Eur. Heart J. 2016, 37, 3232–3245. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Sun, W.; Zhang, Z.; Zheng, Y. The role of Nrf2-mediated pathway in cardiac remodeling and heart failure. Oxid. Med. Cell. Longev. 2014, 2014, 260429. [Google Scholar] [CrossRef] [PubMed]
- Ball, A.M.; Sole, M.J. Oxidative stress and the pathogenesis of heart failure. Cardiol. Clin. 1998, 16, 665–675. [Google Scholar] [CrossRef]
- Dhalla, N.S.; Temsah, R.M.; Netticadan, T. Role of oxidative stress in cardiovascular diseases. J. Hypertens. 2000, 18, 655–673. [Google Scholar] [CrossRef] [PubMed]
- Braunersreuther, V.; Jaquet, V. Reactive oxygen species in myocardial reperfusion injury: From physiopathology to therapeutic approaches. Curr. Pharm. Biotechnol. 2012, 13, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, E.; Kass, D.A. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension 2007, 49, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Granger, D.N.; Kvietys, P.R. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015, 6, 524–551. [Google Scholar] [CrossRef] [PubMed]
- Walters, J.W.; Amos, D.; Ray, K.; Santanam, N. Mitochondrial redox status as a target for cardiovascular disease. Curr. Opin. Pharmacol. 2016, 27, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Giordano, F.J. Oxygen, oxidative stress, hypoxia, and heart failure. J. Clin. Investig. 2005, 115, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Sabri, A.; Hughie, H.H.; Lucchesi, P.A. Regulation of hypertrophic and apoptotic signaling pathways by reactive oxygen species in cardiac myocytes. Antioxid. Redox Signal. 2003, 5, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P. Polyphenols and human health: Prevention of disease and mechanisms of action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [PubMed]
- Benvenuto, M.; Fantini, M.; Masuelli, L.; De Smaele, E.; Zazzeroni, F.; Tresoldi, I.; Calabrese, G.; Galvano, F.; Modesti, A.; Bei, R. Inhibition of ErbB receptors, Hedgehog and NF-kappaB signaling by polyphenols in cancer. Front. Biosci. 2013, 18, 1290–1310. [Google Scholar] [CrossRef]
- Fantini, M.; Benvenuto, M.; Masuelli, L.; Frajese, G.V.; Tresoldi, I.; Modesti, A.; Bei, R. In vitro and in vivo antitumoral effects of combinations of polyphenols, or polyphenols and anticancer drugs: Perspectives on cancer treatment. Int. J. Mol. Sci. 2015, 16, 9236–9282. [Google Scholar] [CrossRef] [PubMed]
- Benvenuto, M.; Mattera, R.; Taffera, G.; Giganti, M.G.; Lido, P.; Masuelli, L.; Modesti, A.; Bei, R. The potential protective effects of polyphenols in asbestos-mediated inflammation and carcinogenesis of mesothelium. Nutrients 2016, 8, E275. [Google Scholar] [CrossRef] [PubMed]
- Marzocchella, L.; Fantini, M.; Benvenuto, M.; Masuelli, L.; Tresoldi, I.; Modesti, A.; Bei, R. Dietary flavonoids: molecular mechanisms of action as anti-inflammatory agents. Recent Pat. Inflamm. Allergy Drug Discov. 2011, 5, 200–220. [Google Scholar] [CrossRef] [PubMed]
- Stedile, N.; Canuto, R.; Col, C.D.; Sene, J.S.; Stolfo, A.; Wisintainer, G.N.; Henriques, J.A.; Salvador, M. Dietary total antioxidant capacity is associated with plasmatic antioxidant capacity, nutrient intake and lipid and DNA damage in healthy women. Int. J. Food Sci. Nutr. 2016, 67, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Izzi, V.; Masuelli, L.; Tresoldi, I.; Sacchetti, P.; Modesti, A.; Galvano, F.; Bei, R. The effects of dietary flavonoids on the regulation of redox inflammatory networks. Front. Biosci. (Landmark Ed.) 2012, 17, 2396–2418. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Micek, A.; Godos, J.; Pajak, A.; Sciacca, S.; Galvano, F.; Giovannucci, E.L. Dietary flavonoid and lignan intake and mortality in prospective cohort studies: Systematic review and dose-response meta-analysis. Am. J. Epidemiol. 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Godos, J.; Lamuela-Raventos, R.; Ray, S.; Micek, A.; Pajak, A.; Sciacca, S.; D’Orazio, N.; Del Rio, D.; Galvano, F. A comprehensive meta-analysis on dietary flavonoid and lignan intake and cancer risk: Level of evidence and limitations. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Yordi, E.G.; Pérez, E.M.; Matos, M.J.; Villares, E.U. Antioxidant and pro-oxidant effects of polyphenolic compounds and structure-activity relationship evidence. In Nutrition, Well-Being and Health; Bouayed, J., Bohn, T., Eds.; InTech: Rijeka, Croatia, 2012; Chapter 2; pp. 23–48. [Google Scholar]
- Halliwell, B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch. Biochem. Biophys. 2008, 476, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, D.E.; Hurst, R.D. Polyphenolic phytochemicals-just antioxidants or much more? Cell. Mol. Life Sci. 2007, 64, 2900–2916. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Visioli, F. Polyphenols and health: Moving beyond antioxidants. J. Berry Res. 2012, 2, 63–71. [Google Scholar]
- Forman, H.J.; Davies, K.J.; Ursini, F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P. Dietary factors, hormesis and health. Ageing Res. Rev. 2008, 7, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F. Xenobiotics and human health: A new view of their pharma-nutritional role. PharmaNutrition 2015, 3, 60–64. [Google Scholar] [CrossRef]
- Calabrese, V.; Cornelius, C.; Dinkova-Kostova, A.T.; Iavicoli, I.; Di Paola, R.; Koverech, A.; Cuzzocrea, S.; Rizzarelli, E.; Calabrese, E.J. Cellular stress responses, hormetic phytochemicals and vitagenes in aging and longevity. Biochim. Biophys. Acta 2012, 1822, 753–783. [Google Scholar] [CrossRef] [PubMed]
- Crespo, M.C.; Tomé-Carneiro, J.; Burgos-Ramos, E.; Loria Kohen, V.; Espinosa, M.I.; Herranz, J.; Visioli, F. One-week administration of hydroxytyrosol to humans does not activate Phase II enzymes. Pharmacol. Res. 2015, 95–96, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Schiano, C.; Vietri, M.T.; Grimaldi, V.; Picascia, A.; De Pascale, M.R.; Napoli, C. Epigenetic-related therapeutic challenges in cardiovascular disease. Trends Pharmacol. Sci. 2015, 36, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Bladé, C.; Baselga-Escudero, L.; Salvadó, M.J.; Arola-Arnal, A. miRNAs, polyphenols, and chronic disease. Mol. Nutr. Food Res. 2013, 57, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Vaiserman, A.M. Hormesis and epigenetics: Is there a link? Ageing Res. Rev. 2011, 10, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Lai, C.S.; Wu, J.C.; Ho, C.T. Epigenetic and disease targets by polyphenols. Curr. Pharm. Des. 2013, 19, 6156–6185. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.L.; Vastolo, V.; Ciccarelli, M.; Albano, L.; Macchia, P.E.; Ungaro, P. Dietary polyphenols and chromatin remodelling. Crit. Rev. Food Sci. Nutr. 2015. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Treatment of cardiovascular pathology with epigenetically active agents: Focus on natural and synthetic inhibitors of DNA methylation and histone deacetylation. Int. J. Cardiol. 2017, 227, 66–82. [Google Scholar] [CrossRef] [PubMed]
- Wojtala, M.; Pirola, L.; Balcerczyk, A. Modulation of the vascular endothelium functioning by dietary components, the role of epigenetics. Biofactors 2017, 43, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, N.; Zhang, Y.; Ran, Y.; Pu, J. Circulating microRNAs are promising novel biomarkers of acute myocardial infarction. Intern. Med. 2011, 50, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandra, Y.; Devanna, P.; Limana, F.; Straino, S.; di Carlo, A.; Brambilla, P.G.; Rubino, M.; Carena, M.C.; Spazzafumo, L.; de Simone, M.; et al. Circulating microRNAs are new and sensitive biomarkers of myocardial infarction. Eur. Heart J. 2010, 31, 2765–2773. [Google Scholar] [CrossRef] [PubMed]
- Menghini, R.; Casagrande, V.; Cardellini, M.; Martelli, E.; Terrinoni, A.; Amati, F.; Vasa-Nicotera, M.; Ippoliti, A.; Novelli, G.; Melino, G.; et al. MicroRNA 217 modulates endothelial cell senescence via silent information regulator 1. Circulation 2009, 120, 1524–1532. [Google Scholar] [CrossRef] [PubMed]
- Fichtlscherer, S.; De, R.S.; Fox, H.; Schwietz, T.; Fischer, A.; Liebetrau, C.; Weber, M.; Hamm, C.W.; Röxe, T.; Müller-Ardogan, M.; et al. Circulating microRNAs in patients with coronary artery disease. Circ. Res. 2010, 107, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Davalos, A. Polyphenols and cardiovascular disease: A critical summary of the evidence. Mini Rev. Med. Chem. 2011, 11, 1186–1190. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Mukherjee, S.; Ahsan, K.; Bagchi, A.; Pacher, P.; Das, D.K. Restoration of altered microRNA expression in the ischemic heart with resveratrol. PLoS ONE 2010, 5, e15705. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, C.; Leoncini, E.; Malaguti, M.; Angelini, S.; Hrelia, P.; Hrelia, S. Role of quercetin in modulating rat cardiomyocyte gene expression profile. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1233–H1243. [Google Scholar] [CrossRef] [PubMed]
- Daubney, J.; Bonner, P.L.; Hargreaves, A.J.; Dickenson, J.M. Cardioprotective and cardiotoxic effects of quercetin and two of its in vivo metabolites on differentiated H9c2 cardiomyocytes. Basic Clin. Pharmacol. Toxicol. 2015, 116, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Park, E.S.; Kang, J.C.; Jang, Y.C.; Park, J.S.; Jang, S.Y.; Kim, D.E.; Kim, B.; Shin, H.S. Cardioprotective effects of rhamnetin in H9c2 cardiomyoblast cells under H2O2-induced apoptosis. J. Ethnopharmacol. 2014, 153, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.J.; Lim, N.R.; Kedikaetswe, A.; Yeap, Y.Y.; Woodman, O.L.; Ng, D.C.; May, C.N. Evidence that the MEK/ERK but not the PI3K/Akt pathway is required for protection from myocardial ischemia-reperfusion injury by 3′,4′-dihydroxyflavonol. Eur. J. Pharmacol. 2015, 758, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Chen, L.; Lu, Q.; Sharma, S.; Li, L.; Morimoto, S.; Wang, G. Quercetin attenuates doxorubicin cardiotoxicity by modulating Bmi-1 expression. Br. J. Pharmacol. 2014, 171, 4440–4454. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Sun, G.; Meng, X.; Wang, H.; Luo, Y.; Qin, M.; Ma, B.; Wang, M.; Cai, D.; Guo, P.; et al. Isorhamnetin protects against doxorubicin-induced cardiotoxicity in vivo and in vitro. PLoS ONE 2013, 8, e64526. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Sun, G.B.; Sun, B.; Wu, Y.; He, L.; Wang, X.; Chen, R.C.; Cao, L.; Ren, X.Y.; Sun, X.B. Kaempferol protects against doxorubicin-induced cardiotoxicity in vivo and in vitro. Toxicology 2012, 292, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Luo, P.; Fu, Y.; Wang, J.; Dai, J.; Shao, J.; Yang, X.; Chang, L.; Weng, Q.; Yang, B.; et al. Dihydromyricetin prevents cardiotoxicity and enhances anticancer activity induced by adriamycin. Oncotarget 2015, 6, 3254–3267. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.; Wang, Z.; Li, X.; Li, X.; Cao, T.; Bi, Y.; Zhou, J.; Chen, X.; Yu, D.; Zhu, L.; et al. Protective effect of quercetin on posttraumatic cardiac injury. Sci. Rep. 2016, 6, 30812. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Peng, Y.; Xu, T.; Yi, X.; Liu, Y.; Luo, Y.; Yin, D.; He, M. The effects of quercetin protect cardiomyocytes from A/R injury is related to its capability to increasing expression and activity of PKCε protein. Mol. Cell. Biochem. 2013, 382, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Liao, Z.; Huang, L.; Liu, D.; Yin, D.; He, M. Kaempferol protects cardiomyocytes against anoxia/reoxygenation injury via mitochondrial pathway mediated by SIRT1. Eur. J. Pharmacol. 2015, 761, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Dusting, G.J.; May, C.N.; Woodman, O.L. 3′,4′-Dihydroxyflavonol reduces infarct size and injury associated with myocardial ischaemia and reperfusion in sheep. Br. J. Pharmacol. 2004, 142, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhang, X.; Cui, Y.; Zhou, H.; Xu, D.; Shan, T.; Zhang, F.; Guo, Y.; Chen, Y.; Wu, D. Taxifolin protects against cardiac hypertrophy and fibrosis during biomechanical stress of pressure overload. Toxicol. Appl. Pharmacol. 2015, 287, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Ozbek, N.; Bali, E.B.; Karasu, C. Quercetin and hydroxytyrosol attenuates xanthine/xanthine oxidase-induced toxicity in H9c2 cardiomyocytes by regulation of oxidative stress and stress-sensitive signaling pathways. Gen. Physiol. Biophys. 2015, 34, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; He, H.; Luo, Y.; Zhou, M.; Yin, D.; He, M. Involvement of Bcl-2 signal pathway in the protective effects of apigenin on anoxia/reoxygenation-induced myocardium injury. J. Cardiovasc. Pharmacol. 2016, 67, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.Y.; Chen, Z.W.; Guo, Y.; Cheng, X.P.; Shao, X. Mechanisms of vitexin preconditioning effects on cultured neonatal rat cardiomyocytes with anoxia and reoxygenation. Am. J. Chin. Med. 2008, 36, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Fan, Y.; Shao, X.; Chen, Z. Vitexin protects against myocardial ischemia/reperfusion injury in Langendorff-perfused rat hearts by attenuating inflammatory response and apoptosis. Food Chem. Toxicol. 2011, 49, 3211–3216. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.H.; Vanden Hoek, T.L.; Qin, Y.; Becker, L.B.; Schumacker, P.T.; Li, C.Q.; Dey, L.; Barth, E.; Halpern, H.; Rosen, G.M.; et al. Baicalein attenuates oxidant stress in cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H999–H1006. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Luk, S.C.; Li, R.A.; Chan, K.K.; Lei, S.W.; Wang, L.; Shen, H.; Leung, G.P.; Lee, S.M. Cytoprotection of baicalein against oxidative stress-induced cardiomyocytes injury through the Nrf2/Keap1 pathway. J. Cardiovasc. Pharmacol. 2015, 65, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.T.; Li, J.; Vanden Hoek, M.S.; Zhu, X.; Li, C.Q.; Huang, H.H.; Hsu, C.W.; Zhong, Q.; Li, J.; Chen, S.J.; et al. Baicalein preconditioning protects cardiomyocytes from ischemia-reperfusion injury via mitochondrial oxidant signaling. Am. J. Chin. Med. 2013, 41, 315–331. [Google Scholar] [CrossRef] [PubMed]
- Tu, I.H.; Yen, H.T.; Cheng, H.W.; Chiu, J.H. Baicalein protects chicken embryonic cardiomyocyte against hypoxia-reoxygenation injury via mu- and delta- but not kappa-opioid receptor signaling. Eur. J. Pharmacol. 2008, 588, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Dreger, H.; Lorenz, M.; Kehrer, A.; Baumann, G.; Stangl, K.; Stangl, V. Characteristics of catechin- and theaflavin-mediated cardioprotection. Exp. Biol. Med. (Maywood) 2008, 233, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.R.; Hsu, C.S.; Lu, C.H.; Chen, W.C.; Chiu, C.H.; Liou, Y.M. Epigallocatechin-3-gallate-mediated cardioprotection by Akt/GSK-3β/caveolin signalling in H9c2 rat cardiomyoblasts. J. Biomed. Sci. 2013, 20, 86. [Google Scholar] [CrossRef] [PubMed]
- Sheng, R.; Gu, Z.L.; Xie, M.L.; Zhou, W.X.; Guo, C.Y. EGCG inhibits cardiomyocyte apoptosis in pressure overload-induced cardiac hypertrophy and protects cardiomyocytes from oxidative stress in rats. Acta Pharmacol. Sin. 2007, 28, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Sheng, R.; Gu, Z.L.; Xie, M.L.; Zhou, W.X.; Guo, C.Y. Epigallocatechin gallate protects H9c2 cardiomyoblasts against hydrogen dioxides- induced apoptosis and telomere attrition. Eur. J. Pharmacol. 2010, 641, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Nie, S.; Xie, M.; Chen, Y.; Li, C.; Zhang, H. A major green tea component, (−)-epigallocatechin-3-gallate, ameliorates doxorubicin-mediated cardiotoxicity in cardiomyocytes of neonatal rats. J. Agric. Food Chem. 2010, 58, 8977–8982. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Lee, H.C.; Bin Sattar, M.M.; Huang, Y.; Bian, J.S. Cardioprotective effects of epigallocatechin-3-gallate against doxorubicin-induced cardiomyocyte injury. Eur. J. Pharmacol. 2011, 652, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, H.; Ramachandran, S.; Waypa, G.B.; Yin, J.J.; Li, C.Q.; Han, M.; Huang, H.H.; Sillard, W.W.; Vanden Hoek, T.L.; et al. Grape seed proanthocyanidins ameliorate Doxorubicin-induced cardiotoxicity. Am. J. Chin. Med. 2010, 38, 569–584. [Google Scholar] [CrossRef] [PubMed]
- Townsend, P.A.; Scarabelli, T.M.; Pasini, E.; Gitti, G.; Menegazzi, M.; Suzuki, H.; Knight, R.A.; Latchman, D.S.; Stephanou, A. Epigallocatechin-3-gallate inhibits STAT-1 activation and protects cardiac myocytes from ischemia/reperfusion-induced apoptosis. FASEB J. 2004, 18, 1621–1623. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.H.; Wojcik, K.R.; Dossumbekova, A.; Hsu, C.; Mehendale, S.R.; Li, C.Q.; Qin, Y.; Sharp, W.W.; Chang, W.T.; Hamann, K.J.; et al. Grape seed proanthocyanidins protect cardiomyocytes from ischemia and reperfusion injury via Akt-NOS signaling. J. Cell. Biochem. 2009, 107, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Hirai, M.; Hotta, Y.; Ishikawa, N.; Wakida, Y.; Fukuzawa, Y.; Isobe, F.; Nakano, A.; Chiba, T.; Kawamura, N. Protective effects of EGCg or GCg, a green tea catechin epimer, against postischemic myocardial dysfunction in guinea-pig hearts. Life Sci. 2007, 80, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Meng, Z. Epigallocatechin-3-gallate protects Na+ channels in rat ventricular myocytes against sulfite. Cardiovasc. Toxicol. 2010, 10, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Meng, Z. Protective effects of epigallocatechin-3-gallate against lead-induced oxidative damage. Hum. Exp. Toxicol. 2011, 30, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Jung, Y.S.; Kim, M.Y.; Yang, S.Y.; Lee, S.; Kim, Y.H. Protective effect of components isolated from Lindera erythrocarpa against oxidative stress-induced apoptosis of H9c2 cardiomyocytes. Phytother. Res. 2011, 25, 1612–1617. [Google Scholar] [CrossRef] [PubMed]
- Quintieri, A.M.; Baldino, N.; Filice, E.; Seta, L.; Vitetti, A.; Tota, B.; de Cindio, B.; Cerra, M.C.; Angelone, T. Malvidin, a red wine polyphenol, modulates mammalian myocardial and coronary performance and protects the heart against ischemia/reperfusion injury. J. Nutr. Biochem. 2013, 24, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Škėmienė, K.; Jablonskienė, G.; Liobikas, J.; Borutaitė, V. Protecting the heart against ischemia/reperfusion-induced necrosis and apoptosis: The effect of anthocyanins. Medicina (Kaunas) 2013, 49, 84–88. [Google Scholar] [PubMed]
- Louis, X.L.; Thandapilly, S.J.; Kalt, W.; Vinqvist-Tymchuk, M.; Aloud, B.M.; Raj, P.; Yu, L.; Le, H.; Netticadan, T. Blueberry polyphenols prevent cardiomyocyte death by preventing calpain activation and oxidative stress. Food Funct. 2014, 5, 1785–1794. [Google Scholar] [CrossRef] [PubMed]
- Ramprasath, T.; Senthamizharasi, M.; Vasudevan, V.; Sasikumar, S.; Yuvaraj, S.; Selvam, G.S. Naringenin confers protection against oxidative stress through upregulation of Nrf2 target genes in cardiomyoblast cells. J. Physiol. Biochem. 2014, 70, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wu, K.; You, Q.; Huang, R.; Li, S.; Wu, K. Naringin inhibits high glucose-induced cardiomyocyte apoptosis by attenuating mitochondrial dysfunction and modulating the activation of the p38 signaling pathway. Int. J. Mol. Med. 2013, 32, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Guo, R.; Yan, H.; Tian, L.; You, Q.; Li, S.; Huang, R.; Wu, K. Naringin inhibits ROS-activated MAPK pathway in high glucose-induced injuries in H9c2 cardiac cells. Basic Clin. Pharmacol. Toxicol. 2014, 114, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Mo, H.; Guo, R.; You, Q.; Huang, R.; Wu, K. Inhibition of the leptin-induced activation of the p38 MAPK pathway contributes to the protective effects of naringin against high glucose-induced injury in H9c2 cardiac cells. Int. J. Mol. Med. 2014, 33, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.C.; Sun, G.B.; Wang, J.; Zhang, H.J.; Sun, X.B. Naringin protects against anoxia/reoxygenation-induced apoptosis in H9c2 cells via the Nrf2 signaling pathway. Food Funct. 2015, 6, 1331–1344. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Ren, D.; Fan, P.; Shen, T.; Lou, H. Protective effects of naringenin-7-O-glucoside on doxorubicin-induced apoptosis in H9c2 cells. Eur. J. Pharmacol. 2008, 581, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Pan, J.; Ren, D.; Cheng, Y.; Fan, P.; Lou, H. Naringenin-7-O-glucoside protects against doxorubicin-induced toxicity in H9c2 cardiomyocytes by induction of endogenous antioxidant enzymes. Food Chem. Toxicol. 2008, 46, 3140–3146. [Google Scholar] [CrossRef] [PubMed]
- Han, X.Z.; Gao, S.; Cheng, Y.N.; Sun, Y.Z.; Liu, W.; Tang, L.L.; Ren, D.M. Protective effect of naringenin-7-O-glucoside against oxidative stress induced by doxorubicin in H9c2 cardiomyocytes. Biosci. Trends 2012, 6, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, Y.; Deng, W.; Dai, J.; Li, F.; Yuan, Y.; Wu, Q.; Zhou, H.; Bian, Z.; Tang, Q. Hesperetin attenuates mitochondria-dependent apoptosis in lipopolysaccharide-induced H9c2 cardiomyocytes. Mol. Med. Rep. 2014, 9, 1941–1946. [Google Scholar] [CrossRef] [PubMed]
- Gang, C.; Qiang, C.; Xiangli, C.; Shifen, P.; Chong, S.; Lihong, L. Puerarin suppresses angiotensin II-induced cardiac hypertrophy by inhibiting NADPH oxidase activation and oxidative stress-triggered AP-1 signaling pathways. J. Pharm. Pharm. Sci. 2015, 18, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, J.; Liu, W.; Liu, N.; Fu, X.; Kwan, H.; Liu, S.; Liu, B.; Zhang, S.; Yu, Z.; et al. Calycosin inhibits oxidative stress-induced cardiomyocyte apoptosis via activating estrogen receptor-α/β. Bioorg. Med. Chem. Lett. 2016, 26, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Niu, H.T.; Wang, P.L.; Lu, J.; Zhao, H.; Liu, S.H.; Zheng, Q.S.; Li, C.G. Cardioprotective effect of licochalcone D against myocardial ischemia/reperfusion injury in Langendorff-perfused rat hearts. PLoS ONE 2015, 10, e0128375. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wang, D.; Yu, B.; Wang, Y.; Ren, H.; Zhang, B.; Wang, Y.; Zheng, Q. Cardioprotection against ischemia/reperfusion by licochalcone B in isolated rat hearts. Oxid. Med. Cell. Longev. 2014, 2014, 134862. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, P.; Zhang, X.; Ma, Y.; Li, W.; Chen, J.M.; Guo, H.M.; Bucala, R.; Zhuang, J.; Li, J. Natural antioxidant-isoliquiritigenin ameliorates contractile dysfunction of hypoxic cardiomyocytes via AMPK signaling pathway. Mediat. Inflamm. 2013, 2013, 390890. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.L.; Wang, J.W.; Guan, Y.; Yin, Y.; Wei, G.; Cui, J.; Zhou, D.; Zhu, Y.R.; Quan, W.; Xi, M.M.; et al. Safflor yellow A protects neonatal rat cardiomyocytes against anoxia/reoxygenation injury in vitro. Acta Pharmacol. Sin. 2013, 34, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.X.; Zhang, Y.; Wang, Y.F.; Li, X.C.; Xiang, M.X.; Bian, C.; Chen, P. Upregulation of heme oxygenase-1 expression by hydroxysafflor yellow A conferring protection from anoxia/reoxygenation-induced apoptosis in H9c2 cardiomyocytes. Int. J. Cardiol. 2012, 160, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Zhong, P.; Wu, L.; Qian, Y.; Fang, Q.; Liang, D.; Wang, J.; Zeng, C.; Wang, Y.; Liang, G. Blockage of ROS and NF-κB-mediated inflammation by a new chalcone L6H9 protects cardiomyocytes from hyperglycemia-induced injuries. Biochim. Biophys. Acta 2015, 1852, 1230–1241. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Muller, C.J.; Louw, J.; Joubert, E.; Salie, R.; Opoku, A.R.; Johnson, R. The cardioprotective effect of an aqueous extract of fermented rooibos (Aspalathus linearis) on cultured cardiomyocytes derived from diabetic rats. Phytomedicine 2014, 21, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.; Dludla, P.; Joubert, E.; February, F.; Mazibuko, S.; Ghoor, S.; Muller, C.; Louw, J. Aspalathin, a dihydrochalcone C-glucoside, protects H9c2 cardiomyocytes against high glucose induced shifts in substrate preference and apoptosis. Mol. Nutr. Food Res. 2016, 60, 922–934. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wu, J.H.; Wang, Q.H.; Shu, Y.L.; Wan, C.W.; Chan, C.O.; Kam-Wah Mok, D.; Chan, S.W. Gui-ling-gao, a traditional Chinese functional food, prevents oxidative stress-induced apoptosis in H9c2 cardiomyocytes. Food Funct. 2013, 4, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Chiarini, A.; Micucci, M.; Malaguti, M.; Budriesi, R.; Ioan, P.; Lenzi, M.; Fimognari, C.; Gallina Toschi, T.; Comandini, P.; Hrelia, S. Sweet chestnut (Castanea sativa Mill.) bark extract: Cardiovascular activity and myocyte protection against oxidative damage. Oxid. Med. Cell Longev. 2013, 2013, 471790. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.E.; Kim, B.; Shin, H.S.; Kwon, H.J.; Park, E.S. The protective effect of hispidin against hydrogen peroxide-induced apoptosis in H9c2 cardiomyoblast cells through Akt/GSK-3β and ERK1/2 signaling pathway. Exp. Cell Res. 2014, 327, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Hollingsworth, A.; Piche, M.; Venkataraman, K.; Kumar, A.; Ross, G.M.; Tai, T.C. Antiapoptotic actions of methyl gallate on neonatal rat cardiac myocytes exposed to H2O2. Oxid. Med. Cell. Longev. 2014, 2014, 657512. [Google Scholar] [CrossRef] [PubMed]
- Ku, H.C.; Lee, S.Y.; Yang, K.C.; Kuo, Y.H.; Su, M.J. Modification of caffeic acid with pyrrolidine enhances antioxidant ability by activating AKT/HO-1 pathway in heart. PLoS ONE 2016, 11, e0148545. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wen, L.; Sun, J.; Bai, W.; Jiao, R.; Hu, Y.; Peng, X.; He, Y.; Ou, S. Cytoprotective mechanism of ferulic acid against high glucose-induced oxidative stress in cardiomyocytes and hepatocytes. Food Nutr. Res. 2016, 60, 30323. [Google Scholar] [CrossRef] [PubMed]
- Atale, N.; Gupta, K.; Rani, V. Protective effect of Syzygium cumini against pesticide-induced cardiotoxicity. Environ. Sci. Pollut. Res. Int. 2014, 21, 7956–7972. [Google Scholar] [CrossRef] [PubMed]
- Chlopcíková, S.; Psotová, J.; Miketová, P.; Sousek, J.; Lichnovský, V.; Simánek, V. Chemoprotective effect of plant phenolics against anthracycline-induced toxicity on rat cardiomyocytes. Part II. caffeic, chlorogenic and rosmarinic acids. Phytother. Res. 2004, 18, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Guan, Y.; Duan, J.; Wei, G.; Zhu, Y.; Quan, W.; Guo, C.; Zhou, D.; Wang, Y.; Xi, M.; et al. Cardioprotective effect of Danshensu against myocardial ischemia/reperfusion injury and inhibits apoptosis of H9c2 cardiomyocytes via Akt and ERK1/2 phosphorylation. Eur. J. Pharmacol. 2013, 699, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, L.; Akinyi, M.; Li, Y.; Duan, Z.; Zhu, Y.; Fan, G. Danshensu protects isolated heart against ischemia reperfusion injury through activation of Akt/ERK1/2/Nrf2 signaling. Int. J. Clin. Exp. Med. 2015, 8, 14793–14804. [Google Scholar] [PubMed]
- Chiu, P.Y.; Chen, N.; Leong, P.K.; Leung, H.Y.; Ko, K.M. Schisandrin B elicits a glutathione antioxidant response and protects against apoptosis via the redox-sensitive ERK/Nrf2 pathway in H9c2 cells. Mol. Cell. Biochem. 2011, 350, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Pang, S.; Yang, N.; Meng, H.; Liu, J.; Zhou, N.; Zhang, M.; Xu, Z.; Gao, W.; Chen, B.; et al. Beneficial effects of schisandrin B on the cardiac function in mice model of myocardial infarction. PLoS ONE 2013, 8, e79418. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Li, Y.; Yang, X.; Yue, Y.; Dou, L.; Wang, Y.; Zhang, W.; Li, X. Protective role of deoxyschizandrin and schisantherin A against myocardial ischemia-reperfusion injury in rats. PLoS ONE 2013, 8, e61590. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Cho, M.; Kim, J.; Kaeberlein, M.; Lee, S.J.; Suh, Y. Syringaresinol protects against hypoxia/reoxygenation-induced cardiomyocytes injury and death by destabilization of HIF-1α in a FOXO3-dependent mechanism. Oncotarget 2015, 6, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Li, Q.; Liu, Y.; Xiong, C.; Li, J.; Zhang, R.; Niu, Y.; Zhao, L.; Wang, Y.; Guo, H. Sesamin ameliorates doxorubicin-induced cardiotoxicity: Involvement of Sirt1 and Mn-SOD pathway. Toxicol. Lett. 2014, 224, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.G.; Ren, Y.N.; Zhao, M.Q.; Tao, S.J.; Kong, X.; Yang, J.R. Effect of serum containing sesamin on angiotensin II-induced apoptosis in rat cardiomyocytes. Zhong Yao Cai 2015, 38, 1013–1017. [Google Scholar] [PubMed]
- Chen, C.J.; Fu, Y.C.; Yu, W.; Wang, W. SIRT3 protects cardiomyocytes from oxidative stress-mediated cell death by activating NF-κB. Biochem. Biophys. Res. Commun. 2013, 430, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.G.; Zhu, W.; Tao, J.P.; Xin, P.; Liu, M.Y.; Li, J.B.; Wei, M. Resveratrol protects cardiomyocytes from oxidative stress through SIRT1 and mitochondrial biogenesis signaling pathways. Biochem. Biophys. Res. Commun. 2013, 438, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Ting, W.J.; Huang, C.Y.; Yang, J.Y.; Lin, W.T. Resveratrol attenuated hydrogen peroxide-induced myocardial apoptosis by autophagic flux. Food Nutr. Res. 2016, 60, 30511. [Google Scholar] [CrossRef] [PubMed]
- Movahed, A.; Yu, L.; Thandapilly, S.J.; Louis, X.L.; Netticadan, T. Resveratrol protects adult cardiomyocytes against oxidative stress mediated cell injury. Arch. Biochem. Biophys. 2012, 527, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Y.P.; Gao, L.; Zhang, P.P.; Zhou, Q.; Xu, Q.F.; Zhou, Z.W.; Guo, K.; Chen, R.H.; Yang, H.T.; et al. Resveratrol protects rabbit ventricular myocytes against oxidative stress-induced arrhythmogenic activity and Ca2+ overload. Acta Pharmacol. Sin. 2013, 34, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Yu, W.; Fu, Y.C.; Wang, X.; Li, J.L.; Wang, W. Resveratrol protects cardiomyocytes from hypoxia-induced apoptosis through the SIRT1-FoxO1 pathway. Biochem. Biophys. Res. Commun. 2009, 378, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Becatti, M.; Taddei, N.; Cecchi, C.; Nassi, N.; Nassi, P.A.; Fiorillo, C. SIRT1 modulates MAPK pathways in ischemic-reperfused cardiomyocytes. Cell. Mol. Life Sci. 2012, 69, 2245–2260. [Google Scholar] [CrossRef] [PubMed]
- Danz, E.D.; Skramsted, J.; Henry, N.; Bennett, J.A.; Keller, R.S. Resveratrol prevents doxorubicin cardiotoxicity through mitochondrial stabilization and the Sirt1 pathway. Free Radic. Biol. Med. 2009, 46, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.Y.; Mukhopadhyay, P.; Mohanraj, R.; Wang, H.; Horváth, B.; Yin, S.; Pacher, P. Resveratrol attenuates azidothymidine-induced cardiotoxicity by decreasing mitochondrial reactive oxygen species generation in human cardiomyocytes. Mol. Med. Rep. 2011, 4, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Li, G.Y.; Liu, Y.; Chai, L.M.; Chen, J.X.; Zhang, Y.; Du, Z.M.; Lu, Y.J.; Yang, B.F. Resveratrol protects against arsenic trioxide-induced cardiotoxicity in vitro and in vivo. Br. J. Pharmacol. 2008, 154, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Yao, Q.; Ke, Z.; Chen, H.; Wu, J.; Liu, C. Resveratrol attenuates high glucose-induced oxidative stress and cardiomyocyte apoptosis through AMPK. Mol. Cell. Endocrinol. 2015, 412, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Wang, W.; Zhabyeyev, P.; Basu, R.; McLean, B.; Fan, D.; Parajuli, N.; DesAulniers, J.; Patel, V.B.; Hajjar, R.J.; et al. Iron-overload injury and cardiomyopathy in acquired and genetic models is attenuated by resveratrol therapy. Sci. Rep. 2015, 5, 18132. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Ding, W.; Liao, Y.; Liu, Y.; Yan, D.; Zhang, Y.; Wang, R.; Zheng, N.; Liu, S.; Liu, J. Polydatin prevents hypertrophy in phenylephrine induced neonatal mouse cardiomyocytes and pressure-overload mouse models. Eur. J. Pharmacol. 2015, 746, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Yang, Y.; Zhou, Y.; Wang, B.; Xiong, H.; Fan, C.; Jiang, S.; Liu, J.; Ma, Z.; Hu, W.; et al. Bakuchiol attenuates myocardial ischemia reperfusion injury by maintaining mitochondrial function: The role of silent information regulator 1. Apoptosis 2016, 21, 532–545. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.Y.; Khan, J.A.; Ryu, S.; Shekhar, R.; Seung, K.B.; Mehta, J.L. Curcumin reduces angiotensin II mediated cardiomyocyte growth via LOX1 inhibition. J. Cardiovasc. Pharmacol. 2010, 55, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kwon, J.S.; Cho, Y.K.; Jeong, M.H.; Cho, J.G.; Park, J.C.; Kang, J.C.; Ahn, Y. Curcumin reduces the cardiac ischemia-reperfusion injury: Involvement of the toll-like receptor 2 in cardiomyocytes. J. Nutr. Biochem. 2012, 23, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Duan, W.; Lin, Y.; Yi, W.; Liang, Z.; Yan, J.; Wang, N.; Deng, C.; Zhang, S.; Li, Y.; et al. SIRT1 activation by curcumin pretreatment attenuates mitochondrial oxidative damage induced by myocardial ischemia reperfusion injury. Free Radic. Biol. Med. 2013, 65, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Yao, Y.; Guo, P.; Wang, T.; Yang, B.; Zhang, Z. Curcumin protects rat heart mitochondria against anoxia-reoxygenation induced oxidative injury. Can. J. Physiol. Pharmacol. 2013, 91, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Zha, W.; Ke, Z.; Min, Q.; Li, C.; Sun, H.; Liu, C. Curcumin protects neonatal rat cardiomyocytes against high glucose-induced apoptosis via PI3K/Akt signalling pathway. J. Diabetes Res. 2016, 2016, 4158591. [Google Scholar] [CrossRef] [PubMed]
- Nehra, S.; Bhardwaj, V.; Kalra, N.; Ganju, L.; Bansal, A.; Saxena, S.; Saraswat, D. Nanocurcumin protects cardiomyoblasts H9c2 from hypoxia-induced hypertrophy and apoptosis by improving oxidative balance. J. Physiol. Biochem. 2015, 71, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Nehra, S.; Bhardwaj, V.; Ganju, L.; Saraswat, D. Nanocurcumin prevents hypoxia induced stress in primary human ventricular cardiomyocytes by maintaining mitochondrial homeostasis. PLoS ONE 2015, 10, e0139121. [Google Scholar] [CrossRef] [PubMed]
- Hardy, N.; Viola, H.M.; Johnstone, V.P.; Clemons, T.D.; Cserne Szappanos, H.; Singh, R.; Smith, N.M.; Iyer, K.S.; Hool, L.C. Nanoparticle-mediated dual delivery of an antioxidant and a peptide against the l-Type Ca2+ channel enables simultaneous reduction of cardiac ischemia-reperfusion injury. ACS Nano 2015, 9, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wu, M.; Tang, L.; Pan, Y.; Liu, Z.; Zeng, C.; Wang, J.; Wei, T.; Liang, G. Novel curcumin analogue 14p protects against myocardial ischemia reperfusion injury through Nrf2-activating anti-oxidative activity. Toxicol. Appl. Pharmacol. 2015, 282, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Tuck, K.L.; Hayball, P.J. Major phenolic compounds in olive oil: Metabolism and health effects. J. Nutr. Biochem. 2002, 13, 636–644. [Google Scholar] [CrossRef]
- Bali, E.B.; Ergin, V.; Rackova, L.; Bayraktar, O.; Küçükboyaci, N.; Karasu, C. Olive leaf extracts protect cardiomyocytes against 4-hydroxynonenal-induced toxicity in vitro: Comparison with oleuropein, hydroxytyrosol, and quercetin. Planta Med. 2014, 80, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.S.; Wang, S.Q. Salvianolic acid B from Salvia miltiorrhiza inhibits tumor necrosis factor-alpha (TNF-alpha)-induced MMP-2 upregulation in human aortic smooth muscle cells via suppression of NAD(P)H oxidase-derived reactive oxygen species. J. Mol. Cell. Cardiol. 2006, 41, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.L.; Lin, F.Y.; Chen, Y.H.; Chiu, J.J.; Shiao, M.S.; Tsai, C.S.; Lin, S.J.; Chen, Y.L. Salvianolic acid B inhibits low-density lipoprotein oxidation and neointimal hyperplasia in endothelium-denuded hypercholesterolaemic rabbits. J. Sci. Food Agric. 2011, 91, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Dutta, M.; Ghosh, A.; Rangari, V.; Jain, G.; Khobragade, S.; Chattopadhyay, A.; Bhowmick, D.; Das, T.; Bandyopadhyay, D. Silymarin protects against copper-ascorbate induced injury to goat cardiac mitochondria in vitro: Involvement of antioxidant mechanism(s). Int. J. Pharm. Pharm. Sci. 2014, 6, 422–429. [Google Scholar]
- Anestopoulos, I.; Kavo, A.; Tentes, I.; Kortsaris, A.; Panayiotidis, M.; Lazou, A.; Pappa, A. Silibinin protects H9c2 cardiac cells from oxidative stress and inhibits phenylephrine-induced hypertrophy: Potential mechanisms. J. Nutr. Biochem. 2013, 24, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Gabrielová, E.; Křen, V.; Jabůrek, M.; Modrianský, M. Silymarin component 2,3-dehydrosilybin attenuates cardiomyocyte damage following hypoxia/reoxygenation by limiting oxidative stress. Physiol. Res. 2015, 64, 79–91. [Google Scholar] [PubMed]
- Esmaeili, M.A.; Sonboli, A. Antioxidant, free radical scavenging activities of Salvia brachyantha and its protective effect against oxidative cardiac cell injury. Food Chem. Toxicol. 2010, 48, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.C.; Xu, X.D.; Zhi Liu, X.; Sun, G.B.; Zhu, Y.D.; Dong, X.; Wang, J.; Zhang, H.J.; Zhang, Q.; Sun, X.B. Total flavonoids from Clinopodium chinense (Benth.) O. Ktze protect against doxorubicin-induced cardiotoxicity in vitro and in vivo. Evid. Based Complement. Altern. Med. 2015, 2015, 472565. [Google Scholar] [CrossRef]
- Chang, W.T.; Shao, Z.H.; Yin, J.J.; Mehendale, S.; Wang, C.Z.; Qin, Y.; Li, J.; Chen, W.J.; Chien, C.T.; Becker, L.B.; et al. Comparative effects of flavonoids on oxidant scavenging and ischemia-reperfusion injury in cardiomyocytes. Eur. J. Pharmacol. 2007, 566, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Akhlaghi, M.; Bandy, B. Preconditioning and acute effects of flavonoids in protecting cardiomyocytes from oxidative cell death. Oxid. Med. Cell. Longev. 2012, 2012, 782321. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; de La Lastra, C.A.; Andres-Lacueva, C.; Aviram, M.; Calhau, C.; Cassano, A.; D’Archivio, M.; Faria, A.; Favé, G.; Fogliano, V.; et al. Polyphenols and human health: A prospectus. Crit. Rev. Food Sci. Nutr. 2011, 51, 524–546. [Google Scholar] [CrossRef] [PubMed]
- Goszcz, K.; Deakin, S.J.; Duthie, G.G.; Stewart, D.; Leslie, S.J.; Megson, I.L. Antioxidants in cardiovascular therapy: Panacea or false hope? Front. Cardiovasc. Med. 2015, 2, 29. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, I.; Kohen, R.; Koren, E. Microbial and host cells acquire enhanced oxidant-scavenging abilities by binding polyphenols. Arch. Biochem. Biophys. 2011, 506, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Venkataraman, K.; Hollingsworth, A.; Piche, M.; Tai, T.C. Polyphenols: Benefits to the cardiovascular system in health and in aging. Nutrients 2013, 5, 3779–3827. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T. Dietary factors affecting polyphenol bioavailability. Nutr. Rev. 2014, 72, 429–452. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [PubMed]
- Barteková, M.; Šimončíková, P.; Fogarassyová, M.; Ivanová, M.; Okruhlicová, Ľ.; Tribulová, N.; Dovinová, I.; Barančík, M. Quercetin improves postischemic recovery of heart function in doxorubicin-treated rats and prevents doxorubicin-induced matrix metalloproteinase-2 activation and apoptosis induction. Int. J. Mol. Sci. 2015, 16, 8168–8185. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Ren, H.; Han, J.; Wang, W.; Zheng, Q.; Wang, D. Protective effects of kaempferol against myocardial ischemia/reperfusion injury in isolated rat heart via antioxidant activity and inhibition of glycogen synthase kinase-3β. Oxid. Med. Cell. Longev. 2015, 2015, 481405. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.; Han, J.; Ren, H.; Yang, W.; Zhang, X.; Zheng, Q.; Wang, D. Cardioprotective effects of astragalin against myocardial ischemia/reperfusion injury in isolated rat heart. Oxid. Med. Cell. Longev. 2016, 2016, 8194690. [Google Scholar] [CrossRef] [PubMed]
- Bhandary, B.; Piao, C.S.; Kim, D.S.; Lee, G.H.; Chae, S.W.; Kim, H.R.; Chae, H.J. The protective effect of rutin against ischemia/reperfusion-associated hemodynamic alteration through antioxidant activity. Arch. Pharm. Res. 2012, 35, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Umarani, V.; Muvvala, S.; Ramesh, A.; Lakshmi, B.V.; Sravanthi, N. Rutin potentially attenuates fluoride-induced oxidative stress-mediated cardiotoxicity, blood toxicity and dyslipidemia in rats. Toxicol. Mech. Methods 2015, 25, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Kar, A. Combined effects of vincristine and quercetin in reducing isoproterenol-induced cardiac necrosis in rats. Cardiovasc. Toxicol. 2015, 15, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Yang, H.; Wang, H.X.; Tian, C.; Liu, Y.; Zeng, X.J.; Gao, E.; Kang, Y.M.; Du, J.; Li, H.H. Inhibition of 12/15 lipoxygenase by baicalein reduces myocardial ischemia/reperfusion injury via modulation of multiple signaling pathways. Apoptosis 2014, 19, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, J.; Hu, J.; Li, X.; Zhang, X.; Li, Z. Apigenin attenuates myocardial ischemia/reperfusion injury via the inactivation of p38 mitogen-activated protein kinase. Mol. Med. Rep. 2015, 12, 6873–6878. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.Y.; Li, S.; Zhen, Y.L.; Wang, Y.N.; Shao, X.; Luo, Z.G. Cardioprotection of vitexin on myocardial ischemia/reperfusion injury in rat via regulating inflammatory cytokines and MAPK pathway. Am. J. Chin. Med. 2013, 41, 1251–1266. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.T.; Qian, L.B.; Zhang, F.J.; Wang, J.; Ai, H.; Tang, L.H.; Wang, H.P. Cardioprotective effects of luteolin on ischemia/reperfusion injury in diabetic rats are modulated by eNOS and the mitochondrial permeability transition pathway. J. Cardiovasc. Pharmacol. 2015, 65, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.H.; Chen, K.P.; Chen, S.X.; Liu, K.Z.; Fan, T.L.; Chen, Y.C. Breviscapine, a traditional Chinese medicine, alleviates myocardial ischaemia reperfusion injury in diabetic rats. Acta Cardiol. 2008, 63, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Akhlaghi, M.; Bandy, B. Dietary green tea extract increases phase 2 enzyme activities in protecting against myocardial ischemia-reperfusion. Nutr. Res. 2010, 30, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Liou, Y.M.; Hsieh, S.R.; Wu, T.J.; Chen, J.Y. Green tea extract given before regional myocardial ischemia-reperfusion in rats improves myocardial contractility by attenuating calcium overload. Pflugers Arch. 2010, 460, 1003–1014. [Google Scholar] [CrossRef] [PubMed]
- Khan, G.; Haque, S.E.; Anwer, T.; Ahsan, M.N.; Safhi, M.M.; Alam, M.F. Cardioprotective effect of green tea extract on doxorubicin-induced cardiotoxicity in rats. Acta Pol. Pharm. 2014, 71, 861–868. [Google Scholar] [PubMed]
- Li, W.; Xu, B.; Xu, J.; Wu, X.L. Procyanidins produce significant attenuation of doxorubicin-induced cardiotoxicity via suppression of oxidative stress. Basic Clin. Pharmacol. Toxicol. 2009, 104, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Sheng, R.; Gu, Z.L.; Xie, M.L. Epigallocatechin gallate, the major component of polyphenols in green tea, inhibits telomere attrition mediated cardiomyocyte apoptosis in cardiac hypertrophy. Int. J. Cardiol. 2013, 162, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Nazimabashir, V.; Manoharan, V.; Miltonprabu, S. Cadmium induced cardiac oxidative stress in rats and its attenuation by GSP through the activation of Nrf2 signaling pathway. Chem. Biol. Interact. 2015, 242, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Huang, L.L.; Yu, T.T.; Zhu, J.H.; Shen, B.; Zhang, Y.; Wang, H.Z.; Gao, S. Effects of oligomeric grape seed proanthocyanidins on heart, aorta, kidney in DOCA-salt mice: Role of oxidative stress. Phytother. Res. 2013, 27, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tan, D.; Shi, L.; Liu, X.; Zhang, Y.; Tong, C.; Song, D.; Hou, M. Blueberry anthocyanins-enriched extracts attenuate cyclophosphamide-induced cardiac injury. PLoS ONE 2015, 10, e0127813. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, C.; Upaganalawar, A.; Balaraman, R. Protection against in vivo focal myocardial ischemia/reperfusion injury-induced arrhythmias and apoptosis by hesperidin. Free Radic Res. 2009, 43, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, Y.O.; Sharma, P.K.; Shrivastava, B.; Ojha, S.; Upadhya, H.M.; Arya, D.S.; Goyal, S.N. Hesperidin produces cardioprotective activity via PPAR-γ pathway in ischemic heart disease model in diabetic rats. PLoS ONE 2014, 9, e111212. [Google Scholar] [CrossRef] [PubMed]
- Rani, N.; Bharti, S.; Manchanda, M.; Nag, T.C.; Ray, R.; Chauhan, S.S.; Kumari, S.; Arya, D.S. Regulation of heat shock proteins 27 and 70, p-Akt/p-eNOS and MAPKs by naringin dampens myocardial injury and dysfunction in vivo after ischemia/reperfusion. PLoS ONE 2013, 8, e82577. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.P.; Kushwaha, S.; Tripathi, D.N.; Jena, G.B. Cardioprotective effects of hesperetin against doxorubicin-induced oxidative stress and DNA damage in rat. Cardiovasc. Toxicol. 2011, 11, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Jiang, D.; Fang, Y.; Zhou, H.; Cheng, Z.; Lin, Y.; Zhang, R.; Zhang, J.; Pu, P.; Liu, Y.; et al. Hesperetin protects against cardiac remodelling induced by pressure overload in mice. J. Mol. Histol. 2013, 44, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, P.; Pugalendi, K.V. Hesperidin, a flavanone glycoside, on lipid peroxidation and antioxidant status in experimental myocardial ischemic rats. Redox Rep. 2010, 15, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Elavarasan, J.; Velusamy, P.; Ganesan, T.; Ramakrishnan, S.K.; Rajasekaran, D.; Periandavan, K. Hesperidin-mediated expression of Nrf2 and upregulation of antioxidant status in senescent rat heart. J. Pharm. Pharmacol. 2012, 64, 1472–1482. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Ashour, M.B.; Abdel-Moneim, A.; Ahmed, O.M. Hesperidin and naringin attenuate hyperglycemia-mediated oxidative stress and proinflammatory cytokine production in high fat fed/streptozotocin-induced type 2 diabetic rats. J. Diabetes Complications 2012, 26, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Wang, X.; Du, G.; Tian, J.; Liu, Y. Calycosin-7-O-β-d-glucoside attenuates ischemia-reperfusion injury in vivo via activation of the PI3K/Akt pathway. Mol. Med. Rep. 2016, 13, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ren, H.B.; Chen, X.L.; Wang, F.; Wang, R.S.; Zhou, B.; Wang, C.; Sun, Y.X.; Wang, Y.J. Puerarin attenuates severe burn-induced acute myocardial injury in rats. Burns 2015, 41, 1748–1757. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Liu, L.; Wang, W.; Han, J.; Ren, H.; Zheng, Q.; Wang, D. Role of licochalcone C in cardioprotection against ischemia/reperfusion injury of isolated rat heart via antioxidant, anti-inflammatory, and anti-apoptotic activities. Life Sci. 2015, 132, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Annapurna, A.; Mudagal, M.P.; Ansari, A.; Rao, A.S. Cardioprotective activity of chalcones in ischemia/reperfusion-induced myocardial infarction in albino rats. Exp. Clin. Cardiol. 2012, 17, 110–114. [Google Scholar] [PubMed]
- Pantsi, W.G.; Marnewick, J.L.; Esterhuyse, A.J.; Rautenbach, F.; van Rooyen, J. Rooibos (Aspalathus linearis) offers cardiac protection against ischaemia/reperfusion in the isolated perfused rat heart. Phytomedicine 2011, 18, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wang, M.; Chen, C.; Le, X.; Sun, S.; Yin, Y. Cardiovascular protection with danshensu in spontaneously hypertensive rats. Biol. Pharm. Bull. 2011, 34, 1596–1601. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Qiao, H.; Li, Y.; Li, L. Protective roles of puerarin and Danshensu on acute ischemic myocardial injury in rats. Phytomedicine 2007, 14, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Pan, Q.; Han, W.; Liu, Z.; Li, L.; Hu, X. Schisandrin B prevents doxorubicin-induced cardiotoxicity via enhancing glutathione redox cycling. Clin. Cancer Res. 2007, 13, 6753–6760. [Google Scholar] [CrossRef] [PubMed]
- Thandavarayan, R.A.; Giridharan, V.V.; Arumugam, S.; Suzuki, K.; Ko, K.M.; Krishnamurthy, P.; Watanabe, K.; Konishi, T. Schisandrin B prevents doxorubicin induced cardiac dysfunction by modulation of DNA damage, oxidative stress and inflammation through inhibition of MAPK/p53 signaling. PLoS ONE 2015, 10, e0119214. [Google Scholar] [CrossRef] [PubMed]
- Li, W.X.; Kong, X.; Zhang, J.X.; Yang, J.R. Long-term intake of sesamin improves left ventricular remodelling in spontaneously hypertensive rats. Food Funct. 2013, 4, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.C.; Kim, K.J.; Kim, Y.M.; Ha, Y.M.; Kim, H.J.; Yun, U.J.; Bae, K.H.; Kim, Y.S.; Kang, S.S.; Seo, H.G.; et al. Anti-apoptotic effect of magnolol in myocardial ischemia and reperfusion injury requires extracellular signal-regulated kinase1/2 pathways in rat in vivo. Exp. Biol. Med. (Maywood) 2008, 233, 1280–1288. [Google Scholar] [CrossRef] [PubMed]
- Dolinsky, V.W.; Chan, A.Y.; Robillard Frayne, I.; Light, P.E.; Des Rosiers, C.; Dyck, J.R. Resveratrol prevents the prohypertrophic effects of oxidative stress on LKB1. Circulation 2009, 119, 1643–1652. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, Q.; Sun, Y.Y.; Xing, Y.F.; Wang, Y.B.; Lu, X.T.; Bai, W.W.; Liu, X.Q.; Zhao, Y.X. Resveratrol-enhanced autophagic flux ameliorates myocardial oxidative stress injury in diabetic mice. J. Cell. Mol. Med. 2014, 18, 1599–1611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Feng, Y.; Qu, S.; Wei, X.; Zhu, H.; Luo, Q.; Liu, M.; Chen, G.; Xiao, X. Resveratrol attenuates doxorubicin-induced cardiomyocyte apoptosis in mice through SIRT1-mediated deacetylation of p53. Cardiovasc. Res. 2011, 90, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Al-Harthi, S.E.; Alarabi, O.M.; Ramadan, W.S.; Alaama, M.N.; Al-Kreathy, H.M.; Damanhouri, Z.A.; Khan, L.M.; Osman, A.M. Amelioration of doxorubicin-induced cardiotoxicity by resveratrol. Mol. Med. Rep. 2014, 10, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Cui, X.H.; Yu, H.L.; Wu, R.; Xu, X.; Gao, J.P. Synergistic effects of polydatin and vitamin C in inhibiting cardiotoxicity induced by doxorubicin in rats. Fundam. Clin. Pharmacol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.; Hu, X.; Zheng, Y.; Wang, S.; Kong, J.; Cai, L. Resveratrol protects against lipopolysaccharide-induced cardiac dysfunction by enhancing SERCA2a activity through promoting the phospholamban oligomerization. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H1051–H1062. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; He, D.; Zhang, Q.; Han, Y.; Jin, S.; Qi, F. Resveratrol protects against Cisplatin-induced cardiotoxicity by alleviating oxidative damage. Cancer Biother. Radiopharm. 2009, 24, 675–680. [Google Scholar] [CrossRef] [PubMed]
- González-Salazar, A.; Molina-Jijón, E.; Correa, F.; Zarco-Márquez, G.; Calderón-Oliver, M.; Tapia, E.; Zazueta, C.; Pedraza-Chaverri, J. Curcumin protects from cardiac reperfusion damage by attenuation of oxidant stress and mitochondrial dysfunction. Cardiovasc. Toxicol. 2011, 11, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Izem-Meziane, M.; Djerdjouri, B.; Rimbaud, S.; Caffin, F.; Fortin, D.; Garnier, A.; Veksler, V.; Joubert, F.; Ventura-Clapier, R. Catecholamine-induced cardiac mitochondrial dysfunction and mPTP opening: Protective effect of curcumin. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H665–H674. [Google Scholar] [CrossRef] [PubMed]
- Soetikno, V.; Sari, F.R.; Sukumaran, V.; Lakshmanan, A.P.; Mito, S.; Harima, M.; Thandavarayan, R.A.; Suzuki, K.; Nagata, M.; Takagi, R.; et al. Curcumin prevents diabetic cardiomyopathy in streptozotocin-induced diabetic rats: Possible involvement of PKC-MAPK signaling pathway. Eur. J. Pharm. Sci. 2012, 47, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Imbaby, S.; Ewais, M.; Essawy, S.; Farag, N. Cardioprotective effects of curcumin and nebivolol against doxorubicin-induced cardiac toxicity in rats. Hum. Exp. Toxicol. 2014, 33, 800–813. [Google Scholar] [CrossRef] [PubMed]
- Andreadou, I.; Sigala, F.; Iliodromitis, E.K.; Papaefthimiou, M.; Sigalas, C.; Aligiannis, N.; Savvari, P.; Gorgoulis, V.; Papalabros, E.; Kremastinos, D.T. Acute doxorubicin cardiotoxicity is successfully treated with the phytochemical oleuropein through suppression of oxidative and nitrosative stress. J. Mol. Cell. Cardiol. 2007, 42, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Andreadou, I.; Mikros, E.; Ioannidis, K.; Sigala, F.; Naka, K.; Kostidis, S.; Farmakis, D.; Tenta, R.; Kavantzas, N.; Bibli, S.I.; et al. Oleuropein prevents doxorubicin-induced cardiomyopathy interfering with signaling molecules and cardiomyocyte metabolism. J. Mol. Cell. Cardiol. 2014, 69, 4–16. [Google Scholar] [CrossRef] [PubMed]
- El-Shitany, N.A.; El-Haggar, S.M.; El-desoky, K. Silymarin prevents adriamycin-induced cardiotoxicity and nephrotoxicity in rats. Food Chem. Toxicol. 2008, 46, 2422–2428. [Google Scholar] [CrossRef] [PubMed]
- Cecen, E.; Dost, T.; Culhaci, N.; Karul, A.; Ergur, B.; Birincioglu, M. Protective effects of silymarin against doxorubicin-induced toxicity. Asian Pac. J. Cancer Prev. 2011, 12, 2697–2704. [Google Scholar] [PubMed]
- El-Awady, el-S.E.; Moustafa, Y.M.; Abo-Elmatty, D.M.; Radwan, A. Cisplatin-induced cardiotoxicity: Mechanisms and cardioprotective strategies. Eur. J. Pharmacol. 2011, 650, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Taghiabadi, E.; Imenshahidi, M.; Abnous, K.; Mosafa, F.; Sankian, M.; Memar, B.; Karimi, G. Protective effect of silymarin against acrolein-induced cardiotoxicity in mice. Evid. Based Complement Altern. Med. 2012, 2012, 352091. [Google Scholar] [CrossRef] [PubMed]
- Muthumani, M.; Prabu, S.M. Silibinin potentially attenuates arsenic-induced oxidative stress mediated cardiotoxicity and dyslipidemia in rats. Cardiovasc. Toxicol. 2014, 14, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Tomé-Carneiro, J.; Visioli, F. Polyphenol-based nutraceuticals for the prevention and treatment of cardiovascular disease: Review of human evidence. Phytomedicine 2016, 23, 1145–1174. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Alam, F.; Solayman, M.; Khalil, M.I.; Kamal, M.A.; Gan, S.H. Dietary phytochemicals: Natural swords combating inflammation and oxidation-mediated degenerative diseases. Oxid. Med. Cell Longev. 2016, 2016, 5137431. [Google Scholar] [CrossRef] [PubMed]
- Magyar, K.; Halmosi, R.; Palfi, A.; Feher, G.; Czopf, L.; Fulop, A.; Battyany, I.; Sumegi, B.; Toth, K.; Szabados, E. Cardioprotection by resveratrol: A human clinical trial in patients with stable coronary artery disease. Clin. Hemorheol. Microcirc. 2012, 50, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, B.; Campen, M.J.; Channell, M.M.; Wherry, S.J.; Varamini, B.; Davis, J.G.; Baur, J.A.; Smoliga, J.M. Resveratrol for primary prevention of atherosclerosis: Clinical trial evidence for improved gene expression in vascular endothelium. Int. J. Cardiol. 2012, 8, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Zunino, S.J.; Peerson, J.M.; Freytag, T.L.; Breksa, A.P.; Bonnel, E.L.; Woodhouse, L.R.; Storms, D.H. Dietary grape powder increases IL-1β and IL-6 production by lipopolysaccharide-activated monocytes and reduces plasma concentrations of large LDL and large LDL-cholesterol particles in obese humans. Br. J. Nutr. 2014, 112, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Zern, T.L.; Wood, R.J.; Greene, C.; West, K.L.; Liu, Y.; Aggarwal, D.; Shachter, N.S.; Fernandez, M.L. Grape polyphenols exert a cardioprotective effect in pre- and postmenopausal women by lowering plasma lipids and reducing oxidative stress. J. Nutr. 2005, 135, 1911–1917. [Google Scholar] [PubMed]
- Vaisman, N.; Niv, E. Daily consumption of red grape cell powder in a dietary dose improves cardiovascular parameters: A double blind, placebo-controlled, randomized study. Int. J. Food Sci. Nutr. 2015, 66, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Mellen, P.B.; Daniel, K.R.; Brosnihan, K.B.; Hansen, K.J.; Herrington, D.M. Effect of muscadine grape seed supplementation on vascular function in subjects with or at risk for cardiovascular disease: A randomized crossover trial. J. Am. Coll. Nutr. 2010, 29, 469–475. [Google Scholar] [CrossRef] [PubMed]
| Polyphenol | Cardiac Damage Inducers | Cell Type | In Vitro Effects | Ref. |
|---|---|---|---|---|
| Quercetin | DOX H2O2 A/R Xanthine/XO | H9c2 cells | ↓ Apoptosis, ROS, LDH release ↓ DNA fragmentation ↓ Bid, p53 and NADPH oxidase ↓ ERK1/2, Akt, p38, JNK, TNF-α ↓ Phospho-ERK1/2 and –Akt ↑ Phospho-c-Jun and -PKCƐ ↓ ∆ψm loss and Ca2+ ↓ Phospho-MAPKAPK-2 and caspase 3 | [45,48,52,53,57] |
| 3′-O-methyl quercetin | H2O2 | H9c2 cells | ↓ Apoptosis and LDH release | [45] |
| Hydroxytyrosol | Xanthine/XO | H9c2 cells | ↑ Phopsho-ERK1/2 and -Hsp-27 ↓ Phospho-c-Jun | [57] |
| Taxifolin | Ang II | Neonatal rat cardiomyocytes | ↓ ROS and hypertrophy | [56] |
| Rhamnetin | H2O2 | H9c2 cells | ↓ Apoptosis and ROS ↑ CAT and MnSOD ↓ Phospho-Akt/GSK-3β, -ERK1/2, -p38 and -JNK | [46] |
| Isorhamnetin | DOX | H9c2 cells | ↓ Apoptosis and ROS ↓ LDH release and lipid peroxidation ↑ Anti-oxidant markers ↓ Bax/Bcl-2 ratio, p53, caspases 9 and 3, PARP and cytochrome c release ↓ Phospho-ERK, -p38 and -JNK | [49] |
| Dihydromyricetin | DOX | Primary myocardial H9c2 cells | ↓ Apoptosis and ROS ↑ GSH ↓ Nuclear damage, caspase 3 and 8, PARP, ∆ψm loss and Bax/Bcl-2 ratio | [51] |
| Kaempferol | DOX A/R | H9c2 cells Neonatal primary rat cardiomyocytes | ↓ Apoptosis and ROS ↓ LDH release ↑ SIRT1, ∆ψm and Bcl-2 ↓ mPTP opening and DNA fragmentation ↓ Phospho-ERK1/2 ↓ p53, cytochrome c release, and caspase 3 and PARP cleavage | [50,54] |
| 3′,4′-dihydroxyflavonol | I/R H2O2 | Cardiomyocytes | ↓ Superoxide ↑ Phospho-ERK, -MEK and -Akt | [47,55] |
| Apigenin | A/R | H9c2 cells | ↓ Apoptosis and ROS ↓ LDH release and cytochrome c release | [58] |
| Apigenin glucoside, vitexin | A/R | Neonatal rat cardiomyocytes | ↓ Apoptosis and ROS ↓ LDH and CK release ↑ Phospho-ERK1/2 | [59] |
| Baicalein | Ipoxia I/R DOX | Chick cardiomyocytes H9c2 cells hESC-CMs | ↓ Apoptosis and ROS ↓ LDH release ↑ Nrf2 pathway and HO-1 ↑ Contractile activity | [61,62,63,64] |
| EGCG | H2O2 I/R DOX Bisulfite/sulfite Lead | Neonatal and adult rat cardiomyocytes H9c2 cells Cultures of cardiomyocytes Rat ventricular myocytes | ↓ Apoptosis and ROS ↓ Cellular damage and citosolic Ca2+ ↓ LDH release and MDA formation ↑ MnSOD, CAT and GSH-Px ↑ HO-1 and caveolin-1 ↓ β-catenin, N-cadherin and Cx43 ↓ p53, p21, caspase 3 and FasR ↓ STAT-1 activation, telomere attrition and TRF2 loss | [65,66,67,68,69,70,72,75,76] |
| EGCG and TF3 | H2O2 | Neonatal rat cardiomyocytes | ↓ Cellular damage ↑ Akt, ERK1/2 and p38 MAPK | [65] |
| (−)-epicatechin, avicularin and quercitrin | BSO | H9c2 cells | ↓ Apoptosis and LDH release | [77] |
| Grape seed proanthocyanidin extract | I/R DOX | Chick cardiomyocytes Primary cultures cardiomyocytes | ↓ Apoptosis and ROS ↑ NO and GSH-GSSG ratio ↓ ∆ψm loss and DNA fragmentation ↑ Contractile activity | [71,73] |
| Malvidin | I/R | Rat cardiomyocytes | ↑ LV pressure, Akt, eNOS, ERK1/2, and phospho-GSK3β | [78] |
| Cyanidin-3-O-glucoside | I/R | Rat cardiomyocytes | ↓ Apoptosis and LDH release | [79] |
| Blueberry phenol fractions | NE | Adult rat cardiomyocytes | ↓ Apoptosis and calpains ↑ SOD and CAT | [80] |
| Narigenin | H2O2 | H9c2 cells | ↓ Apoptosis and lipid peroxidation ↑ GSH-Px, GST, CAT, Nrf2, GCL and NQO-1 | [81] |
| Naringin | High glucose | H9c2 cells | ↓ Apoptosis and ROS ↑ GSH-Px, SOD, CAT and Bcl-2 ↓ ∆ψm loss, p53, Bax, Bad, caspase, release of cytochrome c ↓ ERK1/2, p38 MAPK, JNK and leptin | [82,83,84,85] |
| Naringenin-7-O-glucoside | DOX | H9c2 cells | ↓ Apoptosis and ROS ↑ SOD, CAT, GSH-Px and NQO-1 ↓ CK, LDH, caspase 9 and 3 mRNA ↑ ERK, Nrf2, HO-1 and Bcl-2 | [86,87,88] |
| Hesperetin | LPS | H9c2 cells | ↓ Apoptosis ↑ Bcl-2 ↓ Bax and phopsho-JNK | [89] |
| Puerarin | Ang II | Neonatal murine cardiomyocytes | ↓ ROS ↓ NADPH oxidase ↓ ERK 1/2, JNK and AP-1 | [90] |
| Calycosin | H2O2 | H9c2 cells | ↓ Apoptosis ↑ ERα/β and Akt | [91] |
| Licochalcone D | I/R | Rat cardiomyocytes | ↓ Caspase 3 and PARP ↓ IL-6, TNF-α, CRP, LDH, CK, MDA, NO, NF-κB and p38 MAPK ↑ SOD, GSH/GSSG ratio, eNOS and Akt | [92] |
| Isoliquiritigenin | I/R | Cardiomyocytes | ↓ ROS and mitochondrial potential ↑ AMPK and ERK | [94] |
| Safflor yellow A | A/R | Neonatal rat cardiomyocytes | ↓ LDH, CK, MDA and Bax ↑ SOD, CAT, GSH, GSH-Px and Bcl-2 | [95] |
| Hydroxysafflor yellow A | A/R | H9c2 cells | ↓ Apoptosis ↑ Nrf2 | [96] |
| Chalcone derivative L6H9 | Glucose | H9c2 cells | ↓ ROS, Hypertrophy and fibrosis ↓ Bax, caspase 9 and 3 ↓ IL-6, TNF-α, COX and NF-κB ↑ Nrf2, HO-1, NQO-1 and GCLC | [97] |
| Aspalathin | Hyperglycemia | H9c2 cells | ↓ ROS ↓ DNA nick and ∆ψm loss ↑ GSH, SOD and Bcl-2/Bax ratio | [99] |
| GLG | H2O2 | H9c2 cells | ↓ Apoptosis, ROS and hemolysis ↓ Caspase 3 and nuclear condensation and fragmentation | [100] |
| CSM-bark extract | H2O2 | Neonatal cardiomyocytes | ↓ Apoptosis and ROS | [101] |
| Hispidin | H2O2 | H9c2 cells | ↓ Apoptosis, ROS and LDH release ↓ DNA fragmentation, caspase 3 and Bax ↑ HO-1, CAT, Bcl-2, Akt/GSK3β and ERK 1/2 | [102] |
| Methyl gallate | H2O2 | Neonatal rat cardiomyocytes | ↓ Apoptosis and ROS ↓ DNA damage, caspase 3 and ∆ψm loss ↑ GSH | [103] |
| Pyrrolidinyl caffeamide | H2O2 | HL-1 cells | ↓ Apoptosis and ROS ↑ CAT, MnSOD, HO-1 and phospho-Akt | [104] |
| Ferulic acid | High glucose | Cardiomyocytes | ↓ Apoptosis and ROS ↑ GSH, Nrf2, HO-1 and Keap-1 | [105] |
| MPE | Malathion | H9c2 cells | ↓ ROS, DPPH, ABTS and NO ↑ Integrity of extra cellular matrix components | [106] |
| Hydroxycinnamic acids | DOX | Neonatal rat cardiomyocytes | ↓ Cellular damage and lipid peroxidation | [107] |
| Danshensu | I/R | H9c2 cells | ↓ Apoptosis, ROS, LDH release, CK, MDA and caspase 3 ↑ SOD, CAT, GSH-Px, HO-1, Bcl-2/Bax ratio, PI3K/Akt and ERK1/2 | [108,109] |
| Sch B | I/R | H9c2 cells | ↓ Apoptosis, inflammation and ROS ↓ Bax/Bcl-2 ratio, NF-κB ↑ ERK and Nrf2 | [110,111] |
| Syringaresinol | I/R | H9c2 cells | ↓ ROS, MDA, Bax/Bcl-2 ratio, caspase 3 and HIF-1 ↑ FoxO3 and anti-oxidant markers | [113] |
| Sesamin | DOX Ang II | H9c2 cells | ↓ Apoptosis, ROS and MDA ↓ Caspase 3, p47phox and ∆ψm loss ↑Bcl-2, SOD and T-AOC | [114,115] |
| Resveratrol | H2O2 Hypoxia DOX AZT As2O3 High glucose Iron | H9c2 cells Neonatal rat ventricular cardiomyocytes Human and rat primary cardiomyocytes | ↓ Apoptosis, necrosis, autophagy, mitochondrial dysfunction and cell injury ↓ ROS, NADPH oxidase, LDH release, FoxO1, CaMKII ↓ Bax, phospho-p38 and -JNK ↑ SIRT1, Bcl-2, phospho-Akt and -ERK ↑ SOD, CAT | [116,117,118,119,120,121,122,123,124,125,126,127] |
| Polydatin | Phenylephrine | Neonatal rat cardiomyocytes | ↓ ROS and RhoA/ROCK | [128] |
| Bakuchiol | I/R | Rat cardiomyocytes | ↓ Apoptosis ↑ SIRT1, SDH, cytochrome c oxidase and SOD | [129] |
| Curcumin | TNF-α Peptidoglycan H/R I/R Glucose | Rat cardiomyocytes | ↓ Apoptosis, ROS, NADPH oxidase, MDA, lipid peroxidation and protein carbonylation ↓ Bax, cytochrome c and cardiolipin release, FoxO1, TLR2 and MCP-1 ↑ Bcl-2, SDH, COX, SOD, SIRT1, Akt and phospho-GSK-3β | [131,132,133,134] |
| Nanocurcumin | Hypoxia | H9c2 cells HVCM | ↓ Apoptosis, hypertrophy and ROS ↓ HIF-1α, caspase 3 and 7, p53 translocation ↓ AMPKα, p-300 HAT, LDH, acetyl-CoA and ∆ψm loss ↑ c-Fos, c-Jun and ATP | [135,136] |
| Curcumin analogue 14p | I/R | H9c2 cells | ↓ Apoptosis, ROS and MDA ↑ Nrf2, SOD | [138] |
| Salvianolic acid B | TNF-α | HASMC | ↓ ROS, NADPH oxidase, MMP-2 | [141] |
| Silymarin | Copper-ascorbate | Neonatal rat cardiomyocytes | ↓ ROS, NO, protein carbonylation and lipid peroxidation ↑ mitochondrial function, GSH, GSH-Px, GR, SOD, PDH | [143] |
| Silibinin | H2O2 Phenylephrine | H9c2 cells | ↓ Apoptosis, DNA damage, ROS ↓ ERK and Akt | [144] |
| 2,3-dehydrosilybin | H/R | Neonatal rat cardiomyocytes | ↓ ROS, protein carbonylation and LDH release | [145] |
| TFCC | DOX | H9c2 cells | ↓ Apoptosis, ROS, MDA and LDH release ↓ DNA fragmentation, caspase 3, cytochrome c release, Bax/Bcl-2 ratio, p53, phospho-ERK, -p38 and -JNK ↑ SOD, CAT, GSH-Px, phospho-Akt and PI3K | [147] |
| Polyphenol | In Vivo Model | Protective Effects | Ref. |
|---|---|---|---|
| Quercetin | Mice treated with DOX | ↑ Cardiac function ↓ ROS and lipid peroxidation ↑ Bmi-1 and SOD expression | [48] |
| Rats treated with DOX | ↓ Blood pressure and heart rate increase ↓ Cellular damage ↓ MMP-2 activation and apoptosis ↑ SOD activity | [156] | |
| Vincristine and quercetin | Rats exposed to isoproterenol | ↓ CK-MB, LDH, ALT, cTnT ↓ Lipid peroxidation ↑ SOD, CAT, GR, GSH-Px activities ↓ Heart rate and ST-segment elevation | [161] |
| Taxifolin | Mouse model of TAC | ↓ Pressure overload, fibrosis, ROS, MDA, HNE ↓ Cardiac remodeling and ventricular dysfunction ↓ ANP, BNP, β-MHC expression ↓ Phospho-ERK1/2, phospho-JNK1/2, Smad2 | [56] |
| DiOHF | Sheep model of I/R injury | ↓ ROS, neutrophil accumulation, LVDP, infarct size ↑ Myocardial function | [55] |
| Isorhamnetin | Rats treated with DOX | ↓ Cardiac enzymes, apoptosis, ROS, lipid peroxidation ↑ Anti-oxidant enzymes | [49] |
| Rutin | Rats exposed to sodium fluoride | ↓ Cardiac dysfunction, cardiac serum markers ↓ Lipid peroxidation and DNA fragmentation ↑ SOD, CAT, GSH levels | [160] |
| Dihydromyricetin | Mice treated with DOX | ↑ Survival rate ↓ AST, CK-MB, LDH activities | [51] |
| Kaempferol | Rats treated with DOX | ↑ Body and heart weights, SOD, CAT ↓ LDH levels, apoptosis, mitochondrial damage | [50] |
| Rat model of I/R injury | ↑ Cardiac function, SOD activity, GSH/GSSG ratio ↓ CK, LDH, MDA levels, infarct size, apoptosis | [157] | |
| Astragalin | Rat model of I/R injury | ↑ Cardiac function, SOD activity, GSH/GSSG ratio ↓ CK, LDH, MDA levels, infarct size, apoptosis | [158] |
| Baicalein | Murine model of I/R injury | ↓ Infarct size, apoptosis, pro-inflammatory cytokines ↓ ROS, MDA levels ↑ GSH-Px | [162] |
| Apigenin | Rat model of I/R injury | ↓ Infarct size, apoptosis, CK, LDH, MDA levels ↑ SOD | [163] |
| Vitexin | Rat model of I/R injury | ↑ Cardiac function, SOD activity ↓ Infarct size, apoptosis, inflammatory cytokines ↓ CK, LDH, MDA | [164] |
| Luteolin | Rat model of I/R injury | ↑ Cardiac function, MnSOD activity ↓ LDH, MDA levels | [165] |
| Breviscapine | Rat model of I/R injury | ↓ ICAM-1, ROS, MDA ↑ SOD, GSH-Px activities | [166] |
| Green Tea Exctract (GTE) | Rat model of I/R injury | ↓ Infarct size, apoptosis ↑ GSH, GCL, QR | [167] |
| Rats treated with DOX | ↓ AST, CK, LDH, lipid peroxidation ↑ Cyt P450, GSH, GSH-Px, GR, GST, SOD, CAT | [169] | |
| EGCG, quercetin | Rats with cardiac hypertrophy | ↓ Systolic blood pressure, heart weight indices, MDA ↑ SOD, GSH-Px activities, apoptosis | [67,171] |
| GSP | Rats treated with cadmium | ↓ Cardiac damage, CK-MB, AST, ALT, ALP, LDH ↓ Pro-inflammatory cytokines, apoptosis ↑ GSH-Px, GR, GST, SOD, CAT, G6PD | [172] |
| Procyanidins | Rats treated with DOX | ↑ Cardiac function ↓ Lipid peroxidation | [170] |
| BAE | Rats treated with CTX | ↑ Cardiac function , IL-10, SOD, GSH ↓ Apoptosis, pro-inflammatory cytokines, MDA | [174] |
| Hesperidin | Rat model of I/R injury | ↑ Cardiac function ↓ Apoptosis, oxidative stress | [176] |
| Naringin | Rat model of I/R injury | ↓ CK-MB, LDH, apoptosis, infarct size, inflammation ↑ SOD, GSH-Px | [177] |
| Hesperetin | Rats treated with DOX | ↓ MDA, DNA damage ↑ GSH | [178] |
| Hesperidin | Rats treated with isoproterenol | ↓ Lipid peroxidation ↓ CK, CK-MB, LDH, AST, ALT, cTnI, cTnT ↑ SOD, CAT, GSH-Px, GST, GR | [180] |
| Hesperidin, naringin | HFD/STZ-induced diabetic rats | Prevention of diabetic complications ↓ MDA, NO ↑ SOD, CAT, GSH-Px, GR | [182] |
| Puerarin | Mice treated with Ang II | ↓ Cardiac hypertrophy, HW/BW, LVW/BW | [90] |
| Rats subjected to severe burn | ↓ CK-MB, cTnT, MDA, MPO | [184] | |
| Calycosin-7-O-β- d -glucoside | Rat model of I/R injury | ↑ Cardiac function, SOD activity ↓ Infarct size, CK, LDH, MDA, apoptosis | [183] |
| Chalcone derivative L6H9 | STZ-induced diabetic mice | ↓ Cardiac damage and fibrosis ↓ ROS, TNF-α, IL-6, COX2, Bax ↑ HO-1, NQO-1, GCLC, Bcl-2 | [97] |
| Licochalcone B | Rat model of I/R injury | ↓ Apoptosis, MDA, LDH, CK, TNF-α ↑ LVDP, SOD, GSH/GSSG ratio | [185] |
| Cl-chalcone, F-chalcone | Rat model of I/R injury | ↓ Infarct size, lipid peroxidation, MDA ↑ SOD, CAT | [186] |
| Pyrrolidinyl caffeamide (PLCA) | Rat model of I/R injury | ↓ Troponin, MDA, MPO ↑ Cardiac function, CAT, HO-1, MnSOD | [104] |
| Danshensu | I/R in spontaneously hypertensive rats (SHR) | ↓ Blood pressure increase, arrhythmias, HW/BW ↑ NO content, iNOS activity | [188] |
| Rat model of I/R injury | ↓ Infarct size, CK-MB, cTnI | [108] | |
| Shenge | Rats subjected to LAD | ↓ ST-segment elevation, infarct size ↓ CK-MB, LDH, MDA ↑ SOD activity | [189] |
| Schisandrin B (Sch B) | Rats treated with DOX | ↓ CK, CK-MB, LDH, AST, MDA, MMP ↓ Cardiac damage, cell death ↑ GSH, GSH/GSSG, GR, GST, GSH-Px, SOD | [190] |
| Mice treated with DOX | ↓ Cardiac damage, apoptosis, DNA damage ↓ ROS, MDA, TNF-α, IL-1β, IL-6, MMP-2, MMP-9 ↑ GSH, LV performance | [191] | |
| Mouse model of myocardial infarction (MI) | ↑ Survival rate, heart function, eNOS ↓ Infarct size, TGF-β1, TNF-α, IL-1β, Bax/Bcl-2 | [111] | |
| Rat model of I/R injury | ↑ GSH ↓ LDH | [110] | |
| Magnolol | Rat model of I/R injury | ↓ Infarct size, apoptosis, myocardial dysfunction | [193] |
| Sesamin | SHR rats | ↓ Cardiac fibrosis, systolic blood pressure ↓ HW/BW, LVW/BW, MDA, TGF-β1 ↑ Cardiac total anti-oxidant capability | [192] |
| Rats treated with DOX | Normalization QT intervals, QRS complexes ↑ SIRT1 activation, MnSOD | [114] | |
| Deoxyshizandrin (DSD) + Schisantherin (STA) | Rat model of I/R injury | ↓ Infarct size, LVDP, arrhythmias, MDA ↑ LVSP, SOD | [112] |
| Resveratrol | SHR rats | ↓ H2O2, left ventricular hypertrophy ↑ CAT activity | [119,194] |
| Mice treated with arsenic trioxide (As2O3) | ↓ QT-interval prolongation, cardiac damage, LDH ↑ GSH-Px, CAT, SOD | [125] | |
| Mice treated with LPS | ↑ SERCA2a, Nrf2 ↓ MDA, HNE | [199] | |
| Rats treated with cisplatin | ↓ LDH, CK, MDA ↑ SOD, GSH, GSH-Px, CAT | [200] | |
| Rats treated with DOX | ↓ Cardiac dysfunction, apoptosis, MDA, CK, LDH ↑ SIRT1, GSH | [196,197] | |
| STZ-induced diabetic mice | ↓ Apoptosis, p62 ↑ Cardiac function, SIRT1, autophagy | [195] | |
| Polydatin + vitamin C | Rats treated with DOX | ↓ ROS, MDA, CRP, ST and QT intervals ↑ GSH-Px, SOD | [198] |
| Polydatin | Mice subjected to TAC | ↓ Cardiac hypertrophy | [128] |
| Curcumin | Rat model of I/R | ↓ Lipid peroxidation ↑ Cardiac function, SOD, CAT, GSH, GSH-Px | [201] |
| Rats treated with isoprenaline | ↓ Apoptosis, MPO, MDA ↑ CAT, GSH | [202] | |
| Rat model of I/R | ↑ SIRT1, Bcl-2, SDH, COX ↓ Bax, CK, LDH | [132] | |
| STZ-induced diabetic rats | ↓ MDA, hypertrophy, fibrosis, ventricular dysfunction ↑ GSH-Px | [203] | |
| Curcumin + nebivolol | Rats treated with DOX | ↑ Survival rate, SOD, GSH-Px, Body and heart weights ↓ Cardiac damage, lipid peroxidation, NO ↓ QT and ST intervals | [204] |
| Oleuropein | Rats treated with DOX | ↓ CK, CK-MB, LDH, ALT, AST, apoptosis ↓ MDA, protein carbonyl, nitrotyrosine, iNOS | [205,206] |
| Silymarin | Rats treated with DOX | ↓ CK, LDH, creatinine, urea, MDA ↑ GSH | [207] |
| Rats treated with cisplatin | ↓ LDH, CK, CK-MB, cTnI, MDA ↑ GSH, SOD | [209] | |
| Mice treated with acrolein | ↓ Lipid peroxidation, apoptosis, MDA, cTnI, CK-MB ↑ GSH, SOD, CAT | [210] | |
| Silibinin | Rats treated with arsenic | ↑ Cardiac function, Nrf-2, HO-1 ↑ SOD, CAT, GSH-Px, GST, GR, G6PD ↓ CK-MB, LDH, AST, ALT, ALP, HW/BW | [211] |
| Clinopodium chinense (Benth.) O. Ktze (TFCC) | Rats treated with DOX | ↑ Body and heart weights ↓ CK, AST, LDH, MDA, apoptosis ↑ SOD, CAT, GSH-Px | [147] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mattera, R.; Benvenuto, M.; Giganti, M.G.; Tresoldi, I.; Pluchinotta, F.R.; Bergante, S.; Tettamanti, G.; Masuelli, L.; Manzari, V.; Modesti, A.; et al. Effects of Polyphenols on Oxidative Stress-Mediated Injury in Cardiomyocytes. Nutrients 2017, 9, 523. https://doi.org/10.3390/nu9050523
Mattera R, Benvenuto M, Giganti MG, Tresoldi I, Pluchinotta FR, Bergante S, Tettamanti G, Masuelli L, Manzari V, Modesti A, et al. Effects of Polyphenols on Oxidative Stress-Mediated Injury in Cardiomyocytes. Nutrients. 2017; 9(5):523. https://doi.org/10.3390/nu9050523
Chicago/Turabian StyleMattera, Rosanna, Monica Benvenuto, Maria Gabriella Giganti, Ilaria Tresoldi, Francesca Romana Pluchinotta, Sonia Bergante, Guido Tettamanti, Laura Masuelli, Vittorio Manzari, Andrea Modesti, and et al. 2017. "Effects of Polyphenols on Oxidative Stress-Mediated Injury in Cardiomyocytes" Nutrients 9, no. 5: 523. https://doi.org/10.3390/nu9050523
APA StyleMattera, R., Benvenuto, M., Giganti, M. G., Tresoldi, I., Pluchinotta, F. R., Bergante, S., Tettamanti, G., Masuelli, L., Manzari, V., Modesti, A., & Bei, R. (2017). Effects of Polyphenols on Oxidative Stress-Mediated Injury in Cardiomyocytes. Nutrients, 9(5), 523. https://doi.org/10.3390/nu9050523







