Macronutrient Intake and Risk of Crohn’s Disease: Systematic Review and Dose–Response Meta-Analysis of Epidemiological Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection and Exclusion
2.3. Data Extraction and Quality Assessment
2.4. Statistical Analysis
3. Results
3.1. Study Characteristics
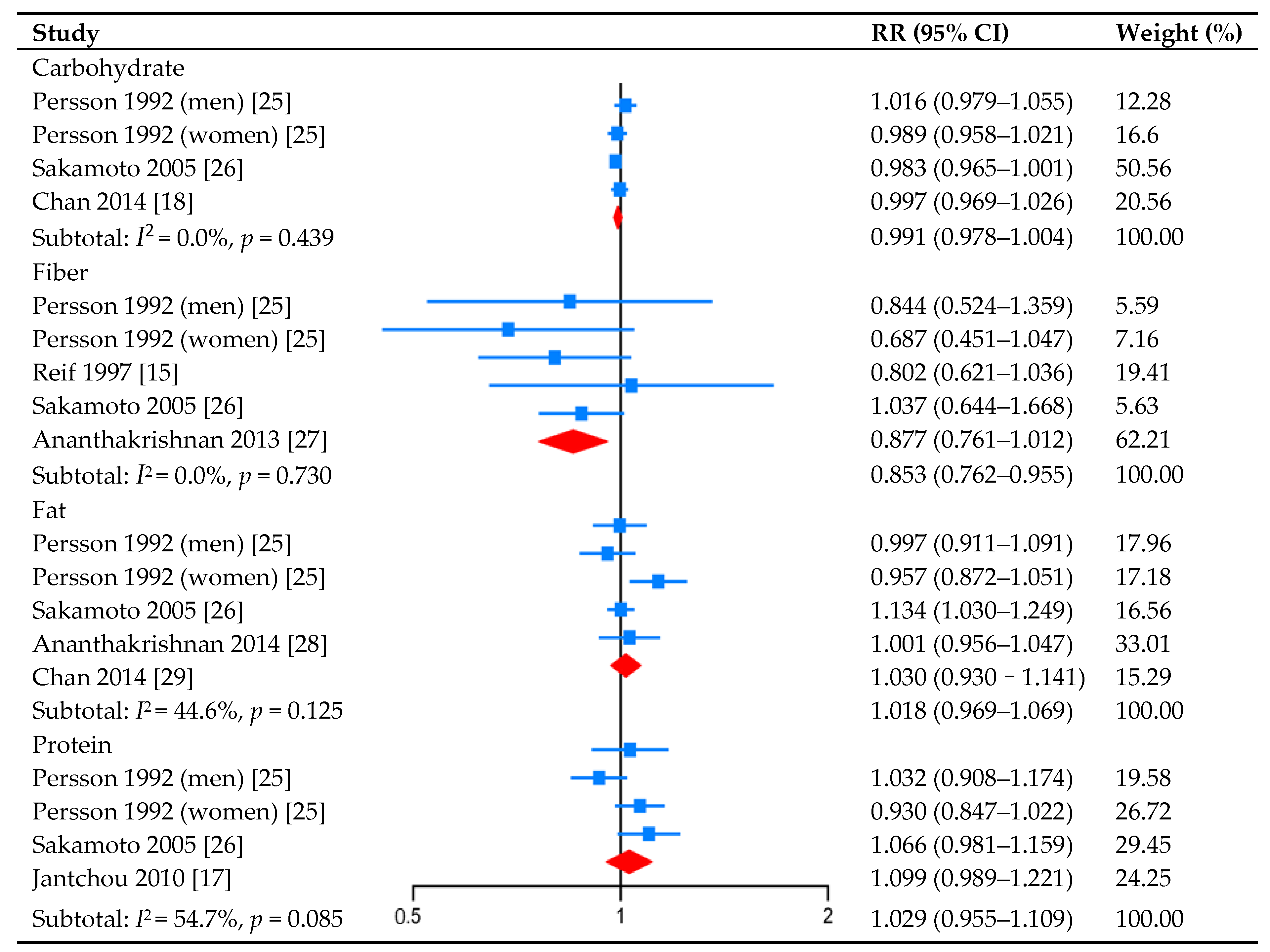
3.2. Carbohydrate Intake and CD Risk
3.3. Fiber Intake and CD Risk
3.4. Fat Intake and CD Risk
3.5. Protein Intake and CD Risk
3.6. Intake of the Nutrients’ Subtypes and CD Risk
3.7. Subgroup Analysis and Publication Bias
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Tang, W.; Ching, J.Y.; Wong, M.; Chow, C.M.; Hui, A.J.; Wong, T.C.; Leung, V.K.; Tsang, S.W.; Yu, H.H.; et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asian-pacific Crohn’s and colitis epidemiology study. Gastroenterology 2013, 145, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, G.R.; Abreu, M.T.; Cohen, R.; Tremaine, W. American Gastroenterological Association Institute medical position statement on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology 2006, 130, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Lin, X.; Zhao, Q.; Li, J. Adverse symptoms with anti-TNF-alpha therapy in inflammatory bowel disease: Systematic review and duration-response meta-analysis. Eur. J. Clin. Pharmacol. 2015, 71, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Anderson, V.; Olsen, A.; Carbonnel, F.; Tjønneland, A.; Vogel, U. Diet and risk of inflammatory bowel disease. Dig. Liver Dis. 2012, 44, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Bernstein, C.N.; Vatn, M.H.; Lakatos, P.L.; Loftus, E.V.; Tysk, C.; O’Morain, C.; Moum, B.; Colombel, J.-F. Geographical variability and environmental risk factors in inflammatory bowel disease. Gut 2013, 62, 630–649. [Google Scholar] [CrossRef] [PubMed]
- Gunasekeera, V.; Mendall, M.A.; Chan, D.; Kumar, D. Treatment of Crohn’s disease with IgG4-gudided exclusion diet: A randomized controlled trial. Dig. Dis. Sci. 2016, 61, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.L.; Limketkai, B.; Medici, V.; Saire, M.M.; Palmer, L.; Bechtold, M. Nutritional strategies in the management of adult patients with inflammatory bowel disease: Dietary considerations from active disease to disease remission. Curr. Gastroenterol. Rep. 2016, 18, 55. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, X.Q.; Wang, W.J.; Zhang, D. Consumption of vegetables and fruit and the risk of inflammatory bowel disease: A meta-analysis. Eur. J. Gastroenterol. Hepatol. 2015, 27, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Lin, X.; Zhao, Q.; Li, J. Fat intake and risk of ulcerative colitis: Systematic review and dose-response meta-analysis of epidemiological studies. J. Gastroenterol. Hepatol. 2017, 32, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Feng, J.R.; Gao, Q.; Ma, M.X.; Lin, X.; Liu, J.; Li, J.; Zhao, Q. Carbohydrate and protein intake and risk of ulcerative colitis: Systematic review and dose-response meta-analysis of epidemiological studies. Clin. Nutr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2011. Available online: http://www.ohri.ca (accessed on 22 April 2017).
- Xu, C.; Han, F.F.; Zeng, X.T.; Liu, T.Z.; Li, S.; Gao, Z.Y. Fat intake is not linked to prostate cancer: A systematic review and dose-response meta-analysis. PLoS ONE 2015, 10, e0131747. [Google Scholar] [CrossRef] [PubMed]
- Bekkering, G.E.; Harris, R.J.; Thomas, S.; Mayer, A.M.; Beynon, R.; Ness, A.R.; Harbord, R.M.; Bain, C.; Smith, G.D.; Sterne, J.A.C. How much of the data published in observational studies of the association between diet and prostate or bladder cancer is usable for meta-analysis? Am. J. Epidemiol. 2008, 167, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Reif, S.; Klein, I.; Lubin, F.; Farbstein, M.; Hallak, A.; Gilat, T. Pre-illness dietary factors in inflammatory bowel disease. Gut 1997, 40, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Orsini, N. From floated to conventional confidence intervals for the relative risks based on published dose-response data. Comput. Methods Progr. Biomed. 2010, 98, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Jantchou, P.; Morois, S.; Clavel-Chapelon, F.; Boutron-Ruault, M.C.; Carbonnel, F. Animal protein intake and risk of inflammatory bowel disease: The E3N prospective study. Am. J. Gastroenterol. 2010, 105, 2195–2201. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.S.; Luben, R.; van Schaik, F.; Oldenburg, B.; Bueno-de-Mesquita, H.B.; Hallmans, G.; Karling, P.; Lindgren, S.; Grip, O.; Key, T.D.; et al. Carbohydrate intake in the etiology of Crohn’s disease and ulcerative colitis. Inflamm. Bowel Dis. 2014, 20, 2013–2021. [Google Scholar] [CrossRef] [PubMed]
- Greenland, S.; Longnecker, M.P. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am. J. Epidemiol. 1992, 135, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Orsini, N.; Bellocco, R.; Greenland, S. Generalized least squares for trend estimation of summarized dose-response data. Stata J. 2006, 6, 40–57. [Google Scholar]
- Harrell, F.E.; Lee, K.L.; Pollock, B.G. Regression models in clinical studies: Determining relationships between predictors and response. J. Natl. Cancer Inst. 1988, 80, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Orsini, N. Multivariate Dose-Response Meta-Analysis: An Update on glst. In Proceedings of the Nordic and Baltic Users Group Meeting, Stockholm, Sweden, 27 September 2013. [Google Scholar]
- Egger, M.; Davey, S.G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analysis. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Persson, P.G.; Ahlbom, A.; Hellers, G. Diet and inflammatory bowel disease: A case-control study. Epidemiology 1992, 3, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, N.; Kono, S.; Wakai, K.; Fukuda, Y.; Satomi, M.; Shimoyama, T.; Inaba, Y.; Miyake, Y.; Sasaki, S.; Okamoto, K.; et al. Dietary risk factors for inflammatory bowel disease a multicenter case-control study in Japan. Inflamm. Bowel Dis. 2005, 11, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Khalili, H.; Konijeti, G.G.; Higuchi, L.M.; de Silva, P.; Korzenik, J.R.; Fuchs, C.S.; Willett, W.C. A prospective study of long-term intake of dietary fiber and risk Crohn’s disease and ulcerative colitis. Gastroenterology 2013, 145, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Khalili, H.; Konijeti, G.G.; Higuchi, L.M.; de Silva, P.; Fuchs, C.S.; Willett, W.C.; Richter, J.M.; Chan, A.T. Long-term intake of dietary fat and risk of ulcerative colitis and Crohn’s disease. Gut 2014, 63, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.S.; Luben, R.; Olsen, A.; Tjonneland, A.; Kaaks, R.; Lindgren, S.; Grip, O.; Bergmann, M.M.; Boeing, H.; Hallmans, G.; et al. Association between high dietary intake of the n-3 polyunsaturated fatty acid docosahexaenoic acid and reduced risk of Crohn’s disease. Aliment. Pharmacol. Ther. 2014, 39, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Russel, M.G.; Engels, L.G.; Muris, J.W.; Limonard, C.B.; Volovics, A.; Brummer, R.-J.M.; Stockbrugger, R.W. ‘Modern life’ in the epidemiology of inflammatory bowel disease: A case-control study with special emphasis on nutritional factors. Eur. J. Gastroenterol. Hepatol. 1998, 10, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Racine, A.; Carbonnel, F.; Chan, S.S.; Hart, A.R.; Bueno-de-Mesquita, H.E.; Oldenburg, B.; van Schaik, F.D.M.; Tjønneland, A.; Olsen, A.; Dahm, C.C.; et al. Dietary patterns and risk of inflammatory bowel diseases in Europe: Results from the EPIC study. Inflamm. Bowel Dis. 2016, 22, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Burisch, J.; Pedersen, N.; Cukovic-Cavka, S.; Turk, N.; Kaimakliotis, I.; Duricova, D.; Bortlik, M.; Shonová, O.; Vind, I.; Avnstrøm, S.; et al. Environmental factors in a population-based inception cohort of inflammatory bowel disease patients in Europe—An ECCO-EpiCom study. J. Crohn’s Colitis 2014, 8, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Neuman, M.G.; Nanau, R.M. Single-nucleotide polymorphisms in inflammatory bowel disease. Transl. Res. 2012, 160, 45–64. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, Y.; Li, F.; Zhang, D. Dietary fiber intake reduces risk of inflammatory bowel disease: Result from a meta-analysis. Nutr. Res. 2015, 35, 753–758. [Google Scholar] [CrossRef] [PubMed]
- To, N.; Gracie, D.J.; Ford, A.C. Systematic review with meta-analysis: The adverse effects of tobacco smoking on the natural history of Crohn’s disease. Aliment. Pharmacol. Ther. 2016, 43, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.H.; Hart, D.A.; Reimer, R.A.; Seerattan, R.A.; Banker, C.W.; Sibole, S.C.; Herzog, W. High-fat high sucrose diet leads to dynamic structural and inflammatory alteration in the rat vastus lateralis muscle. J. Orthop. Res. 2016, 34, 2069–2078. [Google Scholar] [CrossRef] [PubMed]
- Kang, I.; Espin, J.C.; Carr, T.P.; Tomas-Barberan, F.A.; Chung, S. Raspberry seed flour attenuates high-sucrose diet-mediated hepatic stress and adipose tissue inflammation. J. Nutr. Biochem. 2016, 32, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Beilharz, J.E.; Maniam, J.; Morris, M.J. Short-term exposure to a diet high in fat and sugar, or liquid sugar, selectively impairs hippocampal-dependent memory, with differential impacts on inflammation. Behav. Brain Res. 2016, 306, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, S.; Kaser, A.; Blumberg, R.S. Role of endoplasmic reticulum stress and autophagy as interlinking pathways in the pathogenesis of inflammatory bowel disease. Curr. Opin. Gastroenterol. 2015, 31, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Gjymishka, A.; Coman, R.M.; Brusko, T.M.; Glover, S.C. Influence of host immunoregulatory genes, ER stress and gut microbiota on the shared pathogenesis of inflammatory bowel disease and type 1 diabetes. Immunotherapy 2013, 5, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Orsini, N.; Li, R.; Wolk, A.; Khudyakov, P.; Spiegelman, D. Meta-analysis for linear and nonlinear dose-response relations: Examples and evaluation of approximations and software. Am. J. Epidemiol. 2012, 175, 66–73. [Google Scholar] [CrossRef] [PubMed]

| First Author, Year, Area | Study Design | Diagnostic Criteria | Cases/Controls (Age) | Time at Diagnosis (Retrospective Period #) | Exposure Categories (Dietary Assessment) | Risk Estimates (95% CI) | Adjusted Factors |
|---|---|---|---|---|---|---|---|
| Persson, 1992, Sweden (for men) [25] | Population-based case-control | The scoring table suggested by Lennard-Jones | 63/147 (15–79 years) | Within 4 years (5 years ago) | T3 vs. T1 | Relative risk | Age, energy intake |
| Protein | 2.2 (0.7–6.9) | ||||||
| Carbohydrate | 2.1 (0.5–8.1) | ||||||
| Fat | 1.3 (0.4–4.4) | ||||||
| Fiber | 1.2 (0.5–2.6) | ||||||
| (Validated FFQ) | - | ||||||
| Persson, 1992, Sweden (for women) [25] | Population-based case-control | The scoring table suggested by Lennard-Jones | 89/158 (15–79 years) | Within 4 years (5 years ago) | T3 vs. T1 | Relative risk | Age, energy intake |
| Protein | 0.4 (0.2–1.3) | ||||||
| Carbohydrate | 1.0 (0.2–4.3) | ||||||
| Fat | 0.7 (0.2–2.9) | ||||||
| Fiber | 0.4 (0.2–1.0) | ||||||
| (Validated FFQ) | - | ||||||
| Reif, 1997, Israel [15] | Population/clinic-based case-control | - | 33/144 (mean, 29.12/29.45 years) | Within 1 year from onset of symptoms (before the illness and symptoms began) | T3 vs. T1 | Odds ratio | Age, sex, country of origin, residential neighborhood, energy intake |
| Fiber | 0.40 (0.10–1.65) | ||||||
| (Validated FFQ) | - | ||||||
| Sakamoto, 2005, Japan [26] | Hospital-based case-control | The criteria of the Research Committee on Inflammatory bowel disease in Japan | 126/211 (15–34 years) | Within the past 3 years (5 years before the time of the study) | Q4 vs. Q1 | Odds ratio | Age, sex, study area, education, smoking habits |
| Protein | 2.06 (0.99–4.28) | ||||||
| Fat | 2.86 (1.39–5.90) | ||||||
| Carbohydrate | 0.53 (0.27–1.03) | ||||||
| Fiber | 0.90 (0.43–1.86) | ||||||
| (Validated FFQ) | - | ||||||
| Jantchou, 2010, France (for women) [17] | Prospective cohort study | Clinical, radiological, endoscopic and histological criteria | 30/67, 504 (mean, 50.9/52.8 years) | Within a median of 54.5 months (a mean follow up of 10.4 years) | T3 vs. T1 | Hazard ratio | Body weight, energy intake |
| Protein | 3.34 (0.90–12.4) | ||||||
| Carbohydrate | 1.31 (0.42–4.14) | ||||||
| Fat | 0.98 (0.25–3.88) | ||||||
| (Validated FFQ) | - | ||||||
| Ananthakrishnan, 2013, USA (for female registered nurses) [27] | Prospective cohort study | Typical symptoms ≥ 4 weeks; endoscopy; histology; radiography | 269/170, 169 (NHS I: 30–55 years; NHS II: 25–42 years) | With a median age of 54 years at diagnosis (NHS I from 1984 to 2006; NHS II from 1991 to 2007) | Q5 vs. Q1 | Hazard ratio | Age, cohort, smoking, BMI, oral contraceptive use, use of post menopausal hormone therapy, regular use of NSAIDs, regular use of aspirin, energy intake |
| Fiber | 0.59 (0.39–0.90) | ||||||
| (Validated FFQ) | - | ||||||
| Ananthakrishnan, 2014, USA (for female registered nurses) [28] | Prospective cohort study | Typical symptoms ≥ 4 weeks; endoscopy; histology; radiography | 269/170, 169 (NHS I: 30–55 years; NHS II: 25–42 years) | With a median age of 54 years at diagnosis (NHS I from 1884 to 2006; NHS II from 1991 to 2007) | Q5 vs. Q1 | Hazard ratio | Age, cohort, smoking, BMI, oral contraceptive use, use of post menopausal hormone therapy, regular use of NSAIDs, regular use of aspirin, energy intake |
| Fat | 0.98 (0.66–1.45) | ||||||
| (Validated FFQ) | - | ||||||
| Chan, 2014, Europe [18] | Prospective cohort study | Radiology; endoscopy; histology | 110/440 (50.1 years/50.1 years) | More than 18 months after recruitment (from 1991–1998 to 2004–2010) | Q5 vs. Q1 | Odds ratio | Age, sex, center, recruitment date, follow-up period, energy intake, BMI, metabolic rate, physical activity, smoking |
| Carbohydrate | 0.87 (0.24–3.12) | ||||||
| (Validated FFQ) | - | ||||||
| Chan, 2014, Europe [29] | Prospective cohort study | Follow-up questionnaire, in-patient record, histology database, medical note | 73/292 (50.5 years/50.2 years) | More than 18 months after recruitment (from 1991–1998 to 2004) | Q5 vs. Q1 | Odds ratio | Age, sex, center, recruitment date, smoking, total energy intake, BMI, dietary vitamin D and relevant fatty acids |
| Fat | 1.42 (0.26–7.67) | ||||||
| (Validated FFQ) | - |
| Subtypes | Included Studies | RR (95% CI) | I2 (%) |
|---|---|---|---|
| Sugar | Reif 1997 [15]; Chan 2014 [18] | 0.998 (0.969–1.027) | 0.0 |
| Monosaccharide | Persson 1992 (men) [25]; Persson 1992 (women) [25] | 0.971 (0.715–1.317) | 49.9 |
| Fructose | Reif 1997 [15] | 0.843 (0.695–1.023) | - |
| Disaccharide | Persson 1992 (men) [25]; Persson 1992 (women) [25] | 0.988 (0.871–1.121) | 0.0 |
| Sucrose | Persson 1992 (men) [25]; Persson 1992 (women) [25]; Reif 1997 [15] | 1.088 (1.020–1.160) | 0.0 |
| Starch | Chan 2014 [18] | 0.994 (0.946–1.044) | - |
| Fat | |||
| SFA | Sakamoto 2005 [26]; Ananthakrishnan 2014 [28] | 0.980 (0.843–1.140) | 17.2 |
| MUFA | Sakamoto 2005 [26]; Ananthakrishnan 2014 [28] | 1.137 (0.842–1.536) | 78.8 |
| Oleic acid | Ananthakrishnan 2014 [28]; Chan 2014 [29] | 1.015 (0.900–1.144) | 0.0 |
| PUFA | Sakamoto 2005 [26]; Ananthakrishnan 2014 [28] | 1.306 (0.816–2.092) | 76.2 |
| Arachidonic acid | Ananthakrishnan 2014 [28] | 0.000 (0.000–721.226) | - |
| Linoleic acid | Ananthakrishnan 2014 [28]; Chan 2014 [29] | 1.097 (0.871–1.383) | 0.0 |
| α–linoleic acid | Chan 2014 [29] | 0.035 (0.000–3.299) | - |
| DHA | Chan 2014 [29] | 0.004 (0.000–1706.027) # | - |
| EPA | Chan 2014 [29] | 799.371 (0.000–2.36 × 1011) # | - |
| Protein | |||
| Animal protein | Jantchou 2010 [17] | 2.700 (0.690–10.520) * | - |
| Vegetable protein | Jantchou 2010 [17] | 1.040 (0.280–3.800) * | - |
| Subgroup | Carbohydrate | Fibre | Fat | Protein | ||||
|---|---|---|---|---|---|---|---|---|
| RR (95% CI) | I2 (%) | RR (95% CI) | I2 (%) | RR (95% CI) | I2 (%) | RR (95% CI) | I2 (%) | |
| Study design | ||||||||
| Case-control | 0.991 (0.974–1.008) | 19.5 | 0.815 (0.679–0.980) | 0.0 | 1.026 (0.930–1.132) | 70.1 | 1.008 (0.922–1.101) | 57.2 |
| Prospective-cohort | 0.997 (0.969–1.026) | - | 0.877 (0.761–1.012) | - | 1.005 (0.965–1.048) | 0.0 | 1.099 (0.989–1.221) | - |
| Cohort | ||||||||
| Caucasian | 0.999 (0.981–1.018) | 0.0 | 0.844 (0.751–0.947) | 0.0 | 0.997 (0.963–1.033) | 0.0 | 1.015 (0.915–1.126) | 63.8 |
| Asian | 0.983 (0.965–1.001) | - | 1.037 (0.644–1.668) | - | 1.134 (1.030–1.249) | - | 1.066 (0.981–1.159) | - |
| Adjusted for smoking | ||||||||
| Yes | 0.987 (0.972–1.002) | 0.0 | 0.890 (0.776–1.020) | 0.0 | 1.045 (0.970–1.127) | 62.5 | 1.015 (0.915–1.126) | 63.8 |
| No | 1.001 (0.975–1.028) | 14.4 | 0.782 (0.641–0.954) | 0.0 | 0.977 (0.916–1.043) | 0.0 | 1.066 (0.981–1.159) | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, L.; Hu, S.; Chen, P.; Wei, W.; Tan, Y. Macronutrient Intake and Risk of Crohn’s Disease: Systematic Review and Dose–Response Meta-Analysis of Epidemiological Studies. Nutrients 2017, 9, 500. https://doi.org/10.3390/nu9050500
Zeng L, Hu S, Chen P, Wei W, Tan Y. Macronutrient Intake and Risk of Crohn’s Disease: Systematic Review and Dose–Response Meta-Analysis of Epidemiological Studies. Nutrients. 2017; 9(5):500. https://doi.org/10.3390/nu9050500
Chicago/Turabian StyleZeng, Lirong, Sheng Hu, Pengfei Chen, Wenbin Wei, and Yuanzhong Tan. 2017. "Macronutrient Intake and Risk of Crohn’s Disease: Systematic Review and Dose–Response Meta-Analysis of Epidemiological Studies" Nutrients 9, no. 5: 500. https://doi.org/10.3390/nu9050500
APA StyleZeng, L., Hu, S., Chen, P., Wei, W., & Tan, Y. (2017). Macronutrient Intake and Risk of Crohn’s Disease: Systematic Review and Dose–Response Meta-Analysis of Epidemiological Studies. Nutrients, 9(5), 500. https://doi.org/10.3390/nu9050500




