A Combination of Flaxseed Oil and Astaxanthin Improves Hepatic Lipid Accumulation and Reduces Oxidative Stress in High Fat-Diet Fed Rats
Abstract
:1. Introduction
2. Methods
2.1. Chemical Sources
2.2. Animals and Diets
2.3. Tissue Preparation
2.4. Serum Assays
2.5. Liver Histology
2.6. Liver Lipid Content
2.7. Western Blot
2.8. Quantitative Real-Time PCR
2.9. Assay of Hepatic Antioxidant Activity and Lipid Peroxidation
2.10. Protein Measurement
2.11. Statistical Analyses
3. Results
3.1. Food Intake and Body Weight Gain
3.2. Effects of FO and ASX Combination on Hepatic Enzymes in Plasma
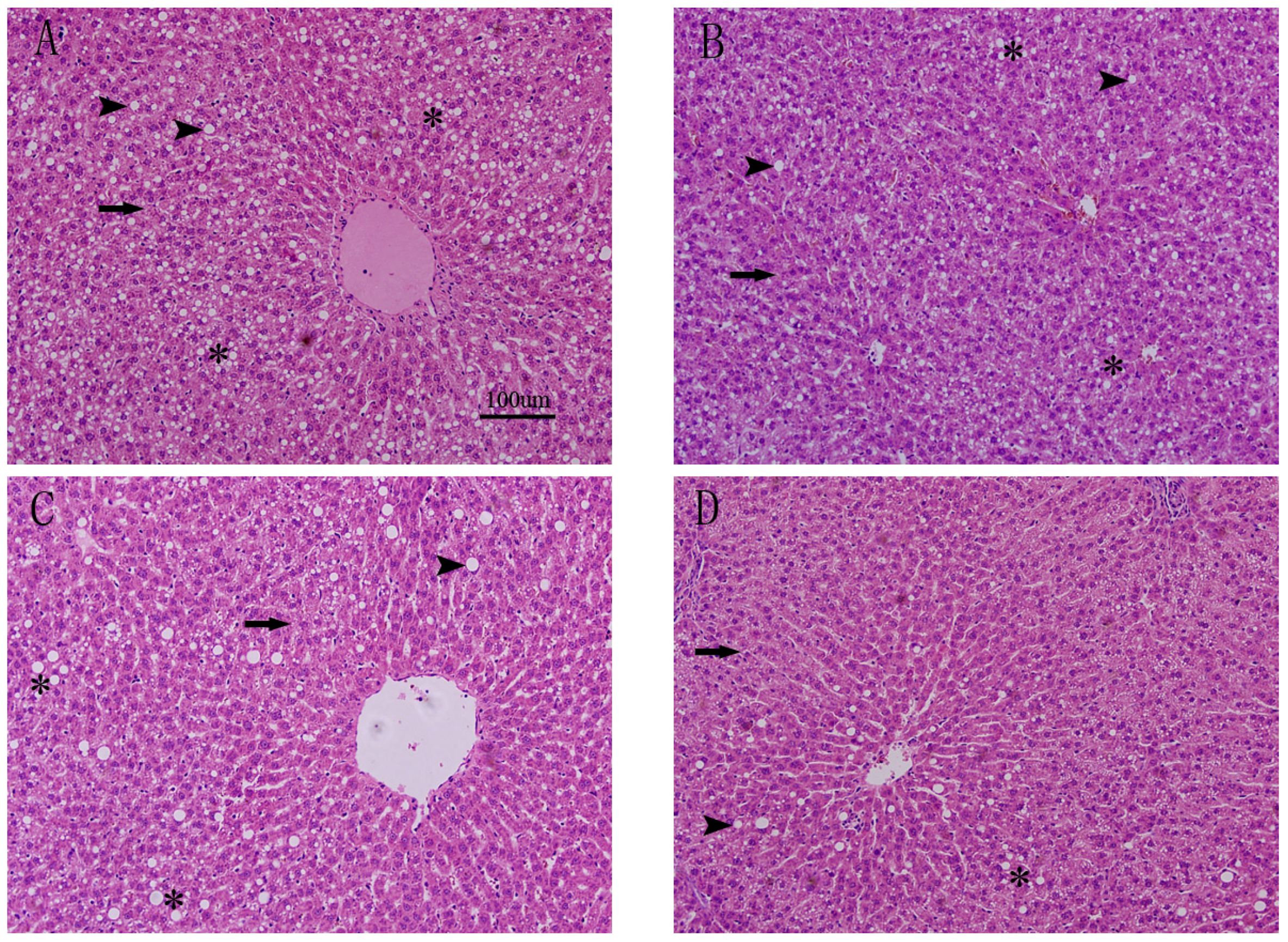
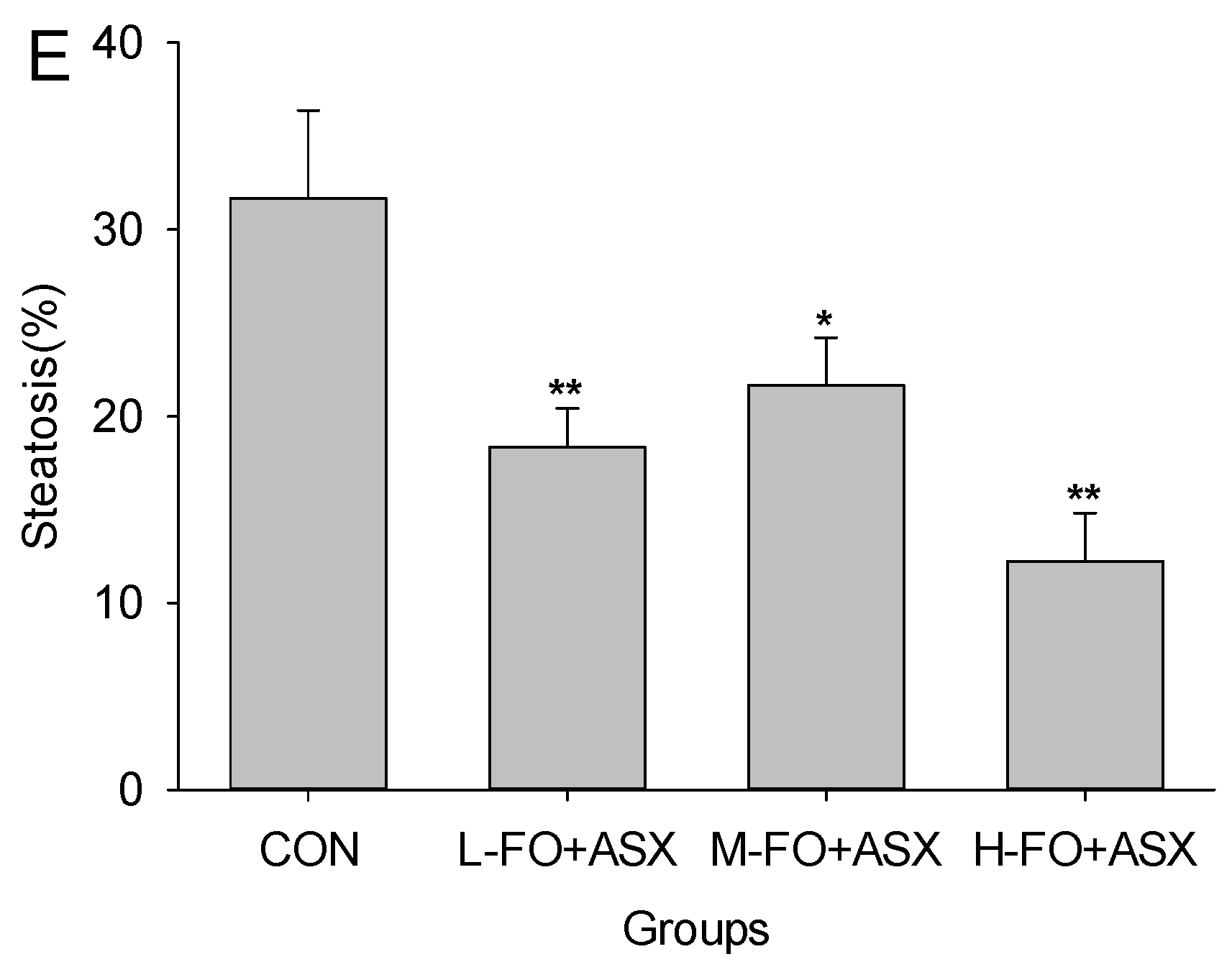
3.3. Effects of FO and ASX on Liver Morphology
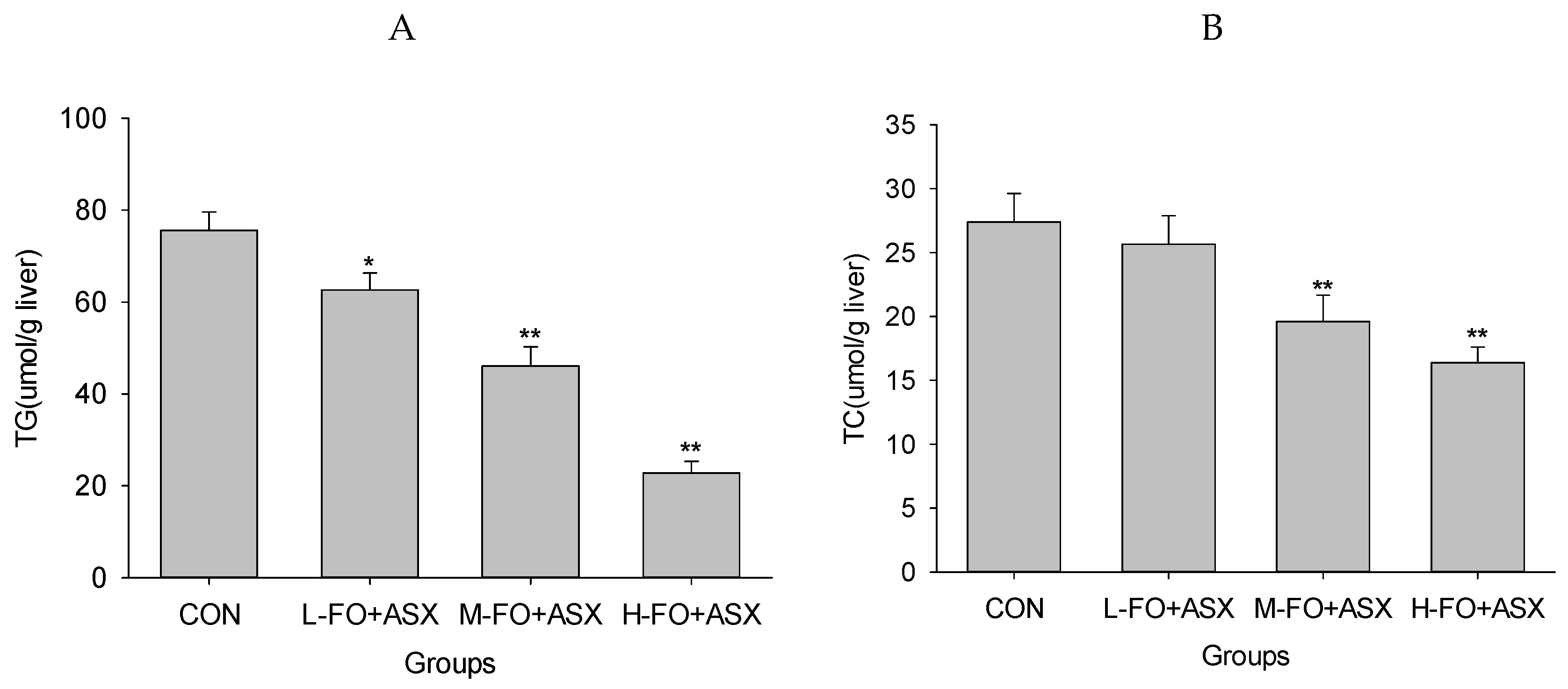
3.4. Effects of FO and ASX on Liver Lipids
3.5. Effects of FO and ASX on Liver Protein Expression
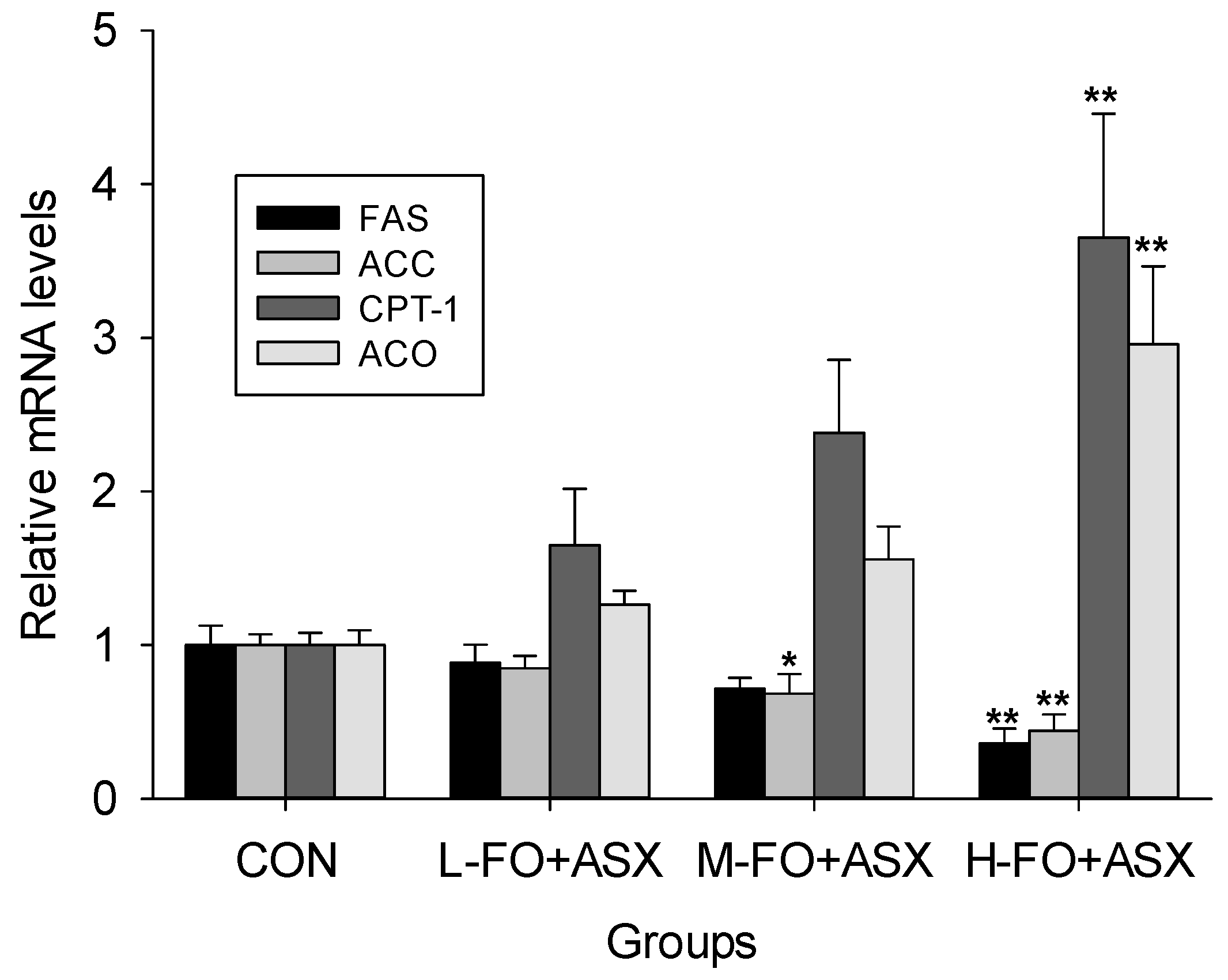
3.6. Effects of FO and ASX on Liver mRNA Expression
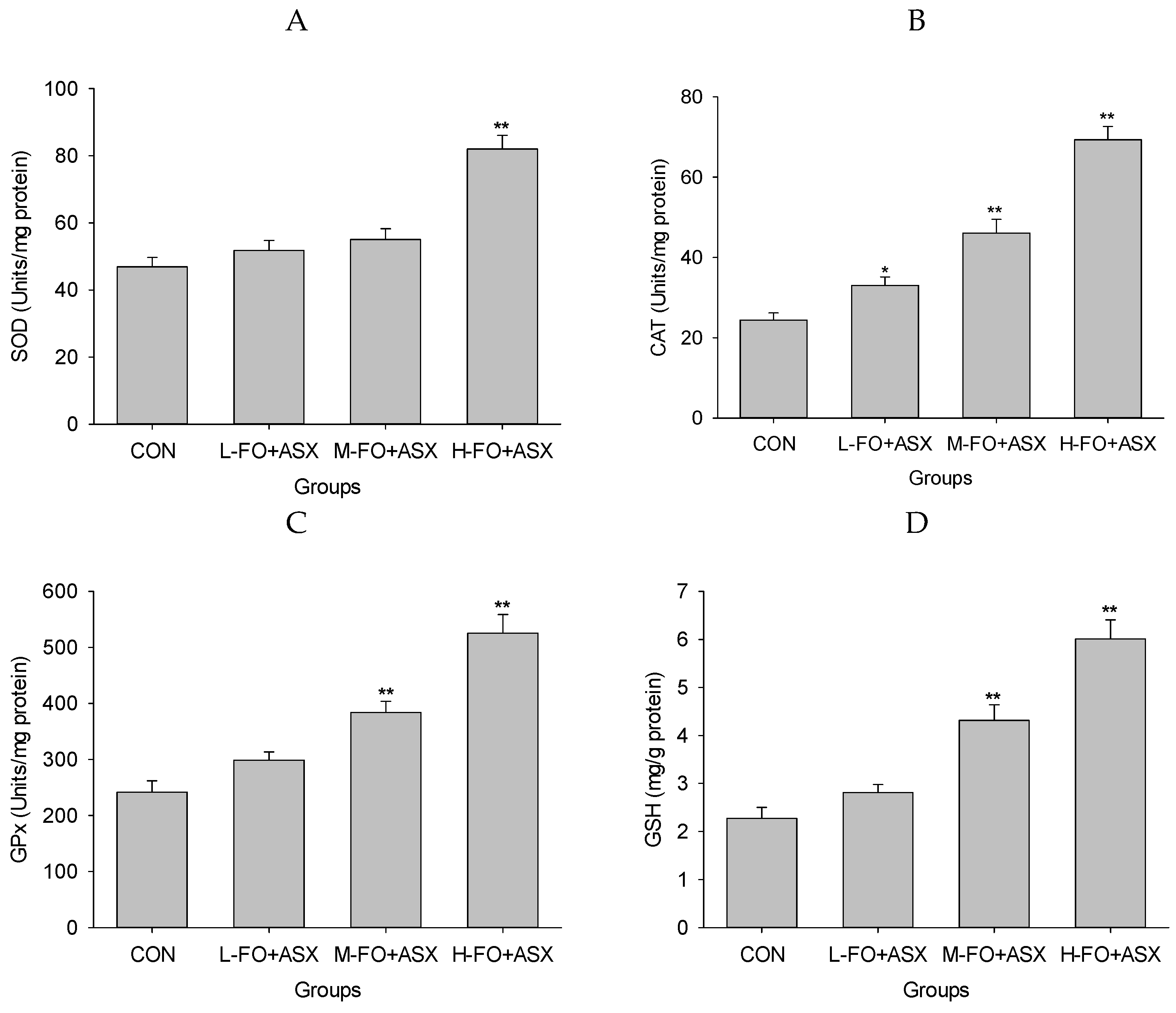
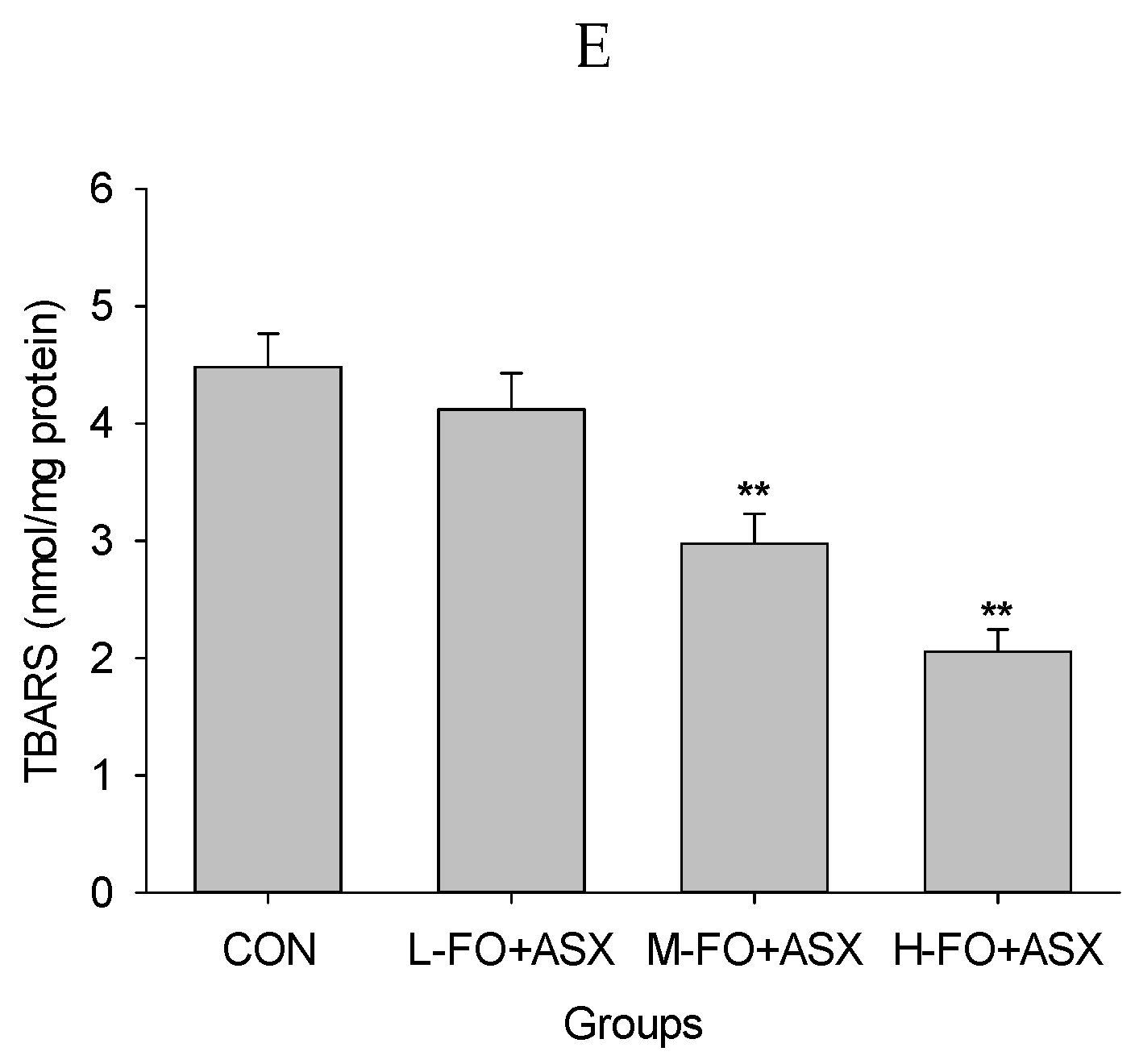
3.7. Effects of FO and ASX on Liver Antioxidant Capacity and Lipid Peroxidation
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vuppalanchi, R.; Chalasani, N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Selected practical issues in their evaluation and management. Hepatology 2009, 49, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.; Borlak, J. Molecular mechanisms and therapeutic targets in steatosis and steatohepatitis. Pharmacol. Rev. 2008, 60, 311–357. [Google Scholar] [CrossRef] [PubMed]
- Amarapurkar, D.; Kamani, P.; Patel, N.; Gupte, P.; Kumar, P.; Agal, S.; Baijal, R.; Lala, S.; Chaudhary, D.; Deshpande, A. Prevalence of non-alcoholic fatty liver disease: Population based study. Ann. Hepatol. 2007, 6, 161–163. [Google Scholar] [PubMed]
- Ciccone, M.M.; Principi, M.; Ierardi, E.; Di Leo, A.; Ricci, G.; Carbonara, S.; Gesualdo, M.; Devito, F.; Zito, A.; Cortese, F.; et al. Inflammatory bowel disease, liver diseases and endothelial function: Is there a linkage? J. Cardiovasc. Med. (Hagerstown) 2015, 16, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Day, C.P.; James, O.F. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Anderson, B.M.; Ma, D.W. Are all n-3 polyunsaturated fatty acids created equal? Lipids Health Dis. 2009, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Holman, R.T. Control of polyunsaturated acids in tissue lipids. J. Am. Coll. Nutr. 1986, 5, 183–211. [Google Scholar] [CrossRef] [PubMed]
- Barcelo-Coblijn, G.; Murphy, E.J. Alpha-linolenic acid and its conversion to longer chain n-3 fatty acids: Benefits for human health and a role in maintaining tissue n-3 fatty acid levels. Prog. Lipid Res. 2009, 48, 355–374. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.F.; Tseng, J.K.; Chang, Y.Y.; Chen, Y.C. Flaxseed oil attenuates nonalcoholic fatty liver of hyperlipidemic hamsters. J. Agric. Food Chem. 2009, 57, 5078–5083. [Google Scholar] [CrossRef] [PubMed]
- Trebušak, T.; Levart, A.; Voljč, M.; Tomažin, U.; Pirman, T. The effect of linseed oil supplementation on performance, fatty acid composition and oxidative status of rabbits. Acta Agric. Slov. 2011, 98, 119–125. [Google Scholar] [CrossRef]
- Hussein, G.; Sankawa, U.; Goto, H.; Matsumoto, K.; Watanabe, H. Astaxanthin, a carotenoid with potential in human health and nutrition. J. Nat. Prod. 2006, 69, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Hussein, G.; Nakamura, M.; Zhao, Q.; Iguchi, T.; Goto, H.; Sankawa, U.; Watanabe, H. Antihypertensive and neuroprotective effects of astaxanthin in experimental animals. Biol. Pharm. Bull. 2005, 28, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Fassett, R.G.; Coombes, J.S. Astaxanthin: A potential therapeutic agent in cardiovascular disease. Mar. Drugs 2011, 9, 447–465. [Google Scholar] [CrossRef] [PubMed]
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, M.M.; Cortese, F.; Gesualdo, M.; Carbonara, S.; Zito, A.; Ricci, G.; De Pascalis, F.; Scicchitano, P.; Riccioni, G. Dietary intake of carotenoids and their antioxidant and anti-inflammatory effects in cardiovascular care. Mediat. Inflamm. 2013, 2013, 782137. [Google Scholar] [CrossRef] [PubMed]
- Bhuvaneswari, S.; Arunkumar, E.; Viswanathan, P.; Anuradha, C.V. Astaxanthin restricts weight gain, promotes insulin sensitivity and curtails fatty liver disease in mice fed a obesity-promoting diet. Process Biochem. 2010, 45, 1406–1414. [Google Scholar] [CrossRef]
- Bhuvaneswari, S.; Yogalakshmi, B.; Sreeja, S.; Anuradha, C.V. Astaxanthin reduces hepatic endoplasmic reticulum stress and nuclear factor-kappab-mediated inflammation in high fructose and high fat diet-fed mice. Cell Stress Chaperones 2014, 19, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Turkez, H.; Geyikoglu, F.; Yousef, M.I.; Togar, B.; Gurbuz, H.; Celik, K.; Akbaba, G.B.; Polat, Z. Hepatoprotective potential of astaxanthin against 2,3,7,8-tetrachlorodibenzo-p-dioxin in cultured rat hepatocytes. Toxicol. Ind. Health 2014, 30, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Takitani, K.; Miyazaki, H.; Yoden, A.; Tamai, H. Children's toxicology from bench to bed—Liver injury (2): Mechanism of antioxidant therapy for nonalcoholic fatty liver disease. J. Toxicol. Sci. 2009, 34, SP223–SP228. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Gao, H.; Zhang, L.; Chen, C.; Yang, W.; Deng, Q.; Huang, Q.; Huang, F. A combination of flaxseed oil and astaxanthin alleviates atherosclerosis risk factors in high fat diet fed rats. Lipids Health Dis. 2014, 13, 63. [Google Scholar] [CrossRef] [PubMed]
- Catta-Preta, M.; Mendonca, L.S.; Fraulob-Aquino, J.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. A critical analysis of three quantitative methods of assessment of hepatic steatosis in liver biopsies. Virchows Arch. 2011, 459, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Kono, Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch. Biochem. Biophys. 1978, 186, 189–195. [Google Scholar] [CrossRef]
- Sazuka, Y.; Tanizawa, H.; Takino, Y. Effect of adriamycin on the activities of superoxide dismutase, glutathione peroxidase and catalase in tissues of mice. Jpn. J. Cancer Res. 1989, 80, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Goth, L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta 1991, 196, 143–151. [Google Scholar] [CrossRef]
- Moron, M.S.; Depierre, J.W.; Mannervik, B. Levels of glutathione, glutathione reductase and glutathione s-transferase activities in rat lung and liver. Biochim. Biophys. Acta 1979, 582, 67–78. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [PubMed]
- Xu, J.; Zhou, X.; Deng, Q.; Huang, Q.; Yang, J.; Huang, F. Rapeseed oil fortified with micronutrients reduces atherosclerosis risk factors in rats fed a high-fat diet. Lipids Health Dis. 2011, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Shimano, H. Sterol regulatory element-binding protein-1 as a dominant transcription factor for gene regulation of lipogenic enzymes in the liver. Trends Cardiovasc. Med. 2000, 10, 275–278. [Google Scholar] [CrossRef]
- Clemenz, M.; Frost, N.; Schupp, M.; Caron, S.; Foryst-Ludwig, A.; Bohm, C.; Hartge, M.; Gust, R.; Staels, B.; Unger, T.; et al. Liver-specific peroxisome proliferator-activated receptor alpha target gene regulation by the angiotensin type 1 receptor blocker telmisartan. Diabetes 2008, 57, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Fujita, T.; Usuda, N.; Cook, W.; Qi, C.; Peters, J.M.; Gonzalez, F.J.; Yeldandi, A.V.; Rao, M.S.; Reddy, J.K. Peroxisomal and mitochondrial fatty acid beta-oxidation in mice nullizygous for both peroxisome proliferator-activated receptor alpha and peroxisomal fatty acyl-coa oxidase. Genotype correlation with fatty liver phenotype. J. Biol. Chem. 1999, 274, 19228–19236. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Gambino, R.; Cassader, M. Recent insights into hepatic lipid metabolism in non-alcoholic fatty liver disease (nafld). Prog. Lipid Res. 2009, 48, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Lieber, C.S.; Leo, M.A.; Mak, K.M.; Xu, Y.; Cao, Q.; Ren, C.; Ponomarenko, A.; DeCarli, L.M. Model of nonalcoholic steatohepatitis. Am. J. Clin. Nutr. 2004, 79, 502–509. [Google Scholar] [PubMed]
- Heinonen, I.; Rinne, P.; Ruohonen, S.T.; Ruohonen, S.; Ahotupa, M.; Savontaus, E. The effects of equal caloric high fat and western diet on metabolic syndrome, oxidative stress and vascular endothelial function in mice. Acta Physiol. 2014, 211, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Omagari, K.; Kato, S.; Tsuneyama, K.; Inohara, C.; Kuroda, Y.; Tsukuda, H.; Fukazawa, E.; Shiraishi, K.; Mune, M. Effects of a long-term high-fat diet and switching from a high-fat to low-fat, standard diet on hepatic fat accumulation in sprague-dawley rats. Dig. Dis. Sci. 2008, 53, 3206–3212. [Google Scholar] [CrossRef] [PubMed]
- Devarshi, P.P.; Jangale, N.M.; Ghule, A.E.; Bodhankar, S.L.; Harsulkar, A.M. Beneficial effects of flaxseed oil and fish oil diet are through modulation of different hepatic genes involved in lipid metabolism in streptozotocin-nicotinamide induced diabetic rats. Genes Nutr. 2013, 8, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Ide, T.; Kobayashi, H.; Ashakumary, L.; Rouyer, I.A.; Takahashi, Y.; Aoyama, T.; Hashimoto, T.; Mizugaki, M. Comparative effects of perilla and fish oils on the activity and gene expression of fatty acid oxidation enzymes in rat liver. Biochim. Biophys. Acta 2000, 1485, 23–35. [Google Scholar] [CrossRef]
- Ide, T. Effect of dietary alpha-linolenic acid on the activity and gene expression of hepatic fatty acid oxidation enzymes. BioFactors 2000, 13, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Murase, T.; Aoki, M.; Tokimitsu, I. Supplementation with alpha-linolenic acid-rich diacylglycerol suppresses fatty liver formation accompanied by an up-regulation of beta-oxidation in zucker fatty rats. Biochim. Biophys. Acta 2005, 1733, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Ide, T.; Murata, M.; Sugano, M. Stimulation of the activities of hepatic fatty acid oxidation enzymes by dietary fat rich in alpha-linolenic acid in rats. J. Lipid Res. 1996, 37, 448–463. [Google Scholar] [PubMed]
- Clouet, P.; Niot, I.; Bezard, J. Pathway of alpha-linolenic acid through the mitochondrial outer membrane in the rat liver and influence on the rate of oxidation. Comparison with linoleic and oleic acids. Biochem. J. 1989, 263, 867–873. [Google Scholar] [CrossRef] [PubMed]
- DeLany, J.P.; Windhauser, M.M.; Champagne, C.M.; Bray, G.A. Differential oxidation of individual dietary fatty acids in humans. Am. J. Clin. Nutr. 2000, 72, 905–911. [Google Scholar] [PubMed]
- Videla, L.A.; Rodrigo, R.; Araya, J.; Poniachik, J. Oxidative stress and depletion of hepatic long-chain polyunsaturated fatty acids may contribute to nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2004, 37, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Choi, H. Dietary alpha-linolenic acid lowers postprandial lipid levels with increase of eicosapentaenoic and docosahexaenoic acid contents in rat hepatic membrane. Lipids 2001, 36, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Morise, A.; Serougne, C.; Gripois, D.; Blouquit, M.F.; Lutton, C.; Hermier, D. Effects of dietary alpha linolenic acid on cholesterol metabolism in male and female hamsters of the lpn strain. J. Nutr. Biochem. 2004, 15, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Scicchitano, P.; Cameli, M.; Maiello, M.; Modesti, P.A.; Muiesan, M.L.; Novo, S.; Palmiero, P.; Saba, P.S.; Pedrinelli, R.; Ciccone, M.M.; et al. Nutraceuticals and dyslipidaemia: Beyond the common therapeutics. J. Funct. Foods 2014, 6, 11–32. [Google Scholar] [CrossRef]
- Moran-Salvador, E.; Lopez-Parra, M.; Garcia-Alonso, V.; Titos, E.; Martinez-Clemente, M.; Gonzalez-Periz, A.; Lopez-Vicario, C.; Barak, Y.; Arroyo, V.; Claria, J. Role for ppargamma in obesity-induced hepatic steatosis as determined by hepatocyte- and macrophage-specific conditional knockouts. FASEB J. 2011, 25, 2538–2550. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Kim, J.Y.; Jun, H.J.; Kim, S.J.; Lee, J.H.; Hoang, M.H.; Hwang, K.Y.; Um, S.J.; Chang, H.I.; Lee, S.J. The natural carotenoid astaxanthin, a ppar-alpha agonist and ppar-gamma antagonist, reduces hepatic lipid accumulation by rewiring the transcriptome in lipid-loaded hepatocytes. Mol. Nutr. Food Res. 2012, 56, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, M.; Koyama, T.; Takahashi, J.; Yazawa, K. Effects of astaxanthin in obese mice fed a high-fat diet. Biosci. Biotechnol. Biochem. 2007, 71, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Seo, J.M.; Nguyen, A.; Pham, T.X.; Park, H.J.; Park, Y.; Kim, B.; Bruno, R.S.; Lee, J. Astaxanthin-rich extract from the green alga haematococcus pluvialis lowers plasma lipid concentrations and enhances antioxidant defense in apolipoprotein e knockout mice. J. Nutr. 2011, 141, 1611–1617. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Pham, T.X.; Wegner, C.J.; Kim, B.; Ku, C.S.; Park, Y.K.; Lee, J.Y. Astaxanthin lowers plasma tag concentrations and increases hepatic antioxidant gene expression in diet-induced obesity mice. Br. J. Nutr. 2014, 112, 1797–1804. [Google Scholar] [CrossRef] [PubMed]
- Jadeja, R.N.; Thounaojam, M.C.; Dandekar, D.S.; Devkar, R.V.; Ramachandran, A.V. Clerodendron glandulosum.Coleb extract ameliorates high fat diet/fatty acid induced lipotoxicity in experimental models of non-alcoholic steatohepatitis. Food Chem. Toxicol. 2010, 48, 3424–3431. [Google Scholar] [CrossRef] [PubMed]
- Raso, G.M.; Esposito, E.; Iacono, A.; Pacilio, M.; Cuzzocrea, S.; Canani, R.B.; Calignano, A.; Meli, R. Comparative therapeutic effects of metformin and vitamin E in a model of non-alcoholic steatohepatitis in the young rat. Eur. J. Pharmacol. 2009, 604, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Shimidzu, N.; Goto, M.; Miki, W. Carotenoids as singlet oxygen quenchers in marine organisms. Fish. Sci. 1996, 62, 134–137. [Google Scholar]
- Krinsky, N.I. Antioxidant functions of carotenoids. Free Radic. Biol. Med. 1989, 7, 617–635. [Google Scholar] [CrossRef]
- Miki, W. Biological functions and activities of animal carotenoids. Pure Appl. Chem. 1991, 63, 141–146. [Google Scholar] [CrossRef]
- McCarty, M.F. Full-spectrum antioxidant therapy featuring astaxanthin coupled with lipoprivic strategies and salsalate for management of non-alcoholic fatty liver disease. Med. Hypotheses 2011, 77, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.M.; Asoh, S.; Hiranuma, H.; Ohsawa, I.; Iio, K.; Satou, A.; Ishikura, M.; Ohta, S. Astaxanthin protects mitochondrial redox state and functional integrity against oxidative stress. J. Nutr. Biochem. 2010, 21, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, R.K.; Baskaran, V. Retinol-deficient rats can convert a pharmacological dose of astaxanthin to retinol: Antioxidant potential of astaxanthin, lutein, and beta-carotene. Can. J. Physiol. Pharmacol. 2010, 88, 977–985. [Google Scholar] [CrossRef] [PubMed]


| Diet | g | kcal |
|---|---|---|
| Nutrient,% | ||
| Protein | 20 | 17 |
| Carbohydrate | 50 | 43 |
| Fat | 20 | 39 |
| Total | 100 | |
| Ingredient | ||
| Casein | 200 | 800 |
| dl-methionine | 3 | 12 |
| Maize starch | 350 | 1400 |
| Sucrose | 150 | 600 |
| Cellulose | 50 | 0 |
| Mineral mixture (AIN-93M) | 35 | 0 |
| Vitamin mixture (AIN-93M) | 10 | 40 |
| Choline bitartrate | 2 | 0 |
| Fat | 200 | 1800 |
| Total | 1000 | 4652 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Rong, S.; Gao, H.; Chen, C.; Yang, W.; Deng, Q.; Huang, Q.; Xiao, L.; Huang, F. A Combination of Flaxseed Oil and Astaxanthin Improves Hepatic Lipid Accumulation and Reduces Oxidative Stress in High Fat-Diet Fed Rats. Nutrients 2017, 9, 271. https://doi.org/10.3390/nu9030271
Xu J, Rong S, Gao H, Chen C, Yang W, Deng Q, Huang Q, Xiao L, Huang F. A Combination of Flaxseed Oil and Astaxanthin Improves Hepatic Lipid Accumulation and Reduces Oxidative Stress in High Fat-Diet Fed Rats. Nutrients. 2017; 9(3):271. https://doi.org/10.3390/nu9030271
Chicago/Turabian StyleXu, Jiqu, Shuang Rong, Hui Gao, Chang Chen, Wei Yang, Qianchun Deng, Qingde Huang, Lingyun Xiao, and Fenghong Huang. 2017. "A Combination of Flaxseed Oil and Astaxanthin Improves Hepatic Lipid Accumulation and Reduces Oxidative Stress in High Fat-Diet Fed Rats" Nutrients 9, no. 3: 271. https://doi.org/10.3390/nu9030271
APA StyleXu, J., Rong, S., Gao, H., Chen, C., Yang, W., Deng, Q., Huang, Q., Xiao, L., & Huang, F. (2017). A Combination of Flaxseed Oil and Astaxanthin Improves Hepatic Lipid Accumulation and Reduces Oxidative Stress in High Fat-Diet Fed Rats. Nutrients, 9(3), 271. https://doi.org/10.3390/nu9030271




