Dietary Patterns and Insomnia Symptoms in Chinese Adults: The China Kadoorie Biobank
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Data Collection
2.3. Dietary Assessment
2.4. Definition of Outcome
2.5. Statistical Analysis
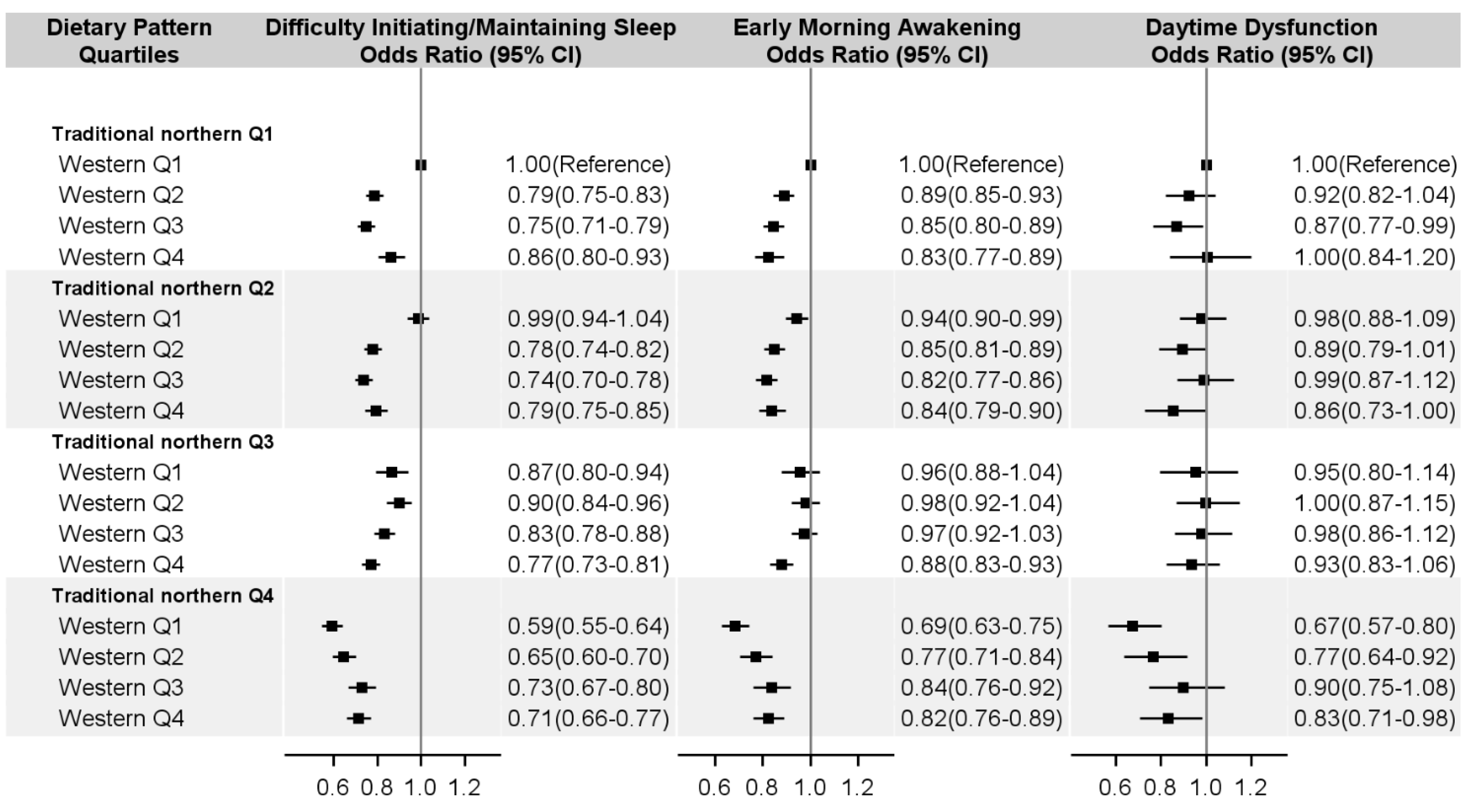
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Parthasarathy, S.; Vasquez, M.M.; Halonen, M.; Bootzin, R.; Quan, S.F.; Martinez, F.D.; Guerra, S. Persistent insomnia is associated with mortality risk. Am. J. Med. 2015, 128, 268.e262–275.e262. [Google Scholar] [CrossRef] [PubMed]
- Lallukka, T.; Podlipskyte, A.; Sivertsen, B.; Andruskiene, J.; Varoneckas, G.; Lahelma, E.; Ursin, R.; Tell, G.S.; Rahkonen, O. Insomnia symptoms and mortality: A register-linked study among women and men from Finland, Norway and Lithuania. J. Sleep Res. 2016, 25, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Cesari, F.; Casini, A.; Macchi, C.; Abbate, R.; Gensini, G.F. Insomnia and risk of cardiovascular disease: A meta-analysis. Eur. J. Prev. Cardiol. 2014, 21, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Laugsand, L.E.; Strand, L.B.; Vatten, L.J.; Janszky, I.; Bjorngaard, J.H. Insomnia symptoms and risk for unintentional fatal injuries—The hunt study. Sleep 2014, 37, 1777–1786. [Google Scholar] [CrossRef] [PubMed]
- Dashti, H.S.; Scheer, F.A.; Jacques, P.F.; Lamon-Fava, S.; Ordovas, J.M. Short sleep duration and dietary intake: Epidemiologic evidence, mechanisms, and health implications. Adv. Nutr. 2015, 6, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, E.; Yatsuya, H.; Uemura, M.; Murata, C.; Otsuka, R.; Toyoshima, H.; Tamakoshi, K.; Sasaki, S.; Kawaguchi, L.; Aoyama, A. Associations of protein, fat, and carbohydrate intakes with insomnia symptoms among middle-aged japanese workers. J. Epidemiol. 2013, 23, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, R.; Asakura, K.; Kobayashi, S.; Suga, H.; Sasaki, S. Low intake of vegetables, high intake of confectionary, and unhealthy eating habits are associated with poor sleep quality among middle-aged female Japanese workers. J. Occup. Health 2014, 56, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, S.; Sakurai, M.; Nakamura, K.; Morikawa, Y.; Miura, K.; Nakashima, M.; Yoshita, K.; Ishizaki, M.; Kido, T.; Naruse, Y.; et al. Associations between rice, noodle, and bread intake and sleep quality in Japanese men and women. PLoS ONE 2014, 9, e105198. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Taylor, A.W.; Zhen, S.; Adams, R.; Appleton, S.; Shi, Z. Soy isoflavone intake and sleep parameters over 5 years among chinese adults: Longitudinal analysis from the Jiangsu Nutrition study. J. Acad. Nutr. Diet. 2016. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wittert, G.; Taylor, A.W.; Adams, R.; Appleton, S.; Shi, Z. Nutrient patterns and chronic inflammation in a cohort of community dwelling middle-aged men. Clin. Nutr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Peuhkuri, K.; Sihvola, N.; Korpela, R. Diet promotes sleep duration and quality. Nutr. Res. 2012, 32, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Chaput, J.P. Sleep patterns, diet quality and energy balance. Physiol. Behav. 2014, 134, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Kurotani, K.; Kochi, T.; Nanri, A.; Eguchi, M.; Kuwahara, K.; Tsuruoka, H.; Akter, S.; Ito, R.; Pham, N.M.; Kabe, I.; et al. Dietary patterns and sleep symptoms in Japanese workers: The Furukawa nutrition and health study. Sleep Med. 2015, 16, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lee, L.; Chen, J.; Collins, R.; Wu, F.; Guo, Y.; Linksted, P.; Peto, R. Cohort profile: The Kadoorie Study of Chronic Disease in China (KSCDC). Int. J. Epidemiol. 2005, 34, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, J.; Collins, R.; Guo, Y.; Peto, R.; Wu, F.; Li, L. China Kadoorie Biobank of 0.5 million people: Survey methods, baseline characteristics and long-term follow-up. Int. J. Epidemiol. 2011, 40, 1652–1666. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Bennett, D.; Li, L.; Whitlock, G.; Guo, Y.; Collins, R.; Chen, J.; Bian, Z.; Hong, L.-S.; Feng, S.; et al. Physical activity and sedentary leisure time and their associations with BMI, waist circumference, and percentage body fat in 0.5 million adults: The China Kadoorie Biobank study. Am. J. Clin. Nutr. 2013, 97, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Walters, E.E.; Kessler, R.C.; Nelson, C.B.; Mroczek, D. Scoring the World Health Organization’s Composite International Diagnostic Interview Short Form (CIDI-SF); World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Millwood, I.Y.; Li, L.; Smith, M.; Guo, Y.; Yang, L.; Bian, Z.; Lewington, S.; Whitlock, G.; Sherliker, P.; Collins, R.; et al. Alcohol consumption in 0.5 million people from 10 diverse regions of China: Prevalence, patterns and socio-demographic and health-related correlates. Int. J. Epidemiol. 2013, 42, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Byrt, T.; Bishop, J.; Carlin, J.B. Bias, prevalence and kappa. J. Clin. Epidemiol. 1993, 46, 423–429. [Google Scholar] [CrossRef]
- Hoehler, F.K. Bias and prevalence effects on kappa viewed in terms of sensitivity and specificity. J. Clin. Epidemiol. 2000, 53, 499–503. [Google Scholar] [CrossRef]
- Wang, D.; He, Y.; Li, Y.; Luan, D.; Zhai, F.; Yang, X.; Ma, G. Joint association of dietary pattern and physical activity level with cardiovascular disease risk factors among Chinese men: A cross-sectional study. PLoS ONE 2013, 8, e66210. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Hall, J.; Byles, J.; Shi, Z. Dietary pattern is associated with obesity in older people in China: Data from China Health and Nutrition Survey (CHNS). Nutrients 2015, 7, 8170–8188. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yao, Y.S.; Dong, Q.; Dong, Y.H.; Liu, J.J.; Yang, L.S.; Huang, F. Characterization and factors associated with sleep quality among rural elderly in China. Arch. Gerontol. Geriatr. 2013, 56, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Meolie, A.L.; Rosen, C.; Kristo, D.; Kohrman, M.; Gooneratne, N.; Aguillard, R.N.; Fayle, R.; Troell, R.; Townsend, D.; Claman, D.; et al. Oral nonprescription treatment for insomnia: An evaluation of products with limited evidence. J. Clin. Sleep Med. 2005, 1, 173–187. [Google Scholar] [PubMed]
- Held, K.; Antonijevic, I.A.; Kunzel, H.; Uhr, M.; Wetter, T.C.; Golly, I.C.; Steiger, A.; Murck, H. Oral Mg(2+) supplementation reverses age-related neuroendocrine and sleep EEG changes in humans. Pharmacopsychiatry 2002, 35, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Zadeh, S.S.; Begum, K. Comparison of nutrient intake by sleep status in selected adults in Mysore, India. Nutr. Res. Pract. 2011, 5, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Uemura, H.; Katsuura-Kamano, S.; Nakamoto, M.; Hiyoshi, M.; Takami, H.; Sawachika, F.; Juta, T.; Arisawa, K. Relationship of dietary factors and habits with sleep-wake regularity. Asia Pac. J. Clin. Nutr. 2013, 22, 457–465. [Google Scholar] [PubMed]
- Zhou, J.; Kim, J.E.; Armstrong, C.L.; Chen, N.; Campbell, W.W. Higher-protein diets improve indexes of sleep in energy-restricted overweight and obese adults: Results from 2 randomized controlled trials. Am. J. Clin. Nutr. 2016, 103, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Espana, R.A.; Scammell, T.E. Sleep neurobiology from a clinical perspective. Sleep 2011, 34, 845–858. [Google Scholar] [PubMed]
- Hashimoto, S.; Kohsaka, M.; Morita, N.; Fukuda, N.; Honma, S.; Honma, K. Vitamin b12 enhances the phase-response of circadian melatonin rhythm to a single bright light exposure in humans. Neurosci. Lett. 1996, 220, 129–132. [Google Scholar] [CrossRef]
- St-Onge, M.P.; Mikic, A.; Pietrolungo, C.E. Effects of diet on sleep quality. Adv. Nutr. 2016, 7, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Del Brutto, O.H.; Mera, R.M.; Ha, J.E.; Gillman, J.; Zambrano, M.; Castillo, P.R. Dietary fish intake and sleep quality: A population-based study. Sleep Med. 2016, 17, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.L.; Dahl, L.; Olson, G.; Thornton, D.; Graff, I.E.; Froyland, L.; Thayer, J.F.; Pallesen, S. Fish consumption, sleep, daily functioning, and heart rate variability. J. Clin. Sleep. Med. 2014, 10, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Tsaluchidu, S.; Cocchi, M.; Tonello, L.; Puri, B.K. Fatty acids and oxidative stress in psychiatric disorders. BMC Psychiatry 2008, 8, S5. [Google Scholar] [CrossRef] [PubMed]
- Wurtman, R.J.; Wurtman, J.J.; Regan, M.M.; McDermott, J.M.; Tsay, R.H.; Breu, J.J. Effects of normal meals rich in carbohydrates or proteins on plasma tryptophan and tyrosine ratios. Am. J. Clin. Nutr. 2003, 77, 128–132. [Google Scholar] [PubMed]
- Fernstrom, J.D.; Wurtman, R.J. Brain serotonin content: Physiological regulation by plasma neutral amino acids. Science 1972, 178, 414–416. [Google Scholar] [CrossRef] [PubMed]
- Lyons, P.M.; Truswell, A.S. Serotonin precursor influenced by type of carbohydrate meal in healthy adults. Am. J. Clin. Nutr. 1988, 47, 433–439. [Google Scholar] [PubMed]
| Food or Beverage Group | Traditional Northern Dietary Pattern | Modern Dietary Pattern |
|---|---|---|
| Rice | −0.84 | 0.20 |
| Wheat | 0.84 | 0.12 |
| Other staple | 0.70 | −0.16 |
| Meat | −0.36 | 0.60 |
| Poultry | −0.35 | 0.55 |
| Fish | −0.35 | 0.52 |
| Eggs | 0.32 | 0.51 |
| Fresh vegetables | −0.06 | 0.20 |
| Soybean | −0.12 | 0.47 |
| Preserved vegetables | −0.15 | 0.13 |
| Fresh fruit | 0.03 | 0.69 |
| Dairy products | 0.23 | 0.63 |
| Beer | 0.06 | 0.20 |
| Rice wine | −0.14 | <0.01 |
| Wine | <0.01 | 0.06 |
| Heavy spirit (≥40%) | −0.08 | <0.01 |
| Light spirit (<40%) | −0.11 | −0.02 |
| Green tea | <0.01 | 0.23 |
| Oolong tea | −0.08 | 0.07 |
| Black tea | −0.19 | −0.04 |
| Other tea | −0.01 | 0.01 |
| Variance explained (%) | 14.58 | 9.74 |
| Characteristics | Traditional Northern Dietary Pattern | Modern Dietary Pattern | ||||
|---|---|---|---|---|---|---|
| Q1 | Q4 | p for Trend | Q1 | Q4 | p for Trend | |
| Age, years | 51.5 | 50.2 | <0.0001 | 51.7 | 50.5 | <0.0001 |
| Female, % | 55.2 | 58.8 | <0.0001 | 64.5 | 57.4 | <0.0001 |
| Urban area, % | 47.9 | 20.4 | <0.0001 | 8.1 | 83.4 | <0.0001 |
| Southern area, % | 98.8 | 0.5 | <0.0001 | 48.2 | 51.3 | <0.0001 |
| Married, % | 91.9 | 91.6 | 0.3487 | 88.7 | 92.0 | <0.0001 |
| High school and above, % | 36.4 | 49.7 | <0.0001 | 29.6 | 77.4 | <0.0001 |
| Household income ≥20,000 CNY/year, % | 56.1 | 19.4 | <0.0001 | 16.9 | 64.8 | <0.0001 |
| Manual worker, % | 58.9 | 73.6 | <0.0001 | 83.2 | 31.1 | <0.0001 |
| Current drinker, % | 21.8 | 10.4 | <0.0001 | 9.9 | 19.9 | <0.0001 |
| Current smoker, % | 31.6 | 26.0 | <0.0001 | 25.1 | 24.6 | 0.2328 |
| Physical activity, MET-h/day | 24.4 | 21.6 | <0.0001 | 22.6 | 19.0 | <0.0001 |
| Depressive symptom, % | 3.0 | 3.0 | 0.0049 | 4.1 | 1.6 | <0.0001 |
| Anxiety symptom, % | 0.5 | 0.7 | 0.0001 | 0.8 | 0.3 | <0.0001 |
| Hypertension, % | 10.5 | 7.9 | <0.0001 | 8.3 | 11.3 | <0.0001 |
| Diabetes, % | 2.1 | 2.6 | <0.0001 | 1.8 | 4.0 | <0.0001 |
| BMI, kg/m2 | 23.4 | 23.9 | <0.0001 | 23.2 | 24.3 | <0.0001 |
| Sleep duration, h | 7.4 | 7.7 | <0.0001 | 7.4 | 7.3 | <0.0001 |
| Energy intake, kcal/day | 1287 | 1372 | <0.0001 | 1151 | 1539 | <0.0001 |
| Quartile of Dietary Pattern Scores | OR Equivalent to 1-SD Increase | |||||
|---|---|---|---|---|---|---|
| Q1 (Low) | Q2 | Q3 | Q4 (High) | p for Trend | ||
| Traditional northern dietary pattern | ||||||
| Difficulty initiating/maintaining sleep | ||||||
| Number of cases (%) | 12,217 (10.2) | 13,626 (11.3) | 11,703 (9.7) | 13,042 (10.8) | ||
| Model 1 | 1.00 (Reference) | 0.98 (0.95–1.00) | 0.95 (0.92–0.98) | 0.77 (0.73–0.81) | <0.0001 | 0.91 (0.89–0.93) |
| Model 2 | 1.00 (Reference) | 0.99 (0.96–1.02) | 0.99 (0.96–1.03) | 0.81 (0.76–0.86) | <0.0001 | 0.93 (0.91–0.95) |
| Early morning awakening | ||||||
| Number of cases (%) | 12,691 (10.5) | 13,360 (11.1) | 11,429 (9.5) | 9433 (7.8) | ||
| Model 1 | 1.00 (Reference) | 0.94 (0.91–0.96) | 0.96 (0.93–0.99) | 0.79 (0.75–0.84) | <0.0001 | 0.93 (0.91–0.95) |
| Model 2 | 1.00 (Reference) | 0.96 (0.93–0.99) | 1.05 (1.01–1.08) | 0.88 (0.83–0.93) | 0.0175 | 0.97 (0.95–1.00) |
| Daytime dysfunction | ||||||
| Number of cases (%) | 1897 (1.6) | 2280 (1.9) | 2092 (1.7) | 2613 (2.2) | ||
| Model 1 | 1.00 (Reference) | 1.00 (0.94–1.07) | 1.01 (0.93–1.09) | 0.83 (0.73–0.93) | 0.004 | 0.93 (0.89–0.98) |
| Model 2 | 1.00 (Reference) | 1.00 (0.93–1.07) | 1.03 (0.95–1.11) | 0.85 (0.75–0.96) | 0.0135 | 0.94 (0.89–0.99) |
| Modern dietary pattern | ||||||
| Difficulty initiating/maintaining sleep | ||||||
| Number of cases (%) | 16,188 (13.5) | 12,690 (10.5) | 11,244 (9.3) | 10,466 (8.7) | ||
| Model 1 | 1.00 (Reference) | 0.88 (0.86–0.91) | 0.82 (0.79–0.84) | 0.78 (0.75–0.81) | <0.0001 | 0.90 (0.89–0.91) |
| Model 2 | 1.00 (Reference) | 0.92 (0.89–0.94) | 0.89 (0.86–0.91) | 0.89 (0.86–0.93) | <0.0001 | 0.95 (0.94–0.97) |
| Early morning awakening | ||||||
| Number of cases (%) | 14,016 (11.7) | 12,483 (10.4) | 11,105 (9.2) | 9309 (7.7) | ||
| Model 1 | 1.00 (Reference) | 0.91 (0.89–0.94) | 0.84 (0.82–0.87) | 0.75 (0.73–0.78) | <0.0001 | 0.89 (0.87–0.90) |
| Model 2 | 1.00 (Reference) | 0.98 (0.95–1.01) | 0.96 (0.93–0.99) | 0.93 (0.90–0.97) | 0.0002 | 0.97 (0.96–0.99) |
| Daytime dysfunction | ||||||
| Number of cases (%) | 3174 (2.6) | 2196 (1.8) | 1831 (1.5) | 1681 (1.4) | ||
| Model 1 | 1.00 (Reference) | 0.97 (0.91–1.02) | 0.94 (0.88–1.00) | 0.83 (0.77–0.90) | <0.0001 | 0.93 (0.90–0.96) |
| Model 2 | 1.00 (Reference) | 1.02 (0.96–1.08) | 1.05 (0.98–1.13) | 1.01 (0.93–1.10) | 0.7166 | 1.01 (0.97–1.04) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.; Shi, Z.; Lv, J.; Guo, Y.; Bian, Z.; Du, H.; Chen, Y.; Tao, R.; Huang, Y.; Chen, J.; et al. Dietary Patterns and Insomnia Symptoms in Chinese Adults: The China Kadoorie Biobank. Nutrients 2017, 9, 232. https://doi.org/10.3390/nu9030232
Yu C, Shi Z, Lv J, Guo Y, Bian Z, Du H, Chen Y, Tao R, Huang Y, Chen J, et al. Dietary Patterns and Insomnia Symptoms in Chinese Adults: The China Kadoorie Biobank. Nutrients. 2017; 9(3):232. https://doi.org/10.3390/nu9030232
Chicago/Turabian StyleYu, Canqing, Zumin Shi, Jun Lv, Yu Guo, Zheng Bian, Huaidong Du, Yiping Chen, Ran Tao, Ying Huang, Junshi Chen, and et al. 2017. "Dietary Patterns and Insomnia Symptoms in Chinese Adults: The China Kadoorie Biobank" Nutrients 9, no. 3: 232. https://doi.org/10.3390/nu9030232
APA StyleYu, C., Shi, Z., Lv, J., Guo, Y., Bian, Z., Du, H., Chen, Y., Tao, R., Huang, Y., Chen, J., Chen, Z., & Li, L. (2017). Dietary Patterns and Insomnia Symptoms in Chinese Adults: The China Kadoorie Biobank. Nutrients, 9(3), 232. https://doi.org/10.3390/nu9030232






