Molecular Bases Underlying the Hepatoprotective Effects of Coffee
Abstract
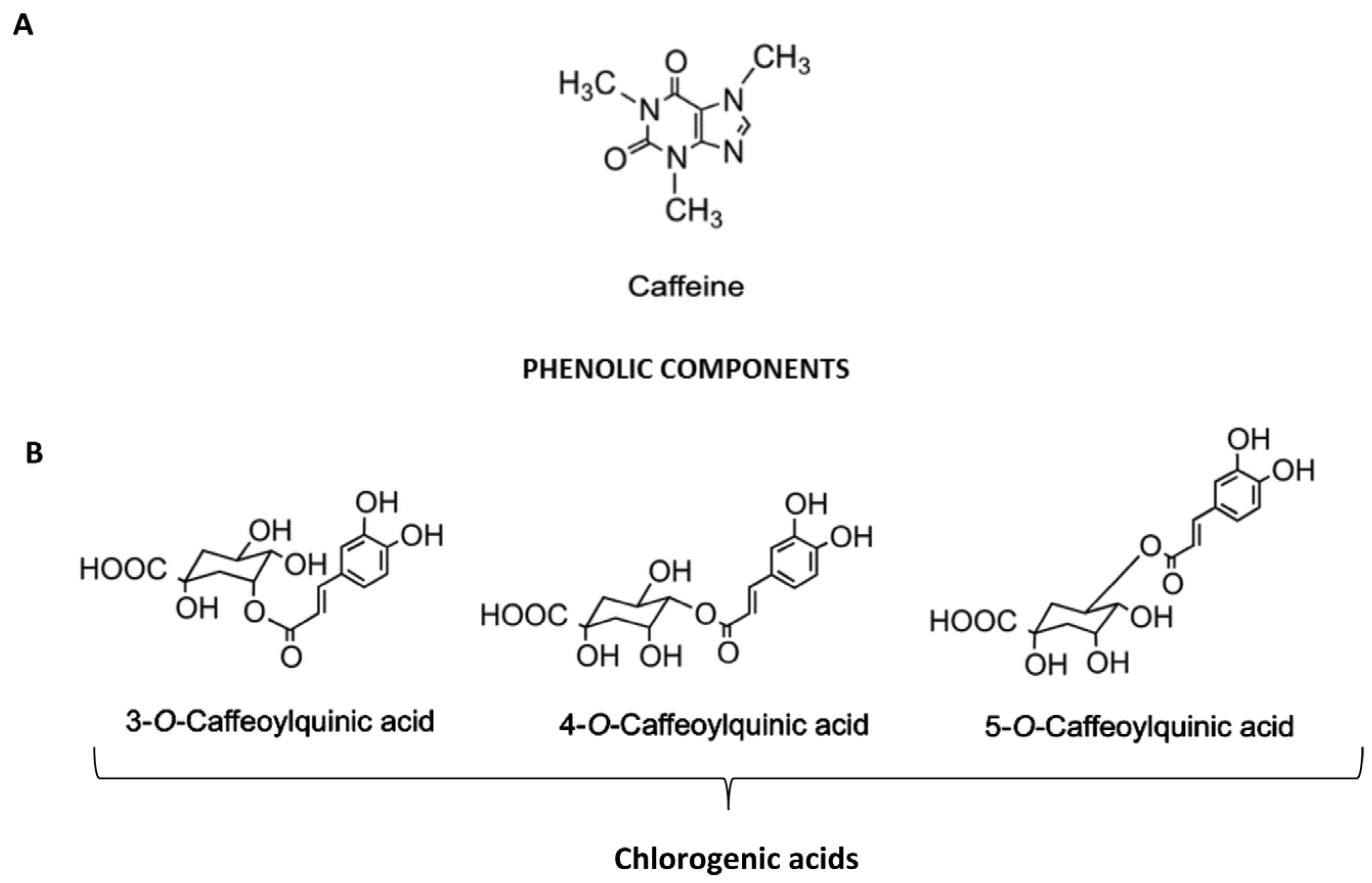
:1. Introduction
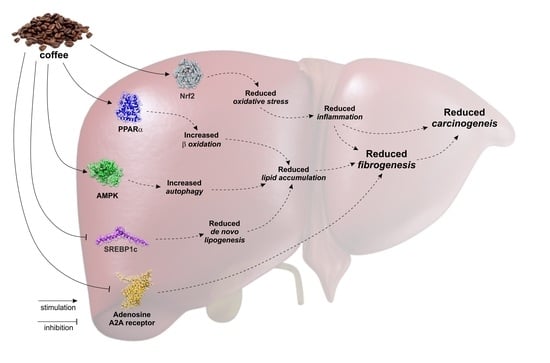
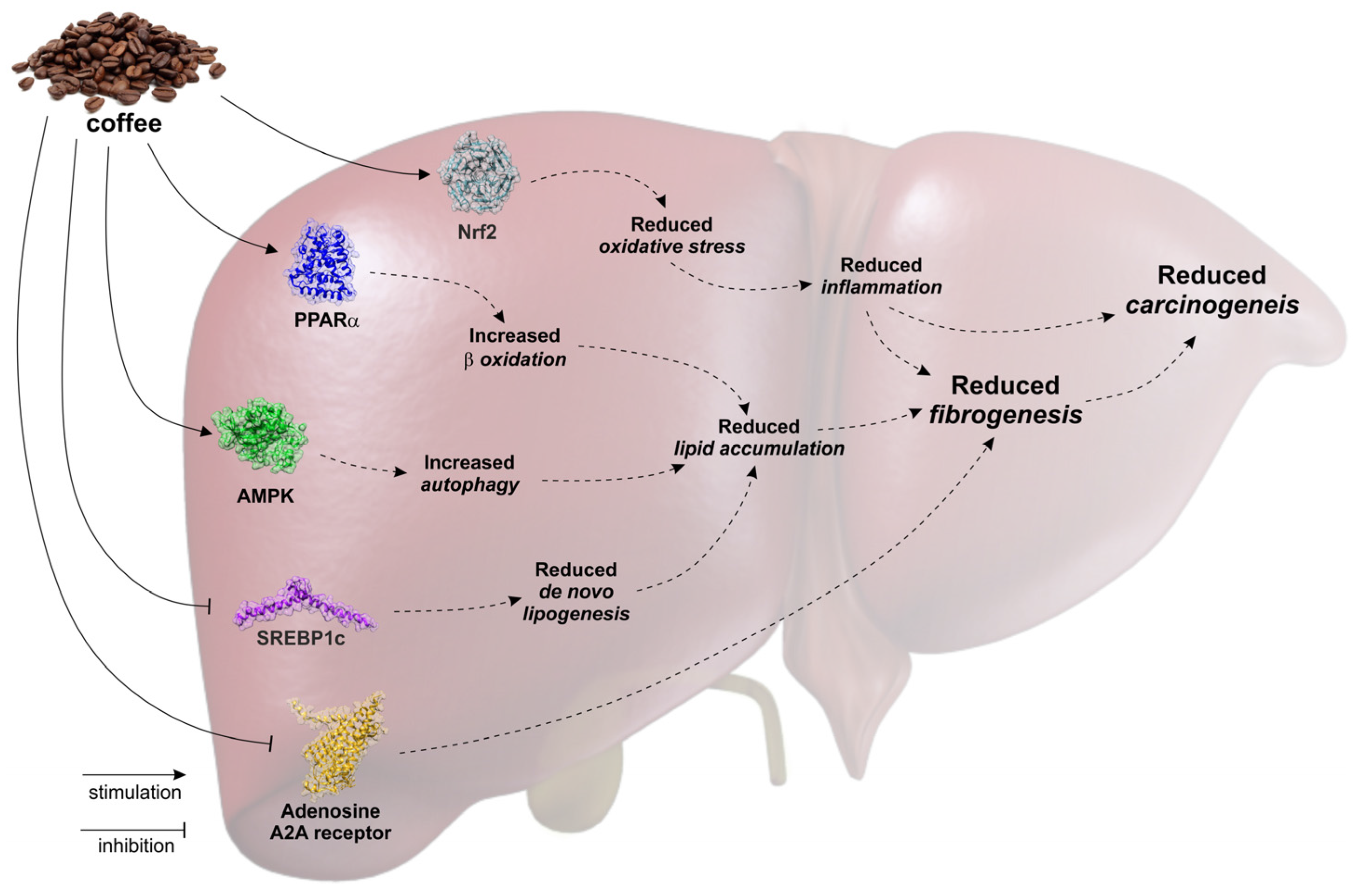
2. Coffee and Liver Steatosis
3. Coffee and Liver Fibrosis/Cirrhosis
4. Coffee and Hepatocellular Carcinoma
5. Nutraceutical/Pharmaceutical Perspective
Author Contributions
Conflicts of Interest
References
- Freedman, N.D.; Park, Y.; Abnet, C.C.; Hollenbeck, A.R.; Sinha, R. Association of coffee drinking with total and cause-specific mortality. N. Engl. J. Med. 2012, 366, 1891–1904. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Satija, A.; Bhupathiraju, S.N.; Hu, Y.; Sun, Q.; Han, J.; Lopez-Garcia, E.; Willett, W.; van Dam, R.M.; Hu, F.B. Association of coffee consumption with total and cause-specific mortality in 3 large prospective cohorts. Circulation 2015, 132, 2305–2315. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Micek, A.; Godos, J.; Sciacca, S.; Pajak, A.; Martinez-Gonzalez, M.A.; Giovannucci, E.L.; Galvano, F. Coffee consumption and risk of all-cause, cardiovascular, and cancer mortality in smokers and non-smokers: A dose-response meta-analysis. Eur. J. Epidemiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Saab, S.; Mallam, D.; Cox, G.A., 2nd; Tong, M.J. Impact of coffee on liver diseases: A systematic review. Liver Int. 2014, 34, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Setiawan, V.W.; Wilkens, L.R.; Lu, S.C.; Hernandez, B.Y.; Le Marchand, L.; Henderson, B.E. Association of coffee intake with reduced incidence of liver cancer and death from chronic liver disease in the us multiethnic cohort. Gastroenterology 2015, 148, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Bhupathiraju, S.N.; Chen, M.; van Dam, R.M.; Hu, F.B. Caffeinated and decaffeinated coffee consumption and risk of type 2 diabetes: A systematic review and a dose-response meta-analysis. Diabetes Care 2014, 37, 569–586. [Google Scholar] [CrossRef] [PubMed]
- Marventano, S.; Salomone, F.; Godos, J.; Pluchinotta, F.; Del Rio, D.; Mistretta, A.; Grosso, G. Coffee and tea consumption in relation with non-alcoholic fatty liver and metabolic syndrome: A systematic review and meta-analysis of observational studies. Clin. Nutr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Zelber-Sagi, S.; Salomone, F.; Webb, M.; Lotan, R.; Yeshua, H.; Halpern, Z.; Santo, E.; Oren, R.; Shibolet, O. Coffee consumption and nonalcoholic fatty liver onset: A prospective study in the general population. Transl. Res. 2015, 165, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Gao, M.; Liu, D. Chlorogenic acid improves high fat diet-induced hepatic steatosis and insulin resistance in mice. Pharm. Res. 2015, 32, 1200–1209. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.A.; Farah, B.L.; Singh, B.K.; Siddique, M.M.; Li, Y.; Wu, Y.; Ilkayeva, O.R.; Gooding, J.; Ching, J.; Zhou, J.; et al. Caffeine stimulates hepatic lipid metabolism by the autophagy-lysosomal pathway in mice. Hepatology 2014, 59, 1366–1380. [Google Scholar] [CrossRef] [PubMed]
- Salomone, F.; Li Volti, G.; Vitaglione, P.; Morisco, F.; Fogliano, V.; Zappala, A.; Palmigiano, A.; Garozzo, D.; Caporaso, N.; D’Argenio, G.; et al. Coffee enhances the expression of chaperones and antioxidant proteins in rats with nonalcoholic fatty liver disease. Transl. Res. 2014, 163, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.W.; Hsu, A.; Tan, B.K. Anti-diabetic and anti-lipidemic effects of chlorogenic acid are mediated by ampk activation. Biochem. Pharmacol. 2013, 85, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Panchal, S.K.; Poudyal, H.; Waanders, J.; Brown, L. Coffee extract attenuates changes in cardiovascular and hepatic structure and function without decreasing obesity in high-carbohydrate, high-fat diet-fed male rats. J. Nutr. 2012, 142, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Murase, T.; Misawa, K.; Minegishi, Y.; Aoki, M.; Ominami, H.; Suzuki, Y.; Shibuya, Y.; Hase, T. Coffee polyphenols suppress diet-induced body fat accumulation by downregulating SREBP-1c and related molecules in C57BL/6J mice. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E122–E133. [Google Scholar] [CrossRef] [PubMed]
- Vitaglione, P.; Morisco, F.; Mazzone, G.; Amoruso, D.C.; Ribecco, M.T.; Romano, A.; Fogliano, V.; Caporaso, N.; D’Argenio, G. Coffee reduces liver damage in a rat model of steatohepatitis: The underlying mechanisms and the role of polyphenols and melanoidins. Hepatology 2010, 52, 1652–1661. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez de Sotillo, D.V.; Hadley, M. Chlorogenic acid modifies plasma and liver concentrations of: Cholesterol, triacylglycerol, and minerals in (fa/fa) Zucker rats. J. Nutr. Biochem. 2002, 13, 717–726. [Google Scholar] [CrossRef]
- Shimoda, H.; Seki, E.; Aitani, M. Inhibitory effect of green coffee bean extract on fat accumulation and body weight gain in mice. BMC Complement. Altern. Med. 2006, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ruiz, C.; Mato, J.M.; Vance, D.; Kaplowitz, N.; Fernandez-Checa, J.C. Acid sphingomyelinase-ceramide system in steatohepatitis: A novel target regulating multiple pathways. J. Hepatol. 2015, 62, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Freedman, N.D.; Everhart, J.E.; Lindsay, K.L.; Ghany, M.G.; Curto, T.M.; Shiffman, M.L.; Lee, W.M.; Lok, A.S.; Di Bisceglie, A.M.; Bonkovsky, H.L.; et al. Coffee intake is associated with lower rates of liver disease progression in chronic hepatitis C. Hepatology 2009, 50, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Cardin, R.; Piciocchi, M.; Martines, D.; Scribano, L.; Petracco, M.; Farinati, F. Effects of coffee consumption in chronic hepatitis c: A randomized controlled trial. Dig. Liver Dis. 2013, 45, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Crozier, T.W.; Stalmach, A.; Lean, M.E.; Crozier, A. Espresso coffees, caffeine and chlorogenic acid intake: Potential health implications. Food Funct. 2012, 3, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Pluchinotta, F.R.; Marventano, S.; Buscemi, S.; Li Volti, G.; Galvano, F.; Grosso, G. Coffee components and cardiovascular risk: Beneficial and detrimental effects. Int. J. Food Sci. Nutr. 2014, 65, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.S.; Montesinos, M.C.; Fernandez, P.; Desai, A.; Delano, D.L.; Yee, H.; Reiss, A.B.; Pillinger, M.H.; Chen, J.F.; Schwarzschild, M.A.; et al. Adenosine A2A receptors play a role in the pathogenesis of hepatic cirrhosis. Br. J. Pharmacol. 2006, 148, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Gressner, O.A.; Lahme, B.; Rehbein, K.; Siluschek, M.; Weiskirchen, R.; Gressner, A.M. Pharmacological application of caffeine inhibits TGF-β-stimulated connective tissue growth factor expression in hepatocytes via PPARγ and SMAD2/3-dependent pathways. J. Hepatol. 2008, 49, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Gressner, O.A.; Lahme, B.; Siluschek, M.; Gressner, A.M. Identification of paraxanthine as the most potent caffeine-derived inhibitor of connective tissue growth factor expression in liver parenchymal cells. Liver Int. 2009, 29, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Klemmer, I.; Yagi, S.; Gressner, O.A. Oral application of 1,7-dimethylxanthine (paraxanthine) attenuates the formation of experimental cholestatic liver fibrosis. Hepatol. Res. 2011, 41, 1094–1109. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.J.; Lee, F.Y.; Wang, S.S.; Hsin, I.F.; Lin, T.Y.; Huang, H.C.; Chang, C.C.; Chuang, C.L.; Ho, H.L.; Lin, H.C.; et al. Caffeine ameliorates hemodynamic derangements and portosystemic collaterals in cirrhotic rats. Hepatology 2015, 61, 1672–1684. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Dai, X.; Yang, W.; Wang, H.; Zhao, H.; Yang, F.; Yang, Y.; Li, J.; Lv, X. Caffeine protects against alcohol-induced liver fibrosis by dampening the cAMP/PKA/CREB pathway in rat hepatic stellate cells. Int. Immunopharmacol. 2015, 25, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guan, W.; Yang, W.; Wang, Q.; Zhao, H.; Yang, F.; Lv, X.; Li, J. Caffeine inhibits the activation of hepatic stellate cells induced by acetaldehyde via adenosine A2A receptor mediated by the cAMP/PKA/SRC/ERK1/2/P38 mapk signal pathway. PLoS ONE 2014, 9, e92482. [Google Scholar] [CrossRef] [PubMed]
- Gordillo-Bastidas, D.; Oceguera-Contreras, E.; Salazar-Montes, A.; Gonzalez-Cuevas, J.; Hernandez-Ortega, L.D.; Armendariz-Borunda, J. Nrf2 and Snail-1 in the prevention of experimental liver fibrosis by caffeine. World J. Gastroenterol. 2013, 19, 9020–9033. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.G.; Jun, D.W.; Kim, E.K.; Saeed, W.K.; Lee, K.N.; Lee, H.L.; Lee, O.Y.; Choi, H.S.; Yoon, B.C. Caffeine attenuates liver fibrosis via defective adhesion of hepatic stellate cells in cirrhotic model. J. Gastroenterol. Hepatol. 2013, 28, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Dong, L.; Jiang, J.; Zhao, J.; Zhao, G.; Dang, X.; Lu, X.; Jia, M. Chlorogenic acid reduces liver inflammation and fibrosis through inhibition of toll-like receptor 4 signaling pathway. Toxicology 2013, 303, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Dong, L.; Dang, X.; Liu, Y.; Jiang, J.; Wang, Y.; Lu, X.; Guo, X. Effect of chlorogenic acid on lps-induced proinflammatory signaling in hepatic stellate cells. Inflamm. Res. 2013, 62, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Furtado, K.S.; Prado, M.G.; Aguiar, E.S.M.A.; Dias, M.C.; Rivelli, D.P.; Rodrigues, M.A.; Barbisan, L.F. Coffee and caffeine protect against liver injury induced by thioacetamide in male wistar rats. Basic Clin. Pharmacol. Toxicol. 2012, 111, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Arauz, J.; Moreno, M.G.; Cortes-Reynosa, P.; Salazar, E.P.; Muriel, P. Coffee attenuates fibrosis by decreasing the expression of TGF-β and CTGF in a murine model of liver damage. J. Appl. Toxicol. 2013, 33, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Dong, L.; Bai, Y.; Zhao, J.; Zhang, Y.; Zhang, L. Chlorogenic acid against carbon tetrachloride-induced liver fibrosis in rats. Eur. J. Pharmacol. 2009, 623, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Bravi, F.; Bosetti, C.; Tavani, A.; Gallus, S.; La Vecchia, C. Coffee reduces risk for hepatocellular carcinoma: An updated meta-analysis. Clin. Gastroenterol. Hepatol. 2013, 11, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Ono, K.; Okauchi, R.; Yagasaki, K. Inhibitory effect of coffee on hepatoma proliferation and invasion in culture and on tumor growth, metastasis and abnormal lipoprotein profiles in hepatoma-bearing rats. J. Nutr. Sci. Vitaminol. (Tokyo) 2004, 50, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Furuse, T.; Yagasaki, K. Inhibitory effect of serum from rats administered with coffee on the proliferation and invasion of rat ascites hepatoma cells. Cytotechnology 1997, 25, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Nishikawa, A.; Shima, H.; Sugie, S.; Shinoda, T.; Yoshimi, N.; Iwata, H.; Mori, H. Inhibitory effects of chlorogenic acid, reserpine, polyprenoic acid (E-5166), or coffee on hepatocarcinogenesis in rats and hamsters. Basic Life Sci. 1990, 52, 429–440. [Google Scholar] [PubMed]
- Furtado, K.S.; Polletini, J.; Dias, M.C.; Rodrigues, M.A.; Barbisan, L.F. Prevention of rat liver fibrosis and carcinogenesis by coffee and caffeine. Food Chem. Toxicol. 2014, 64, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Ferk, F.; Huber, W.W.; Grasl-Kraupp, B.; Speer, K.; Buchmann, S.; Bohacek, R.; Misik, M.; Edelbauer, L.; Knasmuller, S. Protective effects of coffee against induction of DNA damage and pre-neoplastic foci by aflatoxin B1. Mol. Nutr. Food Res. 2014, 58, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Katayama, M.; Donai, K.; Sakakibara, H.; Ohtomo, Y.; Miyagawa, M.; Kuroda, K.; Kodama, H.; Suzuki, K.; Kasai, N.; Nishimori, K.; et al. Coffee consumption delays the hepatitis and suppresses the inflammation related gene expression in the long-evans cinnamon rat. Clin. Nutr. 2014, 33, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Tanaka, T.; Shima, H.; Kuniyasu, T.; Takahashi, M. Inhibitory effect of chlorogenic acid on methylazoxymethanol acetate-induced carcinogenesis in large intestine and liver of hamsters. Cancer Lett. 1986, 30, 49–54. [Google Scholar] [CrossRef]
- Cavin, C.; Marin-Kuan, M.; Langouet, S.; Bezencon, C.; Guignard, G.; Verguet, C.; Piguet, D.; Holzhauser, D.; Cornaz, R.; Schilter, B. Induction of Nrf2-mediated cellular defenses and alteration of phase I activities as mechanisms of chemoprotective effects of coffee in the liver. Food Chem. Toxicol. 2008, 46, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Higgins, L.G.; Cavin, C.; Itoh, K.; Yamamoto, M.; Hayes, J.D. Induction of cancer chemopreventive enzymes by coffee is mediated by transcription factor Nrf2. Evidence that the coffee-specific diterpenes cafestol and kahweol confer protection against acrolein. Toxicol. Appl. Pharmacol. 2008, 226, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Kalthoff, S.; Ehmer, U.; Freiberg, N.; Manns, M.P.; Strassburg, C.P. Coffee induces expression of glucuronosyltransferases by the Aryl hydrocarbon receptor and Nrf2 in liver and stomach. Gastroenterology 2010, 139, 1699–1710. [Google Scholar] [CrossRef] [PubMed]
- Vicente, S.J.; Ishimoto, E.Y.; Torres, E.A. Coffee modulates transcription factor Nrf2 and highly increases the activity of antioxidant enzymes in rats. J. Agric. Food Chem. 2014, 62, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Lecoultre, V.; Carrel, G.; Egli, L.; Binnert, C.; Boss, A.; MacMillan, E.L.; Kreis, R.; Boesch, C.; Darimont, C.; Tappy, L. Coffee consumption attenuates short-term fructose-induced liver insulin resistance in healthy men. Am. J. Clin. Nutr. 2014, 99, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Nardini, M.; Cirillo, E.; Natella, F.; Scaccini, C. Absorption of phenolic acids in humans after coffee consumption. J. Agric. Food Chem. 2002, 50, 5735–5741. [Google Scholar] [CrossRef] [PubMed]
- Rechner, A.R.; Spencer, J.P.; Kuhnle, G.; Hahn, U.; Rice-Evans, C.A. Novel biomarkers of the metabolism of caffeic acid derivatives in vivo. Free Radic. Biol. Med. 2001, 30, 1213–1222. [Google Scholar] [CrossRef]
- Monteiro, M.; Farah, A.; Perrone, D.; Trugo, L.C.; Donangelo, C. Chlorogenic acid compounds from coffee are differentially absorbed and metabolized in humans. J. Nutr. 2007, 137, 2196–2201. [Google Scholar] [PubMed]
- Stalmach, A.; Steiling, H.; Williamson, G.; Crozier, A. Bioavailability of chlorogenic acids following acute ingestion of coffee by humans with an ileostomy. Arch. Biochem. Biophys. 2010, 501, 98–105. [Google Scholar] [CrossRef] [PubMed]
| Author, Year (Reference) | Coffee Compound and Schedule | Experimental Model | Main Findings |
|---|---|---|---|
| Ma Y, 2015 [9] | CGA, 100 mg/kg i.p. twice/wk | C57BL/six mice fed a HFD for 15 weeks and treated with CGA for all 15 weeks or last six weeks | ↓ steatosis and insulin resistance in both preventive and therapeutic arms ↓ Pparγ, Cd36, Fabp4, and Mgat1 ↓ liver and visceral adipose tissue inflammation ↓ F4/80, Cd68, Cd11b, Cd11c, Tnfα, Mcp-1 and Ccr2 |
| Sihna RA, 2014 [10] | Caffeine | C57BL/six mice fed a HFD for four weeks Hepg2 treated with oleic/palmitic acid and caffeine | ↑ autophagic flux in the liver ↑ autophagy in hepatic cells ↓ hepatic steatosis |
| Salomone F, 2014 [11] | Espresso decaffeinated coffee | Wistar rats fed a HFD for 12 weeks and treated with 1.5 mL of decaffeinated espresso coffee (equivalent to six cups) for the last eight weeks | ↑ GSH, PPAR-α ↓ 8-OGH ↓ α-SMA, TGF-β ↑ GSH, PPAR-α |
| Ong Kw, 2013 [12] | Chlorogenic acid | Db/db mice, Hepg2 | ↓ fasting glucose ↓ serum TC, TG ↓ serum FFA ↑ serum adiponectin ↓ liver TC, TG ↓ lipid accumulation in hepatic cells |
| Panchal SK, 2012 [13] | Colombian coffee extract | Wistar rats fed a Western diet for 16 weeks and treated with CE 50 mL/kg of chow (50 mL = 50 g of coffee/100 mL of hot water) for the last eight weeks | ↓ steatosis, inflammation and fibrosis ↑ glucose tolerance ↓ cardiac fibrosis, hypertension |
| Murase T, 2011 [14] | Coffee polyphenols | C57BL/6J mice fed a HFD + CPP (0.5%, 1% of chow) for two, 15 weeks Hepa 1–6 cells treated with CPP for 24 h | ↓ body and liver weight in mice fed 1% CGA group ↓ liver triglycerides and cholesterol in mice fed 1% CGA group ↓ SREBP-1c, FAS, ACC-1, ACC-2, SCD-1 in mice fed 1% CGA and Hepa1-6 cells |
| Vitaglione P, 2010 [15] | Espresso decaffeinated coffee, coffee polyphenols or coffee melanoidins | Wistar rats fed a HFD for 12 weeks and treated with 1.5 mL of decaffeinated espresso coffee (equivalent to six cups) for the last eight weeks | ↓ steatosis, inflammation and fibrosis ↑ liver adiponectin receptor ↓ inflammatory cytokines and oxidative stress |
| Rodriguez de Sotillo DV, 2002 [16] | CGA | Zucker rats fed a standard diet, daily treated with 5 mg/kg bw of CGA for three weeks via intravenous infusion | ↑ glucose tolerance ↓ plasma cholesterol and triglycerides ↓ liver triglycerides |
| Author, Year | Coffee Component | Study Design | Main Findings |
|---|---|---|---|
| Hsu SJ, 2015 [28] | Caffeine 50 mg/kg daily | Sprague-Dawley rats with BDL for four weeks or treated with thioacetamide for eight weeks were administered caffeine at d1 or 15 of study period | ↓ cardiac index, portal pressure and portosystemic shunting ↓ endothelial nitric oxide synthase, vascular endothelial growth factor (VEGF), phospho-VEGFR2 |
| Wang Q, 2015 [29] | Caffeine 5, 10, 20 mg/kg daily | Sprague-Dawley rats treated with alcohol + 20 mg/kg caffeine Rat primary HSC treated with | ↓ AST, ALT ↓ hyaluronic acid, laminin, N-terminal peptide of type III procollagen and type IV collagen ↓ cAMP-PKA-CREB |
| Wang H, 2014 [30] | Caffeine 0.5–8 mM | Rat HSC-T6 treated with 200 µM acetaldehyde for 24–72 h | ↓ Cell viability ↓ procollagen I and III ↓ cAMP-PKA-SRC-ERK1/2 ↓ P38 MAPK |
| Gordillo-Bastidas D, 2013 [31] | Caffeine 15 mg/kg daily | Wistar rats treated with thioacetamide for seven weeks or BDL for 4 weeks | ↓ CTGF, TGF-β, Col-1 ↓ IL-6, IL-1, TNF-α ↑ Nrf-2, SOD, CAT ↓ Snail-1 |
| Shim SG, 2013 [32] | Caffeine 1, 5, 10 mmol | -HSC treated with caffeine -Sprague-Dawley rats treated with thioacetamide for eight weeks | ↑ HSC apoptosis ↓ HSC procollagen type 1c, α-SMA ↓ liver TGF-β, α-SMA |
| Shi H, 2013 [33] | Chlorogenic acid 12.5, 25 and 50 lg/mL | Rat HSC activated by LPS 100 ng/mL treated with CA for 24 h | ↓ ROS ↓IκB-α phosphorylation ↓ MCP-1, IL-6 |
| Shi H, 2013 [34] | Chlorogenic acid | Sprague-Dawley, CCl4 + CGA for eight weeks | ↓ AST, ALT ↓ collagen deposition ↓ α-SMA, collagen I ↓ TLR4 |
| Furtado KS, 2012 [35] | -Conventional coffee -Decaffeinated coffee -Caffeine 0.1% | Wistar rats treated with thioacetamide for eight weeks | ↓ AST, ALT ↓ collagen volume fraction ↓ TGF-β ↓ GST-P positive preneoplastic lesions (only in conventional coffee group) |
| Arauz J, 2013 [36] | -Conventional -Decaffeinated coffee | Wistar rats treated with thioacetamide for eight weeks | ↓ AST, ALT ↓ collagen volume fraction ↓ TGF-β, CTGF, α-SMA, MMP-2 |
| Klemmer I, 2011 [27] | Caffeine metabolite (paraxanthine) 1 mM | Sprague-Dawley rats treated with BDL | ↓ Picrosirius red staining ↓ CTGF, SMAD2 ↓ malondialdehyde |
| Shi H, 2009 [37] | Chlorogenic acid 30, 60 mg/kg | Sprague-Dawley rats treated with CCl4 for eight weeks and CA for eight weeks | ↓histological fibrosis ↓ collagen I, collagen III, α-SMA ↓ TGF-β, VEGF |
| Gressner OA, 2009 [26] | Caffeine metabolite (paraxanthine) 1.25 mM–2.5 mM | HSC treated with TGF-β + caffeine | ↓ CTGF |
| Gressner OA, 2008 [25] | Caffeine 5 mM | Rat hepatocytes treated with cafeine | ↓ CTGF, SMAD2 ↓ SMAD1/3-phosphorylation ↑ PPAR-γ |
| Chan ES, 2006 [24] | Caffeine 50 mg/kg daily orally | C57BL/six mice treated with CCl4 for six weeks or thioacetamide for seven weeks | ↓ AST, ALT ↓ Picrosirius red staining |
| Author, Year | Coffee Compound | Study Design | Main Findings |
|---|---|---|---|
| Furtado KS, 2014 [39] | Regular Coffee Instant coffee Caffeine 0.1% | Wistar rats treated with DEN and CCl4 and administered coffee or caffeine for 24 weeks | ↓ collagen I ↓ size and area of pre-neoplastic lesions ↓ number of neoplastic lesions |
| Ferk F, 2014 [40] | Coffee 25%, 50%, 100% coffee in drinking water | Rats administered coffee treated with aflatoxin B1 0.75 mg/kg b.w. ip and followed-up for 10 weeks | ↓ number of pre-neoplastic foci for all brews ↑ UGT1A |
| Katayama M, 2014 [41] | Coffee | Long Evans Cinnamon rat administered coffee for 25 weeks | ↑ survival ↓ liver inflammatory cytokines ↓ number of pre-neoplastic foci |
| Kalthoff S, 2010 [42] | Coffee | HepG2 and CaCo2 Transgenic mice expressing human UGT1A | ↑ UGT1A isoforms in the liver |
| Higgins LC, 2008 [43] | Coffee 3% or 6% | Nrf2 (+/+) or Nrf2 (−/−) mice fed coffee for five days | ↑ NQO1 and GSTA1 in the liver and gut of Nrf2 (+/+) mice ↑ UGT1A6 and GCLC in the gut of Nrf2 (+/+) mice |
| Cavin C, 2008 [44] | Coffee | Sprague-Dawley rats 0.75%; 1.5%; 3% or 6% coffee | ↑ GSH, HO-1 |
| Miura Y, 2004 [45] | Coffee | Hepatoma-bearing rats given oral administration of instant coffee powder (ICP) solution for two weeks | ↓ tumor growth and metastases dissemination |
| Miura Y, 1997 [38] | Coffee | In vitro effects on a rat hepatoma cell line of sera from rats given oral administration of instant coffee powder (ICP) solution | ↓ proliferation and invasion of hepatoma cells |
| Tanaka T, 1990 [34] | Coffee | Rats administered aminopyrine (0.01%) and sodium nitrite (0.1%) and contemporary drinking coffee solution for 630 days | ↓ incidence of liver tumors ↓ number of hyperplastic liver cell foci |
| Mori H, 1986 [33] | Chlorogenic acid | Syrian golden hamsters given a single intravenous injection of MAM acetate (20 mg/kg body weight) and fed a diet containing 0.025% chlorogenic acid for 24 weeks | ↓ incidence of colon tumors ↓ number of hyperplastic liver cell foci |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salomone, F.; Galvano, F.; Li Volti, G. Molecular Bases Underlying the Hepatoprotective Effects of Coffee. Nutrients 2017, 9, 85. https://doi.org/10.3390/nu9010085
Salomone F, Galvano F, Li Volti G. Molecular Bases Underlying the Hepatoprotective Effects of Coffee. Nutrients. 2017; 9(1):85. https://doi.org/10.3390/nu9010085
Chicago/Turabian StyleSalomone, Federico, Fabio Galvano, and Giovanni Li Volti. 2017. "Molecular Bases Underlying the Hepatoprotective Effects of Coffee" Nutrients 9, no. 1: 85. https://doi.org/10.3390/nu9010085
APA StyleSalomone, F., Galvano, F., & Li Volti, G. (2017). Molecular Bases Underlying the Hepatoprotective Effects of Coffee. Nutrients, 9(1), 85. https://doi.org/10.3390/nu9010085