1. Introduction
Metabolic acidosis is a common complication of chronic kidney disease (CKD) when the glomerular filtration rate falls below 30–40 mL/min/1.73 m
2 [
1,
2,
3]. Current guidelines recommend treatment with alkali when bicarbonate levels are lower than 22 mMol/L [
1] to prevent complications, such as insulin resistance [
4,
5], cardiovascular diseases and progression of CKD, among others [
6,
7].
A correction of metabolic acidosis can be achieved with pharmacological administration of oral alkali as sodium bicarbonate, but also with a diet rich of fruit and vegetables [
8,
9,
10]. In addition, conservative therapy of CKD with low-protein content diets has been revised and reached the scientific rigor of nutritional therapy with the purpose of obtaining a reduction of sodium intake and hypertension [
11,
12], of phosphate intake and serum phosphate levels [
13,
14], of proteinuria [
15,
16], and a delay of dialysis initiation [
17], through a reduced load of catabolites and a better metabolic control [
17,
18,
19]. Hence, as fruit and plant proteins are able to reduce dietetic acid load [
20,
21,
22], our aim is to evaluate whether a very low-protein diet (VLPD) reduces both the acid load in patients with CKD and the need of administrating oral sodium bicarbonate.
2. Materials and Methods
We conducted a case-control study in 146 patients participating in the UBI study—a multicentric, prospective, cohort, randomized, open-label and controlled study, registered in ClinicalTrials.gov (NCT01640119), in which patients were randomized to receive either oral sodium bicarbonate or placebo, in order to evaluate mortality and dialysis risk or doubling of creatinine levels [
23]. All 146 patients were chosen from the experimental arm of the UBI study; therefore, they were all administered oral bicarbonate: 54 out of them assumed a VLPD and 92 case-controls assumed a control diet together with oral bicarbonate. The duration of follow-up was 12 months. We excluded diabetic subjects to avoid bias due to glycemic control complications.
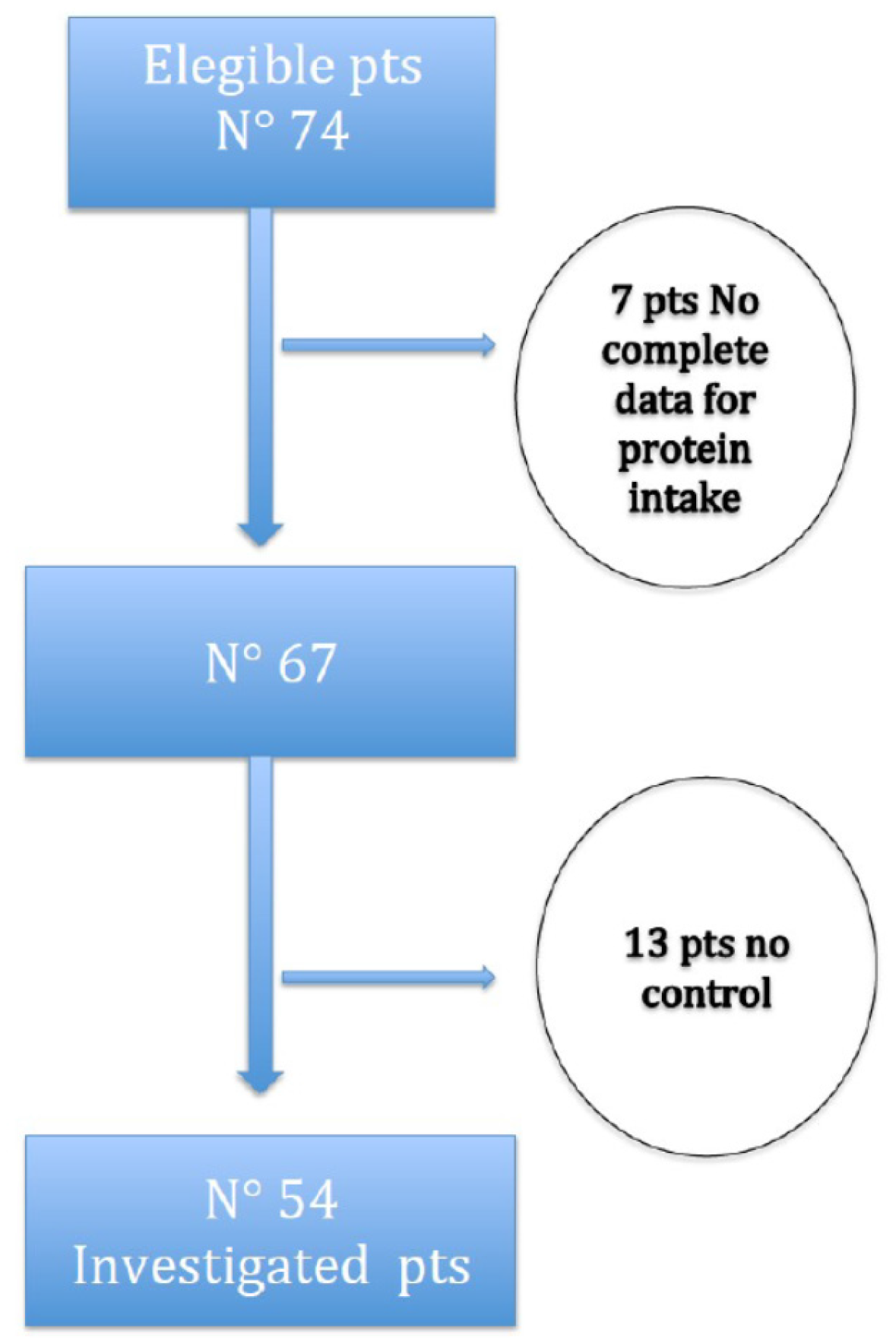
Figure 1 shows the study-flow diagram. Seventy-four subjects assuming VLPD encountered study criteria; 7 of them were excluded because of incompleteness of urinary data needed to calculate protein intake, and 13 because of lack of corresponding controls. Finally, among the included patients (54), only 38 of them had a case-control ratio of 1:2, while the other 16 subjects had a 1:1 ratio.
VLPD consisted of a vegetarian, high-energy diet, with a protein content of 0.3–0.4 g/Kg body weight (BW)/day supplemented with amino-acids and ketoacids in tablets (AlfaKappa
® Fresenius Kabi, Verona, Italia). The main nutritional differences between VLPD and control group diet were plant and animal proteins amount, phosphate and salt content (see
Table 1).
During the course of CKD several metabolic alterations appear (hyperazotemia, metabolic acidosis, hyperpotassiemia, hyperphosphatemia, increase of parathyroid hormone (PTH) levels, intestinal dysbiosis), that are all positively influenced by a nutritional therapy using a VLPD. This diet is characterized by low-protein content with supplements of essential amino-acids and ketoanalogues (proteins used are roughly 90% of plant type), low phosphate content (300–450 mg in VLPD versus 1000–1200 mg in an usual diet), low salt content (80–95 mmol/day in VLPD versus 140–180 mmol/day in a usual diet), and low organic acids and saturated fats amount. The effects of VLPD in terms of reduction or normalization of urea levels in CKD are excellent, but the effects on other biochemical parameters are also worthy of interest [
25].
Every 6 months, study participants performed the common biochemical blood tests and urine parameters (24 h urine urea, creatinine, phosphate, potassium, natrium and protein levels). Particular attention was paid to a correct 24 h urine collection, and all patients were trained in detail how to perform a correct procedure.
Actual protein intake was evaluated according to the formula of Maroni et al.: daily protein intake (g/day) = (6.25 × urinary urea nitrogen mg/day) + (0.031 × body weight-kg) + urinary proteins g/day [
26], using 24-h urinary urea and not dietetic interviews. Twenty-four hour urinary urea excretion together with urinary potassium and phosphate excretion were used to calculate the potential renal acid load (PRAL) and net endogenous acid production (NEAP) every 3 months. PRAL and NEAP, both inversely correlated with serum bicarbonate levels, represent the non-volatile acid load derived from nutrition, estimated by the production of non-volatile acids and bases produced during digestion based on known nutritional content [
27]. PRAL was calculated by the following formula as described by Remer and Manz [
28]: calculated PRAL (mEq/day) = (0.49 × protein intake, g/day) + (0.037 × phosphorus intake, mmol/day) − (0.021 × potassium intake, mmol/day) − ( 0.026 × magnesium, mmol/day) − (0.013 × calcium, mmol/day); NEAP was calculated by the formula described by Frassetto et al. [
29]: NEAP (mEq/day) = (54.5 × protein intake (g/day)/potassium intake, (mmol/day)) − 10.2. Thus, protein intake contained in the two previous formulas was calculated with Maroni formula [
26], and phosphorus and potassium intake were estimated from 24-h urinary phosphate and potassium excretion, representing the actual phosphate and potassium daily intake, in metabolic stable patients.
Finally, the amount of oral bicarbonate needed to maintain bicarbonate plasma levels between 24 and 28 mEq/L was evaluated every 3 months.
4. Results
In VLPD group, the causes of renal disease were hypertensive-vascular nephropathies in 38% of patients, glomerulonephrites in 20%, tubule-interstitial nephropathies in 15%, unknown causes in 27% of patients; in control group the percentages were similar: 41%, 18%, 16%, and 24%, respectively. Moreover, the frequency of cardiovascular complications (angina, infarct, ictus) was 46% in VLPD group, and 42% in control group (p = NS).
Table 2 shows the differences between VLPD and control diet at baseline.
Age and percentage of male sex were not different between the two groups because of the case-control design of the study. On the other hand, VLPD patients had a lower body weight (71.6 ± 13.1 vs. 77.8 ± 14.2 kg;
p < 0.0001). The other biochemical parameters were not different except for urinary creatinine (69.8 ± 29.1 in VLPD vs. 99 ± 32.7 µmol/day in control group;
p < 0.0001), as consequence of different body weights and residual renal function in the two groups (26 ± 12 mL/min in VLPD group vs. 39 ± 14 mL/min in control group;
p < 0.0001). There were no statistical differences between the two groups regarding systolic blood pressure (SBP), diastolic blood pressure (DBP), protein and phosphate intake, urinary natrium, potassium, phosphate and urea nitrogen, NEAP, and PRAL (
Table 2).
Table 3 shows differences at 6 and 12 months of the same parameters seen in
Table 2. VLPD patients showed at 6 and also 12 months a significant reduction of SBP (
p < 0.0001), DBP (
p < 0.001), plasma urea (
p < 0.0001) protein intake (
p < 0.0001), calcemia (
p < 0.0001), phosphatemia (
p < 0.0001), phosphate intake (
p < 0.0001), urinary natrium (
p < 0.0001), urinary potassium (
p < 0.002), and urinary phosphate (
p < 0.0001). At six months potassemia was higher in VLPD group than in controls (
p < 0.001), but not at 12 months (patients were not administrated potassium binders, and the correction of hyperpotassemia at 12 months was mostly as a consequence of a physiological correction of metabolic acidosis).
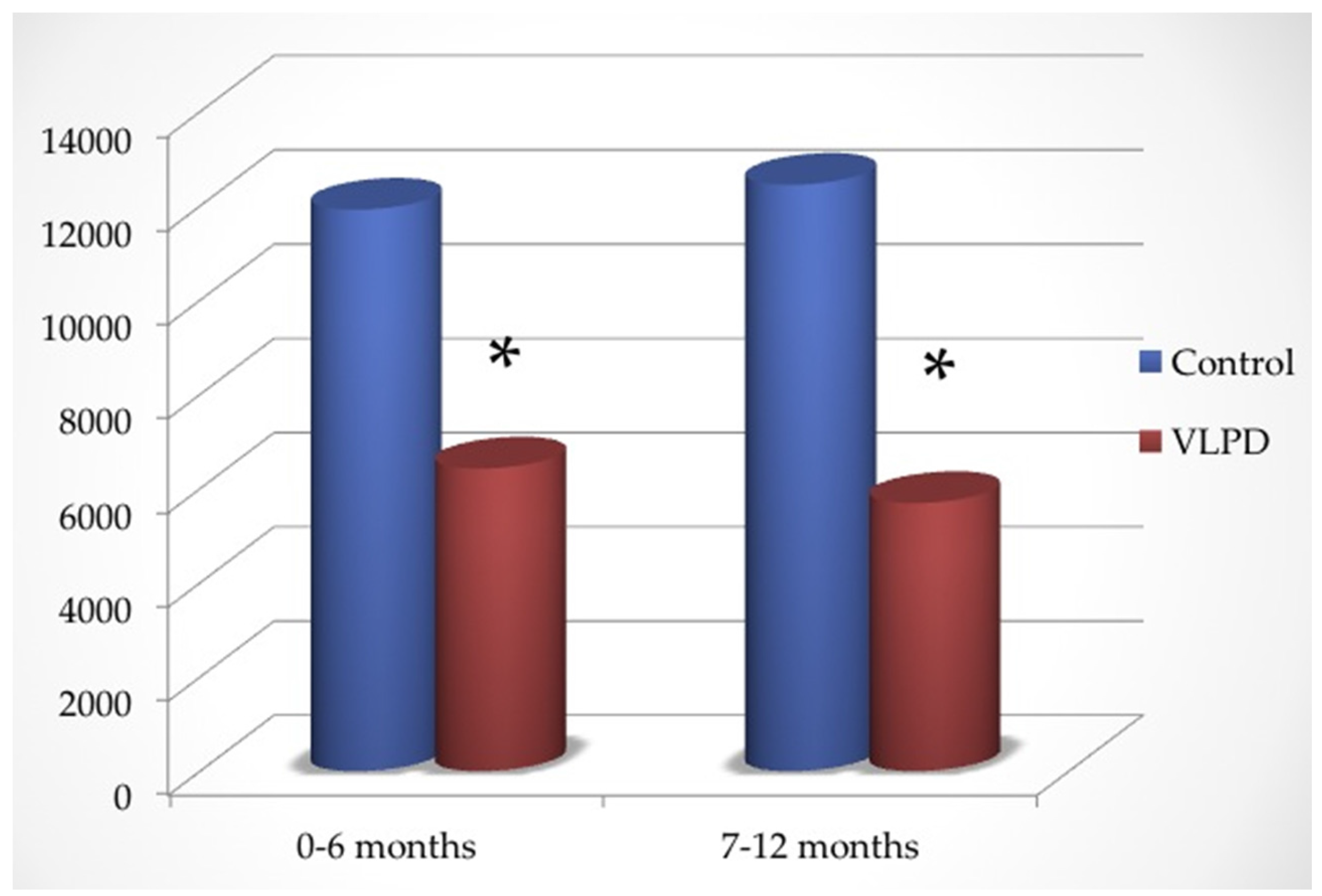
As shown in
Figure 2, in the first six months the dose of oral bicarbonate administered was 0.86 ± 0.31 (controls) versus 0.51 ± 0.33 mmol/kg/day (VLPD group) (
p < 0.0001), while in the second part of follow-up it was 0.91 ± 0.42 (controls) versus 0.48 ± 0.35 mmol/kg/day (VLPD group) (
p < 0.0001).
Total oral bicarbonate administered in the first half of follow-up was 11,919 ± 297 mmol in controls and 6426 ± 224 mmol in VLPD patients, while in the second half of follow-up it was 12,448 ± 451 in controls and 5962 ± 374 mmol in VLPD patients (
Figure 2).
Therefore, during the follow-up VLPD reduced the amount of oral bicarbonate of 30–37 mEq/day. (
Table 3).
In VLPD group, NEAP dropped from 71 ± 37 mEq/day to 33 ± 16 mEq/day (after six months) and to 25 ± 11 mEq/day (after 12 months) (p < 0.001), while in control patients it remained unchanged (from 73 ± 35 mEq/day to 71 ± 39 mEq/day after six months and to 77 ± 41 mEq/day after 12 months (p = NS). Similarly, in VLPD patients PRAL reduced from 22 ± 9 mEq/day to −4.5 ± 4.1 mEq/day after six months and to −13 ± 6 mEq/day after 12 months (p < 0.001). It was unchanged in control patients (24 ± 13 mEq/day vs. 22 ± 9 mEq/day vs. 34 ± 11 mEq/day respectively; p = NS). Therefore, in VLPD patients NEAP diminished of 53% after six months (p < 0.0001) and of 67% after 12 months (p < 0.0001); PRAL decreased of 120% after six months (p < 0.0001) and of 138% after 12 months (p < 0.0001).
5. Discussion
Beneficial effects of a correction of metabolic acidosis has been described in several studies. In 2010, Menon showed in a post-hoc analysis of MDRD study that low plasma bicarbonate levels increased the risk of outcomes such as renal death and mortality [
30]. Wesson et al. showed the paramount role of a diet rich in fruit and vegetables, not only from the nutritional point of view, but also in the nephrology field, because it ensures an amount of alkali that are needed in CKD [
31,
32,
33,
34,
35,
36,
37,
38,
39,
40,
41].
The fact that the acid load linked to animal proteins is higher than that linked to plant proteins is already known in the scientific community [
42,
43,
44]. Moe et al. showed that the use of only plant proteins, compared to animal proteins, was able to reduce daily serum and urinary phosphate levels in eight subjects, the load of sodium, calcium and phosphorus being equal [
14]. Therefore, D’Alessandro et al.’s phosphorus pyramid seems useful as tool for dietary phosphate management [
13]. Moreover, several studies showed that the use of animal proteins compared to fruit and plant proteins induced a worsening of hard outcomes such as mortality and CKD progression [
39,
44]; then, nutritional therapy has to be deeply analyzed, and nutritional prescription has to be focused not only on reduction of protein content but also on proteins’ quality [
45].
Our study has some limitations: (1) it is a post-hoc analysis of a previous study, but with a case-control design that should reduce some bias; (2) the small number of patients (again, the case-control study has the property of amplifying the number of studied subjects); and (3) a follow-up of only 12 months.
A strength of our study is the direct measure of protein intake to calculate NEAP and PRAL with the Maroni formula based on 24-h urinary urea nitrogen instead of protein intake estimated by diary interviews, and direct measure of urinary phosphate and potassium. Our data showed that VLPD (containing high quantity of fruit and vegetables and with a very low-proteins amount supplemented with essential amino-acids and ketoanalogues of non-essential amino-acids) significantly reduced NEAP and PRAL. Essential amino-acids contained in VLPD respond to metabolic need ensuring a positive nitrogen balance, thus avoiding malnutrition, while ketoanalogues allow a significant reduction of serum urea levels using urea nitrogen to transform the ketoanalgous in the corresponding no-essential amino acid, as shown in our previous studies [
46,
47].
The importance of serum urea reduction in CKD patients, somewhat neglected in recent decades, has now also been reconsidered because of its role in the etiopathogenesis of cyanate increase [
47] and of intestinal microbial flora alteration in CKD patients [
48]. Finally, a higher intake of fruit and vegetables with diet has not to be considered alarming in relation to hyperpotassiemia as long as patients have a good urine output because alkali introduction (contained in fruit and vegetables) facilitates intracellular potassium shift. Moreover, there was no difference in nutritional status before and after the study in the two groups. In fact, BMI did not change in both groups: 28 ± 4 kg/m
2 and 27 ± 4 kg/m
2 in VLPD group, and 30 ± 5 kg/m
2 and 29 ± 4 kg/m
2 in controls, before and after the study, respectively. Similarly, serum albumin was also unchanged: 38 ± 7 g/L and 38 ± 3 g/L in VLPD group, and 40 ± 5 g/L and 38 ± 3 g/L in controls, respectively.
In previous studies, VLPD produced a significant decrease of proteinuria, reducing phosphate burden and renal disease progression in CKD patients, also in patients treated with angiotensin converting enzyme-inhibitors, who did not achieve a satisfying proteinuria reduction with the drug [
15]. The same happened in our subjects, who showed a proteinuria reduction from 424 mg to 11 mg/day after 12 months of follow-up.
Furthermore, several studies showed that VLPD reduces oxidative stress in both experimental animals [
49,
50] and in humans [
51].
We did not find any depressive symptoms in our patients nor in previous published studies.
It notable that the increase of creatinine clearance from 26 to 30 mL/min after 12 months (renal function gain of 4 mL/min in one year) together with an improvement of nutritional indexes in VLPD group compared to a reduction of creatinine clearance from 39 to 30 mL/min in one year in control group (renal function loss of 9 mL/min) is suggestive of the efficacy of VLPD as nutritional therapy.
In conclusion, nutritional therapy of CKD, which has always taken into consideration a lower protein, salt, and phosphate intake, should also be adopted to correct metabolic acidosis, an important target in the treatment of CKD patients. Correction of metabolic acidosis is not only the aim of VLPD, but also of a vegetarian diet [
37,
38,
48,
52,
53].
We provide useful indications regarding acid load of food and drinks (see
Table 4A–E) that may be used as an acid load dietary “traffic light” similar to the phosphorus pyramid of D’Alessandro et al. [
13].
The green light indicates that food can be consumed every day, while the yellow light means a two or three times a week consumption, and the red light an occasional food consumption. Of course, when using the “traffic light” information, one must always consider that the phosphorus content in plant foods have a lower absorption than that contained in foods of animal origin [
12,
14]; thus, animal foods with low phosphate content can lead to a higher acid load than vegetables with higher phosphate content, because of its higher intestinal absorption. Similarly, fear of potassium in fruits and vegetables in CKD is overrated, especially in CKD patients-4 and CKD-5 with retained urine flow. In fact, in these patients, hyperkalemia can be often determined by the use of aldosterone antagonists (especially for high doses) or, to a lesser degree, of inhibitors of the renin angiotensin aldosterone system drugs, or a combination of both [
32,
33].
Finally, nutritional therapy of CKD should take into account not only a low-protein diet, but also a rational, complex and integrated control of sodium, phosphorus and acids intake with food [
18,
52,
54]. This means that a reduced intake of proteins is not the only important determinant of nutritional therapy of CKD but also the quality of proteins assumed and the amount of calories (no less than 30–34 kilocalories/body weight/day) in order to maintain a good metabolic and nutritional status and avoid malnutrition [
55,
56,
57].