Impact of Probiotic Administration on Serum C-Reactive Protein Concentrations: Systematic Review and Meta-Analysis of Randomized Control Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Selection Criteria
2.3. Data Extraction and Management
2.4. Quality Assessment
2.5. Data Synthesis
2.6. Publication Bias
3. Results
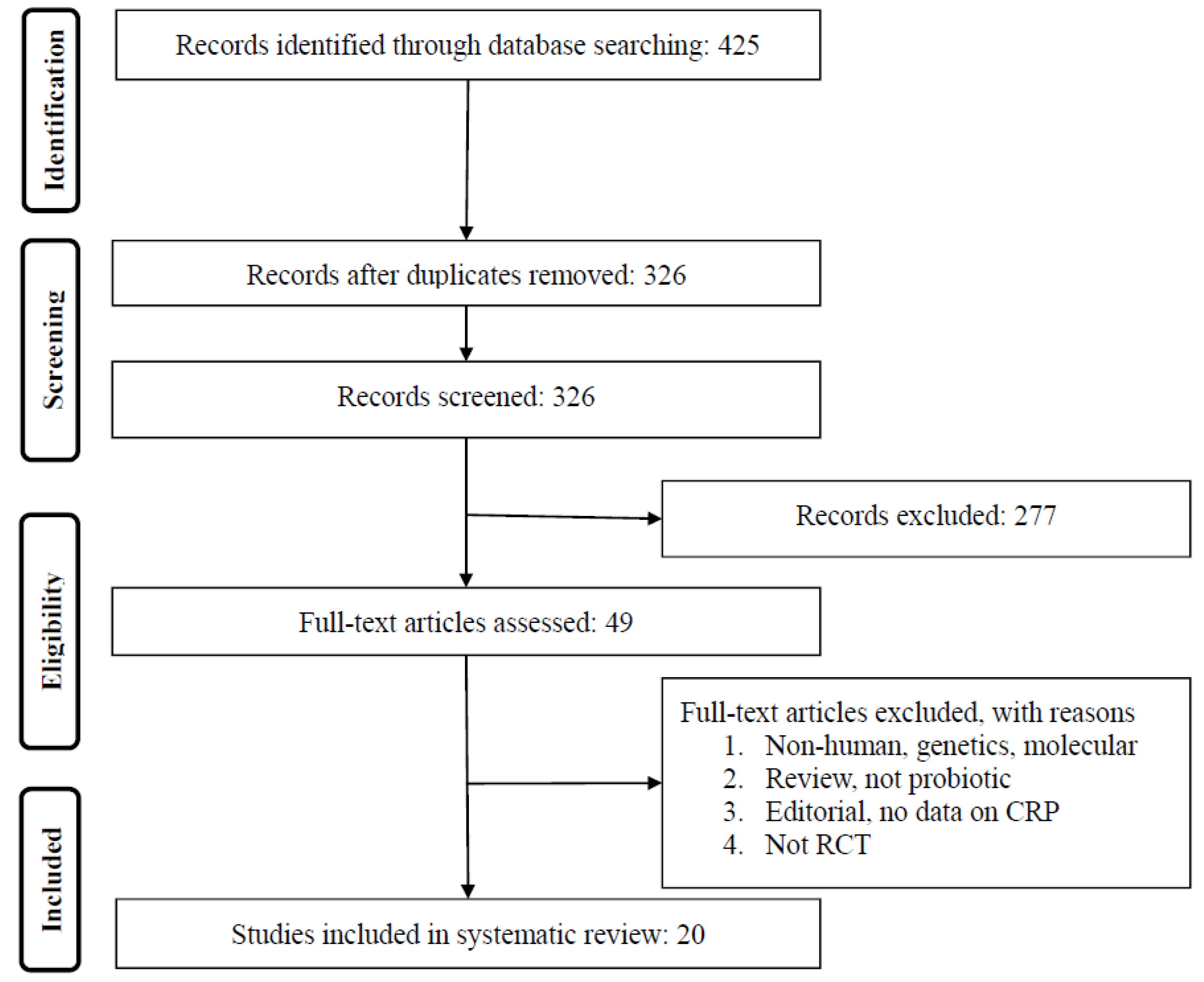
3.1. Summary of Searches and Study Selection Process
3.2. Risk of Bias Assessment
3.3. Characteristics of the Eligible Studies
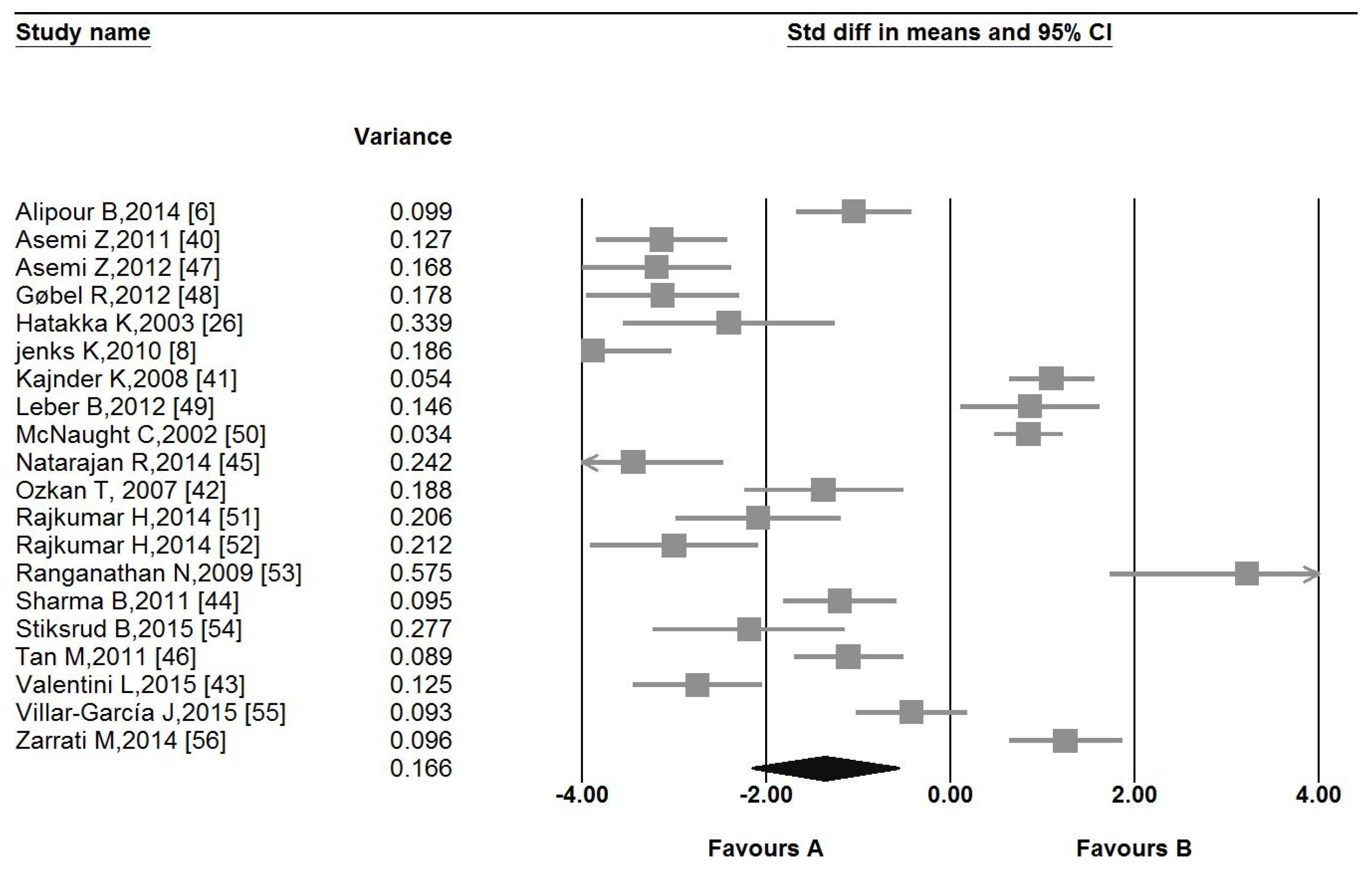
3.4. Pooled Estimate of the Effect of Probiotic Administration on CRP
3.5. Sensitivity Analysis
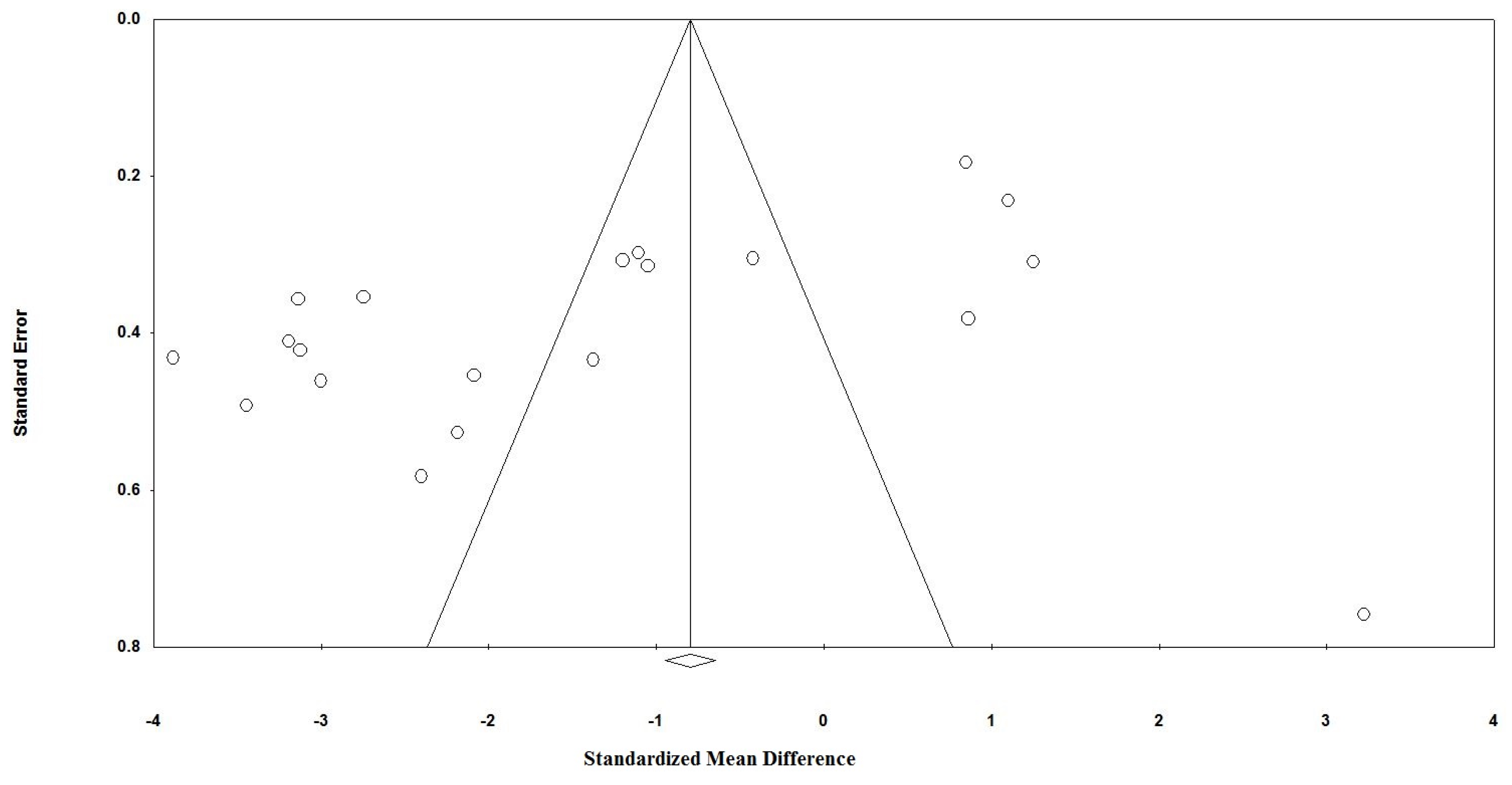
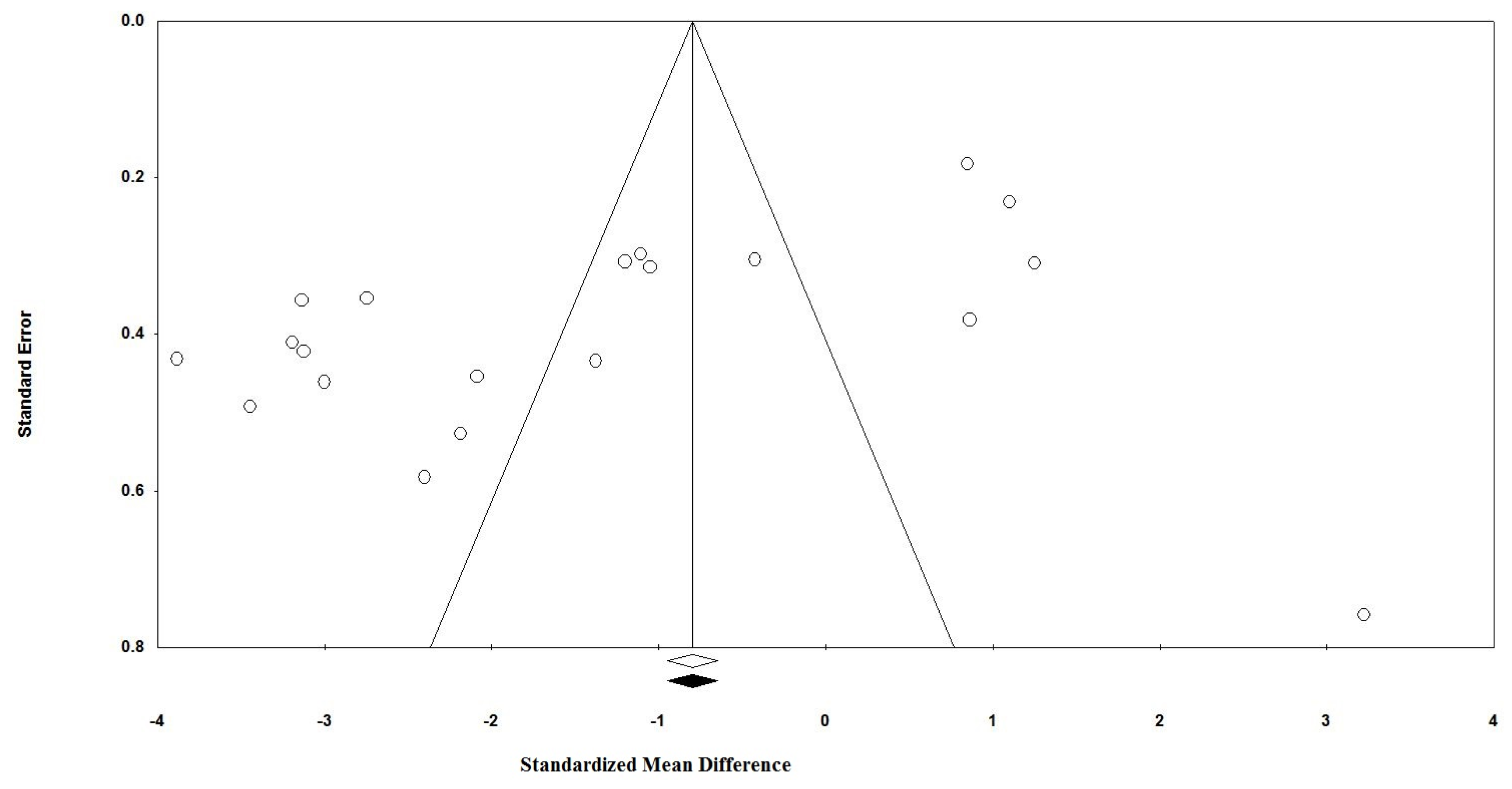
3.6. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ganguly, N.K.; Bhattacharya, S.K.; Sesikeran, B.; Nair, G.B.; Ramakrishna, B.S.; Sachdev, H.P.S.; Batish, V.K.; Kanagasabapathy, A.S.; Muthuswamy, V.; Kathuria, S.C.; et al. ICMR-DBT Guidelines for Evaluation of Probiotics in Food. Indian J. Med. Res. 2011, 134, 22–25. [Google Scholar]
- Mazidi, M.; Rezaie, P.; Kengne, A.P.; Mobarhan, M.G.; Ferns, G.A. Gut microbiome and metabolic syndrome. Diabetes Metab. Syndr. 2016, 10, S150–S157. [Google Scholar] [CrossRef] [PubMed]
- Schrezenmeir, J.; de Vrese, M. Probiotics, prebiotics, and synbiotics—Approaching a definition. Am. J. Clin. Nutr. 2001, 73, 361s–364s. [Google Scholar] [PubMed]
- Roberfroid, M.B. Prebiotics and probiotics: Are they functional foods? Am. J. Clin. Nutr. 2000, 71, 1682S–1687S. [Google Scholar] [PubMed]
- Ali, A.A.; Velasquez, M.T.; Hansen, C.T.; Mohamed, A.I.; Bhathena, S.J. Effects of soybean isoflavones, probiotics, and their interactions on lipid metabolism and endocrine system in an animal model of obesity and diabetes. J. Nutr. Biochem. 2004, 15, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Alipour, B.; Homayouni-Rad, A.; Vaghef-Mehrabany, E.; Sharif, S.K.; Vaghef-Mehrabany, L.; Asghari-Jafarabadi, M.; Nakhjavani, M.R.; Mohtadi-Nia, J. Effects of Lactobacillus casei supplementation on disease activity and inflammatory cytokines in rheumatoid arthritis patients: A randomized double-blind clinical trial. Int. J. Rheum. Dis. 2014, 17, 519–527. [Google Scholar] [PubMed]
- Saez-Lara, M.J.; Robles-Sanchez, C.; Ruiz-Ojeda, F.J.; Plaza-Diaz, J.; Gil, A. Effects of probiotics and synbiotics on obesity, insulin resistance syndrome, type 2 diabetes and non-alcoholic fatty liver disease: A review of human clinical trials. Int. J. Mol. Sci. 2016, 17, 928. [Google Scholar] [CrossRef] [PubMed]
- Jenks, K.; Stebbings, S.; Burton, J.; Schultz, M.; Herbison, P.; Highton, J. Probiotic therapy for the treatment of spondyloarthritis: A randomized controlled trial. J. Rheumatol. 2010, 37, 2118–2125. [Google Scholar] [CrossRef] [PubMed]
- Haffner, S.M. The metabolic syndrome: Inflammation, diabetes mellitus, and cardiovascular disease. Am. J. Cardiol. 2006, 97, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Heidari-Bakaboli, A.; Khayyatzadeh, S.S.; Azarpazhooh, M.R.; Nematy, M.; Safarian, M.; Esmaeili, H.; Parizadeh, S.M.; Ghayour-Mobarhan, M.; Kengne, A.P.; et al. Dietary cholesterol, but not dietary fatty acid intake, varies with serum hs-CRP concentrations in individuals free of any history of cardiovascular disease. Eur. J. Clin. Nutr. 2016, 70, 1454–1457. [Google Scholar] [CrossRef] [PubMed]
- Hermsdorff, H.H.M.; Zulet, M.Á.; Abete, I.; Martínez, J.A. A legume-based hypocaloric diet reduces proinflammatory status and improves metabolic features in overweight/obese subjects. Eur. J. Nutr. 2011, 50, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M.; Gibbs, A.L.; Mehling, C.; Chiasson, J.-L.; Connelly, P.W.; Josse, R.G.; Leiter, L.A.; Maheux, P.; Rabasa-Lhoret, R.; Rodger, N.W. The Canadian trial of carbohydrates in diabetes (CCD), a 1-y controlled trial of low-glycemic-index dietary carbohydrate in type 2 diabetes: No effect on glycated hemoglobin but reduction in C-reactive protein. Am. J. Clin. Nutr. 2008, 87, 114–125. [Google Scholar] [PubMed]
- Rizzo, M.; Rizvi, A.A.; Rini, G.B.; Berneis, K. The therapeutic modulation of atherogenic dyslipidemia and inflammatory markers in the metabolic syndrome: What is the clinical relevance? Acta Diabetol. 2009, 46, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fredrikson, G.N.; Hedblad, B.; Nilsson, J.-A.; Alm, R.; Berglund, G.; Nilsson, J. Association between diet, lifestyle, metabolic cardiovascular risk factors, and plasma C-reactive protein levels. Metabolism 2004, 53, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Selvin, E.; Paynter, N.P.; Erlinger, T.P. The effect of weight loss on C-reactive protein: A systematic review. Arch. Intern. Med. 2007, 167, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Nakano, S.; Kuboki, K.; Matsumoto, T.; Nishimura, C.; Yoshino, G. Small, dense ldl and high-sensitivity C-reactive protein (hs-CRP) in metabolic syndrome with type 2 diabetes mellitus. J. Atheroscler. Thromb. 2010, 17, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Karimi, E.; Rezaie, P.; Ferns, G.A. Treatment with glp1 receptor agonists reduce serum CRP concentrations in patients with type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. J. Diabetes Its Complicat. 2016. [Google Scholar] [CrossRef]
- Khayyatzadeh, S.S.; Moohebati, M.; Mazidi, M.; Avan, A.; Tayefi, M.; Parizadeh, S.M.; Ebrahimi, M.; Heidari-Bakavoli, A.; Azarpazhooh, M.R.; Esmaily, H.; et al. Nutrient patterns and their relationship to metabolic syndrome in Iranian adults. Eur. J. Clin. Invest. 2016, 46, 840–852. [Google Scholar] [CrossRef] [PubMed]
- Erlinger, T.P.; Miller, E.R.; Charleston, J.; Appel, L.J. Inflammation modifies the effects of a reduced-fat low-cholesterol diet on lipids results from the dash-sodium trial. Circulation 2003, 108, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Amdekar, S.; Singh, V.; Singh, R.; Sharma, P.; Keshav, P.; Kumar, A. Lactobacillus casei reduces the inflammatory joint damage associated with collagen-induced arthritis (CIA) by reducing the pro-inflammatory cytokines: Lactobacillus casei: Cox-2 inhibitor. J. Clin. Immunol. 2011, 31, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.D.; McNaught, C.E.; Jain, P.K.; MacFie, J. Randomised clinical trial of synbiotic therapy in elective surgical patients. Gut 2004, 53, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Shoaei, T.; Heidari-Beni, M.; Tehrani, H.G.; feizi, A.; Esmaillzadeh, A.; Askari, G. Effects of probiotic supplementation on pancreatic β-cell function and C-reactive protein in women with polycystic ovary syndrome: A randomized double-blind placebo-controlled clinical trial. Int. J. Prev. Med. 2015, 6, 27. [Google Scholar] [PubMed]
- Mazidi, M.; Rezaie, P.; Ferns, G.A.; Gao, H.K. Impact of different types of tree nut, peanut, and soy nut consumption on serum C-reactive protein (CRP): A systematic review and meta-analysis of randomized controlled clinical trials. Medicine 2016, 95, e5165. [Google Scholar] [CrossRef] [PubMed]
- Asemi, Z.; Zare, Z.; Shakeri, H.; Sabihi, S.S.; Esmaillzadeh, A. Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Ann. Nutr. Metab. 2013, 63, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, S.K.; El-Bedewy, M.M. Effect of probiotics on pro-inflammatory cytokines and NF-κB activation in ulcerative colitis. World J. Gastroenterol. WJG 2010, 16, 4145–4151. [Google Scholar] [CrossRef] [PubMed]
- Hatakka, K.; Martio, J.; Korpela, M.; Herranen, M.; Poussa, T.; Laasanen, T.; Saxelin, M.; Vapaatalo, H.; Moilanen, E.; Korpela, R. Effects of probiotic therapy on the activity and activation of mild rheumatoid arthritis—A pilot study. Scand. J. Rheumatol. 2003, 32, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The prisma statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Phan, K.; Tian, D.H.; Cao, C.; Black, D.; Yan, T.D. Systematic review and meta-analysis: Techniques and a guide for the academic surgeon. Ann. Cardiothorac. Surg. 2015, 4, 112–122. [Google Scholar] [PubMed]
- Mazidi, M.; Gao, H.K.; Rezaie, P.; Ferns, G.A. The effect of ginger supplementation on serum C-reactive protein, lipid profile and glycaemia: A systematic review and meta-analysis. Food Nutr. Res. 2016, 60, 32613. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Green, S.E. Cochrane Handbook for Systematic Reviews of Interventions, version 5.0.2; The Cochrane Collaboration: London, UK, 2009. [Google Scholar]
- Mazidi, M.; Rezaie, P.; Karimi, E.; Kengne, A.P. The effects of bile acid sequestrants on lipid profile and blood glucose concentrations: A systematic review and meta-analysis of randomized controlled trials. Int. J. Cardiol. 2017, 227, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Sutton, A.J.; Abrams, K.R.; Jones, D.R.; Sheldon, T.A.; Song, F. Methods for Meta-Analysis in Medical Research; John Wiley & Sons: West Sussex, UK, 2000. [Google Scholar]
- Mazidi, M.; Rezaie, P.; Vatanparast, H.; Kengne, A.P. Effect of statins on serum vitamin D concentrations: A systematic review and meta-analysis. Eur. J. Clin. Investig. 2016. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, G.; Bacchetti, T.; Sahebkar, A. Effect of statin therapy on paraoxonase-1 status: A systematic review and meta-analysis of 25 clinical trials. Prog. Lipid Res. 2015, 60, 50–73. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A. Are curcuminoids effective C-reactive protein-lowering agents in clinical practice? Evidence from a meta-analysis. Phytother. Res. 2014, 28, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Serban, M.C.; Mikhailidis, D.P.; Toth, P.P.; Muntner, P.; Ursoniu, S.; Mosterou, S.; Glasser, S.; Martin, S.S.; Jones, S.R.; et al. Head-to-head comparison of statins versus fibrates in reducing plasma fibrinogen concentrations: A systematic review and meta-analysis. Pharmacol. Res. 2016, 103, 236–252. [Google Scholar] [CrossRef] [PubMed]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.; Higgins, J.; Rothstein, H. Comprehensive Meta-Analysis, version 2; Biostat. Inc.: Englewood Cliffs, NJ, USA, 2005. [Google Scholar]
- Asemi, Z.; Jazayeri, S.; Najafi, M.; Samimi, M.; Mofid, V.; Shidfar, F.; Foroushani, A.R.; Shahaboddin, M.E. Effects of daily consumption of probiotic yoghurt on inflammatory factors in pregnant women: A randomized controlled trial. Pak. J. Biol. Sci. 2011, 14, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Kajander, K.; Myllyluoma, E.; Rajilic-Stojanovic, M.; Kyronpalo, S.; Rasmussen, M.; Jarvenpaa, S.; Zoetendal, E.G.; de Vos, W.M.; Vapaatalo, H.; Korpela, R. Clinical trial: Multispecies probiotic supplementation alleviates the symptoms of irritable bowel syndrome and stabilizes intestinal microbiota. Aliment. Pharmacol. Ther. 2008, 27, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, T.B.; Sahin, E.; Erdemir, G.; Budak, F. Effect of saccharomyces boulardii in children with acute gastroenteritis and its relationship to the immune response. J. Int. Med. Res. 2007, 35, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Valentini, L.; Pinto, A.; Bourdel-Marchasson, I.; Ostan, R.; Brigidi, P.; Turroni, S.; Hrelia, S.; Hrelia, P.; Bereswill, S.; Fischer, A.; et al. Impact of personalized diet and probiotic supplementation on inflammation, nutritional parameters and intestinal microbiota—The “ristomed project”: Randomized controlled trial in healthy older people. Clin. Nutr. 2015, 34, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Srivastava, S.; Singh, N.; Sachdev, V.; Kapur, S.; Saraya, A. Role of probiotics on gut permeability and endotoxemia in patients with acute pancreatitis: A double-blind randomized controlled trial. J. Clin. Gastroenterol. 2011, 45, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, R.; Pechenyak, B. Randomized controlled trial of strain-specific probiotic formulation (renadyl) in dialysis patients. Biomed. Res. Int. 2014, 2014, 568571. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Zhu, J.C.; Du, J.; Zhang, L.M.; Yin, H.H. Effects of probiotics on serum levels of Th1/Th2 cytokine and clinical outcomes in severe traumatic brain-injured patients: A prospective randomized pilot study. Crit. Care 2011, 15, R290. [Google Scholar] [CrossRef] [PubMed]
- Asemi, Z.; Hashemi, T.; Karamali, M.; Samimi, M.; Esmaillzadeh, A. Effects of vitamin D supplementation on glucose metabolism, lipid concentrations, inflammation, and oxidative stress in gestational diabetes: A double-blind randomized controlled clinical trial. Am. J. Clin. Nutr. 2013, 98, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Gobel, R.J.; Larsen, N.; Jakobsen, M.; Molgaard, C.; Michaelsen, K.F. Probiotics to adolescents with obesity: Effects on inflammation and metabolic syndrome. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Leber, B.; Tripolt, N.J.; Blattl, D.; Eder, M.; Wascher, T.C.; Pieber, T.R.; Stauber, R.; Sourij, H.; Oettl, K.; Stadlbauer, V. The influence of probiotic supplementation on gut permeability in patients with metabolic syndrome: An open label, randomized pilot study. Eur. J. Clin. Nutr. 2012, 66, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- McNaught, C.E.; Woodcock, N.P.; MacFie, J.; Mitchell, C.J. A prospective randomised study of the probiotic Lactobacillus plantarum 299v on indices of gut barrier function in elective surgical patients. Gut 2002, 51, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, H.; Kumar, M.; Das, N.; Kumar, S.N.; Challa, H.R.; Nagpal, R. Effect of probiotic Lactobacillus Salivarius ubl s22 and prebiotic fructo-oligosaccharide on serum lipids, inflammatory markers, insulin sensitivity, and gut bacteria in healthy young volunteers: A randomized controlled single-blind pilot study. J. Cardiovasc. Pharmacol. Ther. 2015, 20, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, H.; Mahmood, N.; Kumar, M.; Varikuti, S.R.; Challa, H.R.; Myakala, S.P. Effect of probiotic (vsl#3) and omega-3 on lipid profile, insulin sensitivity, inflammatory markers, and gut colonization in overweight adults: A randomized, controlled trial. Mediat. Inflamm. 2014, 2014, 348959. [Google Scholar]
- Ranganathan, N.; Friedman, E.A.; Tam, P.; Rao, V.; Ranganathan, P.; Dheer, R. Probiotic dietary supplementation in patients with stage 3 and 4 chronic kidney disease: A 6-month pilot scale trial in Canada. Curr. Med. Res. Opin. 2009, 25, 1919–1930. [Google Scholar] [CrossRef] [PubMed]
- Stiksrud, B.; Nowak, P.; Nwosu, F.C.; Kvale, D.; Thalme, A.; Sonnerborg, A.; Ueland, P.M.; Holm, K.; Birkeland, S.E.; Dahm, A.E.; et al. Reduced levels of d-dimer and changes in gut microbiota composition after probiotic intervention in hiv-infected individuals on stable art. J. Acquir. Immune Defic. Syndr. 2015, 70, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Villar-Garcia, J.; Hernandez, J.J.; Guerri-Fernandez, R.; Gonzalez, A.; Lerma, E.; Guelar, A.; Saenz, D.; Sorli, L.; Montero, M.; Horcajada, J.P.; et al. Effect of probiotics (saccharomyces boulardii) on microbial translocation and inflammation in HIV-treated patients: A double-blind, randomized, placebo-controlled trial. J. Acquir. Immune Defic. Syndr. 2015, 68, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Zarrati, M.; Salehi, E.; Nourijelyani, K.; Mofid, V.; Zadeh, M.J.; Najafi, F.; Ghaflati, Z.; Bidad, K.; Chamari, M.; Karimi, M.; et al. Effects of probiotic yogurt on fat distribution and gene expression of proinflammatory factors in peripheral blood mononuclear cells in overweight and obese people with or without weight-loss diet. J. Am. Coll. Nutr. 2014, 33, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, J.R.; Campbell, L.V. Relationship between inflammation, insulin resistance and type 2 diabetes: ‘Cause or effect’? Curr. Diabetes Rev. 2006, 2, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Mazloom, Z.; Yousefinejad, A.; Dabbaghmanesh, M.H. Effect of probiotics on lipid profile, glycemic control, insulin action, oxidative stress, and inflammatory markers in patients with type 2 diabetes: A clinical trial. Iran. J. Med. Sci. 2013, 38, 38–43. [Google Scholar] [PubMed]
- Sugawara, G.; Nagino, M.; Nishio, H.; Ebata, T.; Takagi, K.; Asahara, T.; Nomoto, K.; Nimura, Y. Perioperative synbiotic treatment to prevent postoperative infectious complications in biliary cancer surgery: A randomized controlled trial. Ann. Surg. 2006, 244, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Furrie, E.; Macfarlane, S.; Kennedy, A.; Cummings, J.H.; Walsh, S.V.; O’Neil, D.A.; Macfarlane, G.T. Synbiotic therapy (Bifidobacterium longum/synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: A randomised controlled pilot trial. Gut 2005, 54, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Thushara, R.M.; Gangadaran, S.; Solati, Z.; Moghadasian, M.H. Cardiovascular benefits of probiotics: A review of experimental and clinical studies. Food Funct. 2016, 7, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, N.; Caicedo, R.; Neu, J. Alive and dead Lactobacillus rhamnosus gg decrease tumor necrosis factor-alpha-induced interleukin-8 production in caco-2 cells. J. Nutr. 2005, 135, 1752–1756. [Google Scholar] [PubMed]
- Gill, H.S.; Rutherfurd, K.J.; Cross, M.L.; Gopal, P.K. Enhancement of immunity in the elderly by dietary supplementation with the probiotic Bifidobacterium Lactis hn019. Am. J. Clin. Nutr. 2001, 74, 833–839. [Google Scholar] [PubMed]
- Taghizadeh, M.; Asemi, Z. Effects of synbiotic food consumption on glycemic status and serum hs-CRP in pregnant women: A randomized controlled clinical trial. Hormones 2014, 13, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Gaur, P.; Aggarwal, A. Effect of probiotics on clinical and immune parameters in enthesitis-related arthritis category of juvenile idiopathic arthritis. Clin. Exp. Immunol. 2016, 185, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Jiang, X.Y.; Zhou, L.S.; Song, J.H.; Zhang, X. Effects of probiotics on intestinal mucosa barrier in patients with colorectal cancer after operation: Meta-analysis of randomized controlled trials. Medicine 2016, 95, e3342. [Google Scholar] [CrossRef] [PubMed]
- Nuesch, E.; Trelle, S.; Reichenbach, S.; Rutjes, A.W.; Tschannen, B.; Altman, D.G.; Egger, M.; Juni, P. Small study effects in meta-analyses of osteoarthritis trials: Meta-epidemiological study. BMJ 2010, 341, c3515. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Gavaghan, D.; Egger, M. Publication and related bias in meta-analysis: Power of statistical tests and prevalence in the literature. J. Clin. Epidemiol. 2000, 53, 1119–1129. [Google Scholar] [CrossRef]
| First Author, Reference | Country | Total Sample Size (% Female) | Study Design | Probiotics Features | Delivery Method | Duration (Weeks) | Age (Years) | C-reactive Protein (CRP) Assay | Background Disease | Sample Size |
|---|---|---|---|---|---|---|---|---|---|---|
| Alipour B, 2014 [6] | Iran | 44 (100%) | Randomized, double-blind, placebo-controlled trial, | 108 colony forming units (CFU) of L. casei 01 and maltodextrin | Orally | 8 weeks | Test: 44.29; Control: 41.14 | Turbidometric assay | Rheumatoid arthritis | 30 |
| Asemi Z, 2011 [40] | Iran | 35 (100%) | Prospective, randomized, single-blinded clinical trial | Probiotic yogurt enriched with Lactobacillus acidophilus and Bifidobacterium animalis | Orally | 9 weeks | Test: 24.2; Control: 25.7 | Enzyme-linked immunosorbent assay (ELISA) | Healthy | 37 |
| Asemi Z, 2013 [47] | Iran | 54 (70%) | Randomized double-blinded controlled clinical trial | Lactobacillus acidophilus (2 × 109 CFU), L. casei (7 × 109 CFU), L. rhamnosus (1.5 × 109 CFU), L. bulgaricus (2 × 108 CFU), Bifidobacterium breve (2 × 1010 CFU), B. longum (7 × 109 CFU), Streptococcus thermophilus (1.5 × 109 CFU) | Orally | 8 weeks | Test: 52.59; Control: 50.51 | ELISA | Diabetic patients | 54 |
| Gobel R, 2012 [48] | Denmark | 50 (Test: 52%; Control: 59.2) | Double-blinded, randomized, placebo controlled intervention study | L. salivarius (1010 CFU) | Orally | 12 weeks | 12–15 | Specific high-sensitivity CRP | Obese | 50 |
| Hattakka K, 2003 [26] | Finland | 21 (Test: 100%; Control: 61.5%) | Randomized, double-blind, placebo-controlled study | Lactobacillus rhamnosus, 5 × 109 CFU | Orally | 12 weeks | Test: 50; Control: 53 | ------- | Rheumatoid arthritis | 21 |
| Jenks K, 2010 [8] | New Zealand | 62 (Test: 41%; Control: 32%) | Randomized controlled trial | Streptococcus salivarius, Bifidobacterium lactis, and Lactobacillus acidophilus | Orally | 12 weeks | Test: 45.5; Control: 41.1 | ELISA | Spondyloarthritis | 63 |
| Kajander K, 2007 [41] | Finland | 86 (Test: 5%; Control: 91%) | Randomized double-blind, placebo-controlled, | L. rhamnosus GG, L. rhamnosus, P. freudenreichii ssp. shermanii JS, 107 CFU | Orally | 5 months | Test: 50; Control: 46 | Particle-enhanced immunoturbidimetric assay | Irritable bowel syndrome patients | 86 |
| Leber B, 2012 [49] | Austria | 30 (Test: 30.7%; Control: 40%) | An open label, randomized pilot study | L. casei Shirota, 6.5 × 109 CFU | Orally | 3 months | Test: 51.5; Control: 54.5 | ----- | Metabolic syndrome | 28 |
| Mc Naught C, 2002 [50] | Sweden | 130 (Test: 39%; Control: 44.6%) | Prospective and randomized | Lactobacillus plantarum, 5 × 107 CFU | Orally | 2 weeks | Test: 68; Control: 69 | ----- | Surgical patients | 129 |
| Natarajan R, 2014 [45] | USA | 41 (16.7%) | Randomized, double-blind, placebo-controlled crossover study | Renadyl | Orally | 6 months | 29–79 | ----- | End-stage renal disease | ----- |
| Ozkan T, 2007 [42] | Turkey | 27 (44.4%) | Prospectively | Saccharomyces boulardii | Orally | 7 days | 6 months to 10 years | ELISA | Healthy | 27 |
| Rajkumar H, 2014 [51] | India | 30 (53.8%) | A randomized controlled single-blind pilot study | Lactobacillus salivarius, 2 × 109 CFU | Orally | 6 weeks | 20–25 | dbc-hs Krishgen | Healthy | 45 |
| Rajkumar H, 2014 [52] | India | 40 (50%) | A randomized, controlled trial | Lyophilized Bifidobacteria, Lactobacilli, and Streptococcus thermophilus, 112.5 × 109 CFU | Orally | 6 weeks | 40–60 | dbc-hs Krishgen | Healthy | 60 |
| Ranganathan N, 2009 [53] | Canada | 16 (30.7%) | Pilot scale trial | L. acidophilus B. longum and S. thermophilus, 1.5 × 1010 CFU | Orally | 6 months | 54 | ----- | Chronic kidney disease | 16 |
| Sharma B, 2011 [44] | India | 50 (Test: 57.6%; Control: 50%) | A double-blind randomized placebo-controlled trial | Lactobacillus acidophilus, Bifidobacterium longus, Bifidobacterium bifidum, and Bifidobacterium infantalis | Oral, nasojejunal, alternatively, nasogastric | 7 days | Test: 40.19; Control: 41 | ELISA | Acute pancreatitis | 50 |
| Stiksrud B, 2015 [54] | Norway | 24 (Test: 28.6%; Control: 100%) | Randomized in a double-blind | Lactobacillus rhamnosus, Bifidobacterium animalis subsp. lactis and Lactobacillus acidophilus La-5, 108 CFU | Orally | 8 weeks | Test: 50.3; Control: 52.5 | ----- | Patients on antiretroviral therapy | 32 |
| Tan M, 2011 [46] | China | 52 (Test: 26.9%; Control: 19.2%) | A prospective, randomized pilot study | Bifidobacterium longum, Lactobacillus bulgaricus, and Streptococcus thermophilus, 109 CFU | nasogastric tube | 21 days | Test: 40.5; Control: 40.8 | ELISA | Traumatic brain injury | 26 |
| Valentini L, 2015 [43] | France, Germany, Italy | 62 (53.2%) | Randomized controlled trial | Bifidobacterium infantis, Bifidobacterium longum, Bifidobacterium breve, Lactobacillus acidophilus, Lactobacillus delbrückii ssp. bulgaricus, Lactobacillus paracasei, Lactobacillus plantarum | Orally | 8 weeks | 65–85 | ELISA | Healthy | 62 |
| Villar Garcia J, 2015 [55] | Spain | 44 (Test: 9.1%; Control: 22.7%) | A single-center, randomized, double-blind, placebo-controlled pilot study | S. boulardii, 6 × 107 CFU | Orally | 12 weeks | Test: 49.45; Control: 45.5 | Immulite chemiluminescent immunometric assay | HIV-1–infected patients with virologic suppression | 44 |
| Zarrati M, 2014 [56] | Iran | 50 (68%) | Randomized double-blind controlled clinical trial | Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium lactis, 108 CFU | Orally | 8 weeks | 20–50 | ----- | Overweight and obese individuals | 75 |
| Factors | Results of the Pooled Estimate |
|---|---|
| IL10 | −1.65 pg/dL, (95% CI −3.45 to 0.14) |
| TNF-α | −0.45 pg/mL, (95% CI −1.38 to 0.48) |
| IL1β | −1.07 pg/dL, (95% CI −1.55 to −0.59) |
| TG | −0.92 mg/dL, (95% CI −1.22 to −0.62) |
| TC | −0.58 mg/dL, (95% CI −0.84 to −0.32) |
| LDL | −1.36 mg/dL, (95% CI −1.70 to −1.02) |
| HDL | 0.51 mg/dL, (95% CI 0.19 to 0.83) |
| FBG | −0.75 mg/dL, (95% CI −1.11 to −0.38) |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazidi, M.; Rezaie, P.; Ferns, G.A.; Vatanparast, H. Impact of Probiotic Administration on Serum C-Reactive Protein Concentrations: Systematic Review and Meta-Analysis of Randomized Control Trials. Nutrients 2017, 9, 20. https://doi.org/10.3390/nu9010020
Mazidi M, Rezaie P, Ferns GA, Vatanparast H. Impact of Probiotic Administration on Serum C-Reactive Protein Concentrations: Systematic Review and Meta-Analysis of Randomized Control Trials. Nutrients. 2017; 9(1):20. https://doi.org/10.3390/nu9010020
Chicago/Turabian StyleMazidi, Mohsen, Peyman Rezaie, Gordon A. Ferns, and Hassan Vatanparast. 2017. "Impact of Probiotic Administration on Serum C-Reactive Protein Concentrations: Systematic Review and Meta-Analysis of Randomized Control Trials" Nutrients 9, no. 1: 20. https://doi.org/10.3390/nu9010020
APA StyleMazidi, M., Rezaie, P., Ferns, G. A., & Vatanparast, H. (2017). Impact of Probiotic Administration on Serum C-Reactive Protein Concentrations: Systematic Review and Meta-Analysis of Randomized Control Trials. Nutrients, 9(1), 20. https://doi.org/10.3390/nu9010020






