Platycodon grandiflorus Root Extract Attenuates Body Fat Mass, Hepatic Steatosis and Insulin Resistance through the Interplay between the Liver and Adipose Tissue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Platycodon grandiflorus Root Extract
2.2. Animals and Diets
3. Results
3.1. Composition of Platycosides in PGE
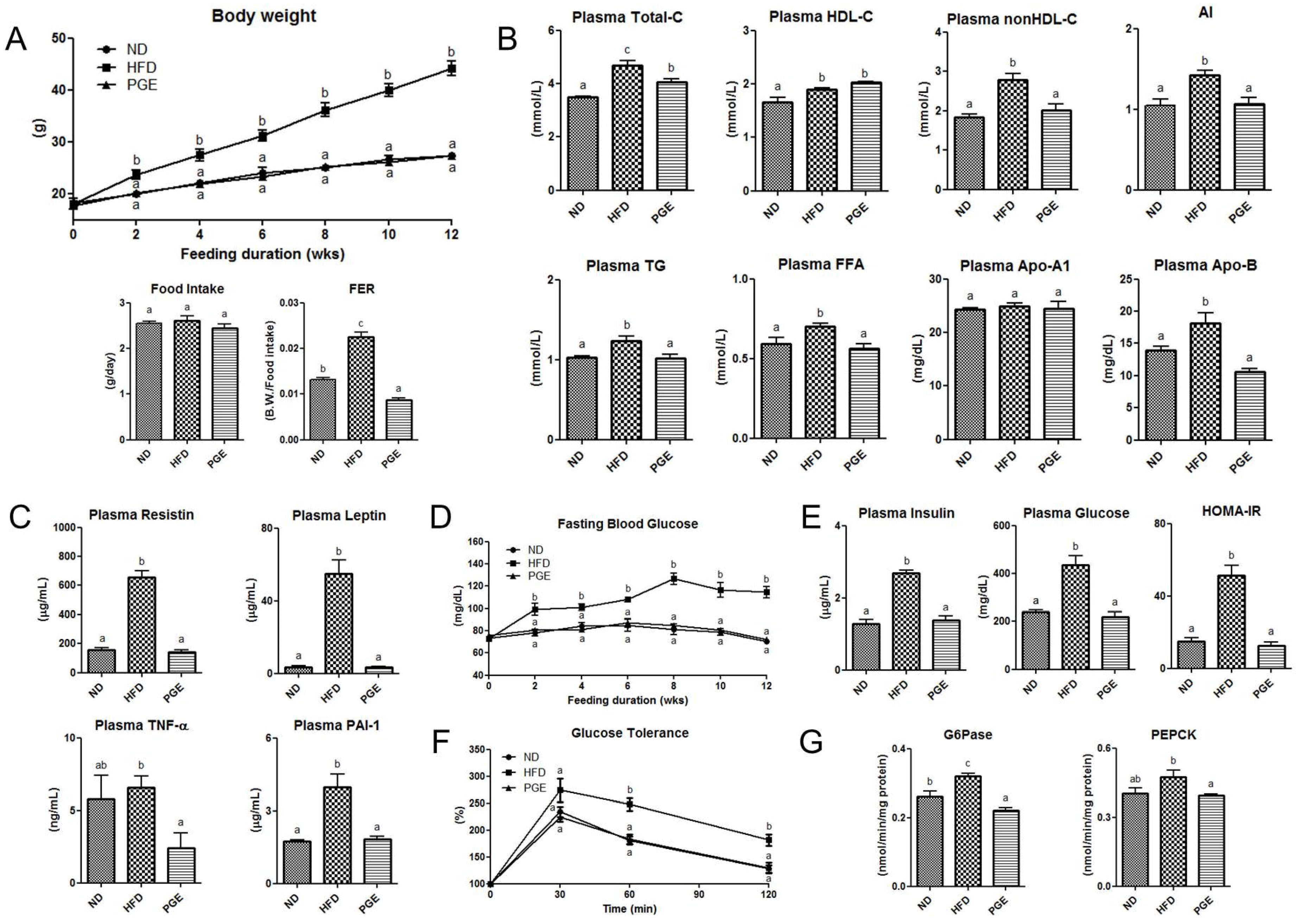
3.2. PGE Supplement Lowered Body Weight Gain and Improved Plasma Lipid Profiles and Adipokine Levels in DIO Mice
3.3. PGE Improved Insulin Resistance and Glucose Tolerance by Modulating Activities of Hepatic Glucose-Regulating Enzymes in DIO Mice
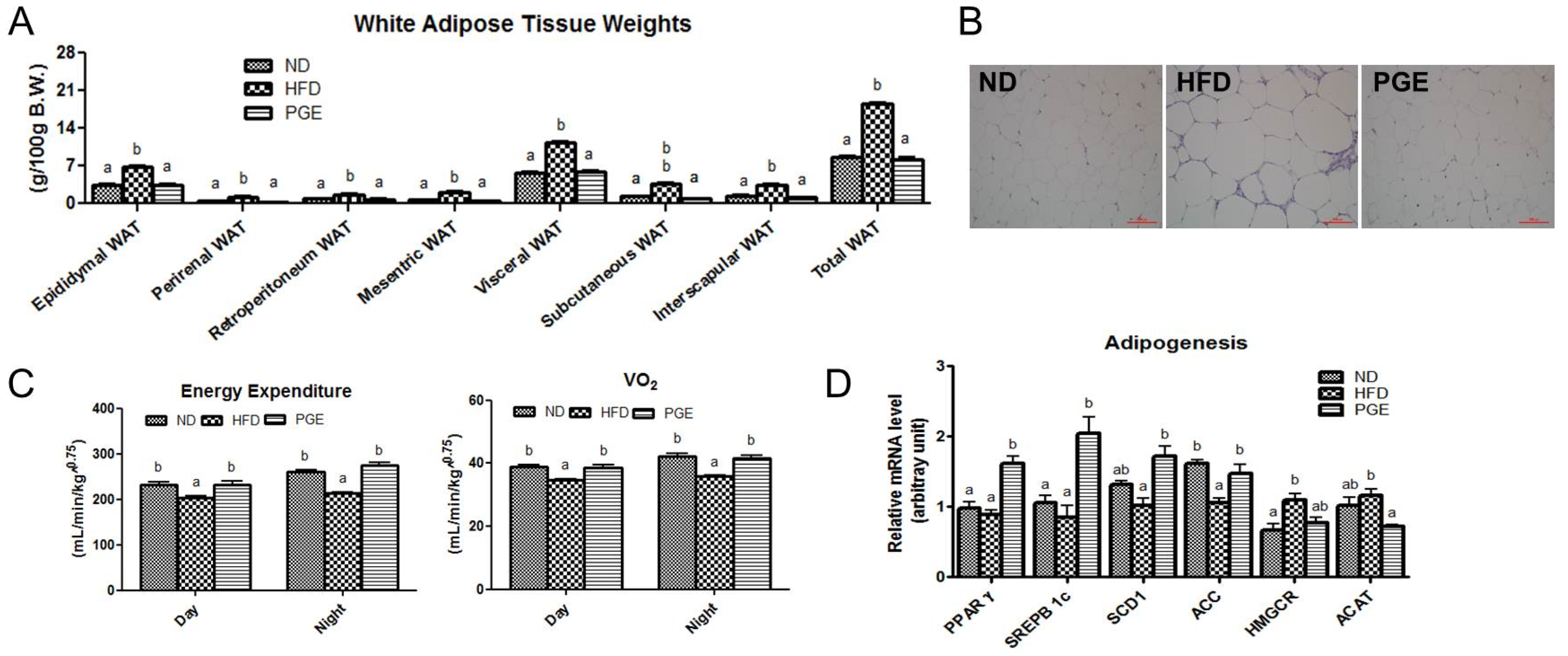
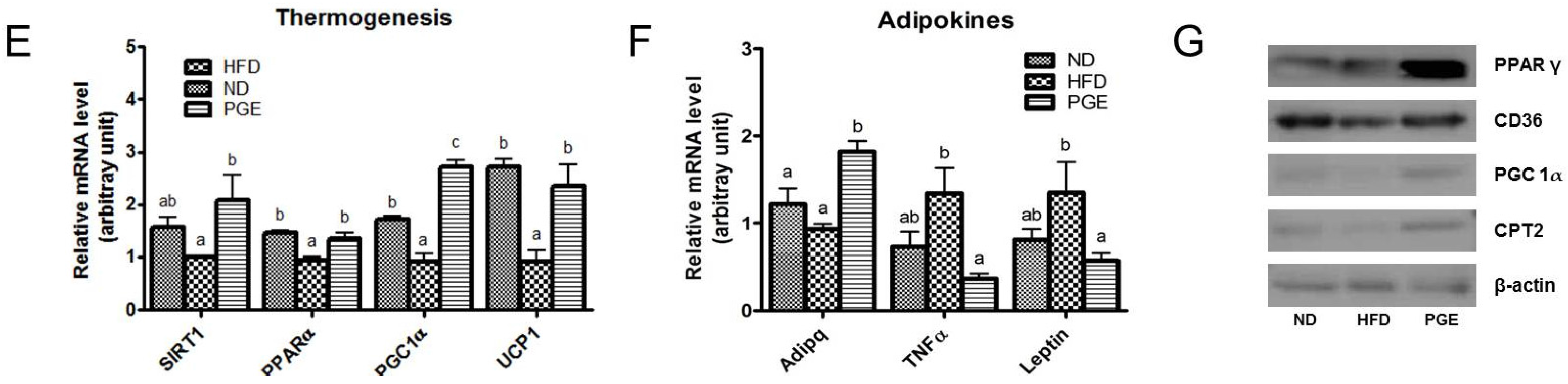
3.4. PGE Supplement Decreased Body Fat Mass by Increasing Fatty Acid Oxidation-Related Gene Expression and Energy Expenditure in DIO Mice
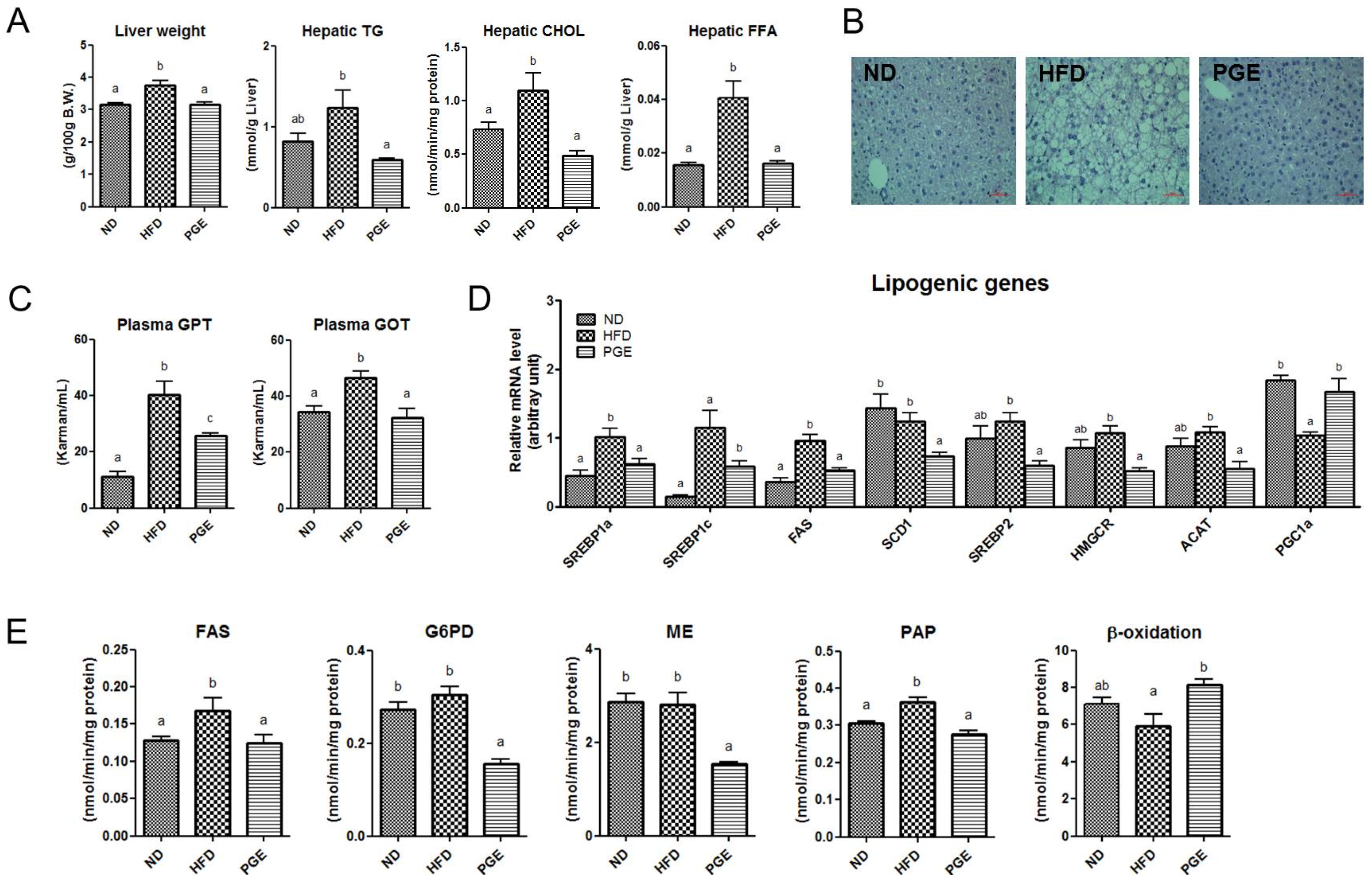
3.5. PGE Supplement Lowered the Levels of Hepatic Lipids and Lipotoxicity Markers by Altering Hepatic Lipogenic Gene Expression and Enzyme Activities in DIO Mice
4. Discussion
4.1. PGE Normalized Body Weight Gain and Fat Mass and Increased Expression of Browning Markers in eWAT
4.2. PGE Lowered Inflammatory Adipokines and Improved Insulin Resistance and Hepatic Steatosis by Alteration of Lipogenic Gene Expression and Glucose Metabolism
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ACAT | acetyl-CoA acetyltransferase |
| ACC | acetyl-CoA carboxylase |
| BAT | brown adipose tissue |
| FAS | fatty acid synthase |
| HFD | high-fat diet |
| HMGCR | 3-hydroxy-3-methylglutaryl-CoA reductase |
| PPARγ | peroxisome proliferator-activated receptor gamma |
| PGC1α | PPARγ coactivator-1α |
| PGE | Platycodon grandiflorus root ethanol extract |
| SCD1 | stearoyl-CoA desaturase1 |
| SIRT1 | sirtuin1 |
| SREBP | sterol regulatory element-binding proteins |
| TNF-α | tumor necrosis factor alpha |
| UCP1 | uncoupling protein 1 |
| WAT | white adipose tissue |
| eWAT | epididydimal white adipose tissue |
References
- Akagiri, S.; Naito, Y.; Ichikawa, H.; Mizushima, K.; Takagi, T.; Handa, O.; Kokura, S.; Yoshikawa, T. Bofutsushosan, an oriental herbal medicine, attenuates the weight gain of white adipose tissue and the increased size of adipocytes associated with the increase in their expression of uncoupling protein 1 in high-fat diet-fed male KK/Ta mice. J. Clin. Biochem. Nutr. 2008, 42, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Patil, I.Y.; Jiang, T.; Sancheti, H.; Walsh, J.P.; Stiles, B.L.; Yin, F.; Cadenas, E. High-fat diet induces hepatic insulin resistance and impairment of synaptic plasticity. PLoS ONE 2015, 10, e0128274. [Google Scholar] [CrossRef] [PubMed]
- Chalkiadaki, A.; Guarente, L. High-fat diet triggers inflammation-induced cleavage of SIRT1 in adipose tissue to promote metabolic dysfunction. Cell. Metab. 2012, 16, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Choi, M.-S.; Cha, B.Y.; Woo, J.T.; Park, Y.B.; Kim, S.R.; Jung, U.J. Long-term supplementation of honokiol and magnolol ameliorates body fat accumulation, insulin resistance, and adipose inflammation in high-fat fed mice. Mol. Nutr. Food Res. 2013, 57, 1988–1998. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Choi, M.-S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6182–6223. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.-Y.; Jung, U.J.; Park, T.; Yun, J.W.; Choi, M.-S. Luteolin attenuates hepatic steatosis and insulin resistance through the interplay between the liver and adipose tissue in diet-induced obese mice. Diatetes 2015, 64, 1–12. [Google Scholar]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1a. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Teng, R.; Di, L.; Rogers, H.; Wu, H.; Kopp, J.B.; Noguchi, C.T. PPARα and Sirt1 Mediate Erythropoietin Action in Increasing Metabolic Activity and Browning of White Adipocytes to Protect against Obesity and Metabolic Disorders. Diabetes 2013, 62, 4122–4131. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Choi, M.-S.; Seo, K.-I.; Lee, H.I.; Lee, J.H.; Kim, M.J.; Lee, M.K. Platycodi radix saponin inhibits α-glucosidase in vitro and modulates hepatic glucose-regulating enzyme activities in C57BL/KsJ -db/db mice. Arch. Pharm. Res. 2014, 37, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Du, X.; Zhang, Y.; Wang, R. Platycodin D, a triterpenoid saponin from Platycodon grandiflorum, induces G2/M arrest and apoptosis in human hepatoma HepG2 cells by modulating the PI3K/Akt pathway. Tumour Biol. 2014, 35, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Yang, D.; Zhang, C.; Zhang, N.; Li, M.; Liu, Y. Platycodon grandiflorus—An ethnopharmacological, phytochemical and pharmacological review. J. Ethnopharmacol. 2015, 164, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.; Martin, T.L.; Surwit, R.S.; Robidoux, J. Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: Physiological and molecular characteristics. Physiol. Behav. 2004, 81, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Wang, Y.; Lu, S.F.; Hong, H.; Fu, S.; He, S.; Li, Q.; Yue, J.; Xu, B.; Zhu, B.M. Acupuncture promotes white adipose tissue browning by inducing UCP1 expression on DIO mice. BMC Complement. Altern. Med. 2014, 501, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vergnes, L.; Reue, K. Adaptive Thermogenesis in White Adipose Tissue: Is Lactate the New Brown (ing)? Diabetes 2014, 63, 3175–3176. [Google Scholar] [CrossRef] [PubMed]
- Cariou, B.; Charbonnel, B.; Staels, B. Thiazolidinediones and PPARγ agonists: Time for a reassessment. Trends. Endocrinol. Metab. 2012, 23, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.T.; Lerin, C.; Haas, W.; Gygi, S.P.; Spiegelman, B.M.; Puigserver, P. Nutrient control of glucose homeostasis through a complex of PGC-1 alpha and SIRT1. Nature 2005, 434, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef]
- Qian, S.-W.; Tang, Y.; Li, X.; Liu, Y.; Zhang, Y.Y.; Huang, H.Y.; Xue, R.D.; Yu, H.Y.; Guo, L.; Gao, H.D.; et al. BMP4-mediated brown fat-like changes in white adipose tissue alter glucose and energy homeostasis. Proc. Natl. Acad. Sci. USA 2013, 110, E798–E807. [Google Scholar] [CrossRef] [PubMed]
- Rachid, T.L.; Penna-de-Carvalho, A.; Bringhenti, I.; Aguila, M.B.; Mandarim-de-Lacerda, C.A.; Souza-Mello, V. Fenofibrate (PPAR alpha agonist) induces beige cell formation in subcutaneous white adipose tissue from diet-induced male obese mice. Mol. Cell. Endocrinol. 2015, 402, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Samuel, V.T.; Petersen, K.F.; Shulman, G.I. Lipid-induced insulin resistance: Unravelling the mechanism. Lancet 2010, 375, 2267–2277. [Google Scholar] [CrossRef]
- Tsuchida, A.; Yamauchi, T.; Takekawa, S.; Hada, Y.; Ito, Y.; Maki, T.; Kadowaki, T. Peroxisome proliferator-activated receptor (PPAR) alpha activation increases adiponectin receptors and reduces obesity-related inflammation in adipose tissue: Comparison of activation of PPARalpha, PPARgamma, and their combination. Diabetes 2005, 54, 3358–3370. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.P.; Soni, S.; Parikh, P.; Gosai, J.; Chruvattil, R.; Gupta, S. Swertiamarin: An active lead from Enicostemma littorale regulates hepatic and adipose tissue gene expression by targeting PPAR-γ and improves insulin sensitivity in experimental NIDDM rat model. Evid. Based Complement. Altern. Med. 2013. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Waki, H.; Terauchi, Y.; Kubota, N.; Hara, K.; Mori, Y.; Ide, T.; Murakami, K.; Tsuboyama-Kasaoka, N.; et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001, 7, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.; Catalano, K.J.; Ananthnarayan, S.; Kim, S.P.; Van Citters, G.W.; Dea, M.K.; Bergman, R.N. Molecular evidence supporting the portal theory: A causative link between visceral adiposity and hepatic insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E454–E461. [Google Scholar] [CrossRef] [PubMed]
- Lomonaco, R.; Ortiz-Lopez, C.; Orsak, B.; Webb, A.; Hardies, J.; Darland, C.; Finch, J.; Gastaldelli, A.; Harrison, S.; Tio, F.; et al. Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in obese patients with nonalcoholic fatty liver disease. Hepatology 2012, 55, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.J.; Choi, J.-Y.; Ryu, R.; Lee, J.; Cho, S.-J.; Kwon, E.-Y.; Lee, M.-K.; Liu, K.-H.; Rina, Y.; Sung, M.-K.; et al. Platycodon grandiflorus Root Extract Attenuates Body Fat Mass, Hepatic Steatosis and Insulin Resistance through the Interplay between the Liver and Adipose Tissue. Nutrients 2016, 8, 532. https://doi.org/10.3390/nu8090532
Kim YJ, Choi J-Y, Ryu R, Lee J, Cho S-J, Kwon E-Y, Lee M-K, Liu K-H, Rina Y, Sung M-K, et al. Platycodon grandiflorus Root Extract Attenuates Body Fat Mass, Hepatic Steatosis and Insulin Resistance through the Interplay between the Liver and Adipose Tissue. Nutrients. 2016; 8(9):532. https://doi.org/10.3390/nu8090532
Chicago/Turabian StyleKim, Ye Jin, Ji-Young Choi, Ri Ryu, Jeonghyeon Lee, Su-Jung Cho, Eun-Young Kwon, Mi-Kyung Lee, Kwang-Hyeon Liu, Yu Rina, Mi-Kyung Sung, and et al. 2016. "Platycodon grandiflorus Root Extract Attenuates Body Fat Mass, Hepatic Steatosis and Insulin Resistance through the Interplay between the Liver and Adipose Tissue" Nutrients 8, no. 9: 532. https://doi.org/10.3390/nu8090532
APA StyleKim, Y. J., Choi, J.-Y., Ryu, R., Lee, J., Cho, S.-J., Kwon, E.-Y., Lee, M.-K., Liu, K.-H., Rina, Y., Sung, M.-K., & Choi, M.-S. (2016). Platycodon grandiflorus Root Extract Attenuates Body Fat Mass, Hepatic Steatosis and Insulin Resistance through the Interplay between the Liver and Adipose Tissue. Nutrients, 8(9), 532. https://doi.org/10.3390/nu8090532





