Protective Effect of Garlic on Cellular Senescence in UVB-Exposed HaCaT Human Keratinocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Garlic Extract
2.2. Antioxidant Activity
2.3. Cell Culture and UV Irradiation
2.4. ROS Production
2.5. Quantitative Real Time RT-PCR
2.6. MMP-1 Production
2.7. Cytokine Determination
2.8. Senescence-Associated β-Galactosidase (SA-β-gal) Histochemical Staining
2.9. SIRT1 Activity
2.10. Analysis of Organosulfur Compound
2.11. Statistical Analysis
3. Results
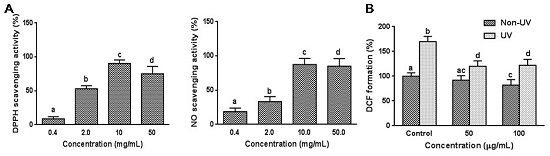
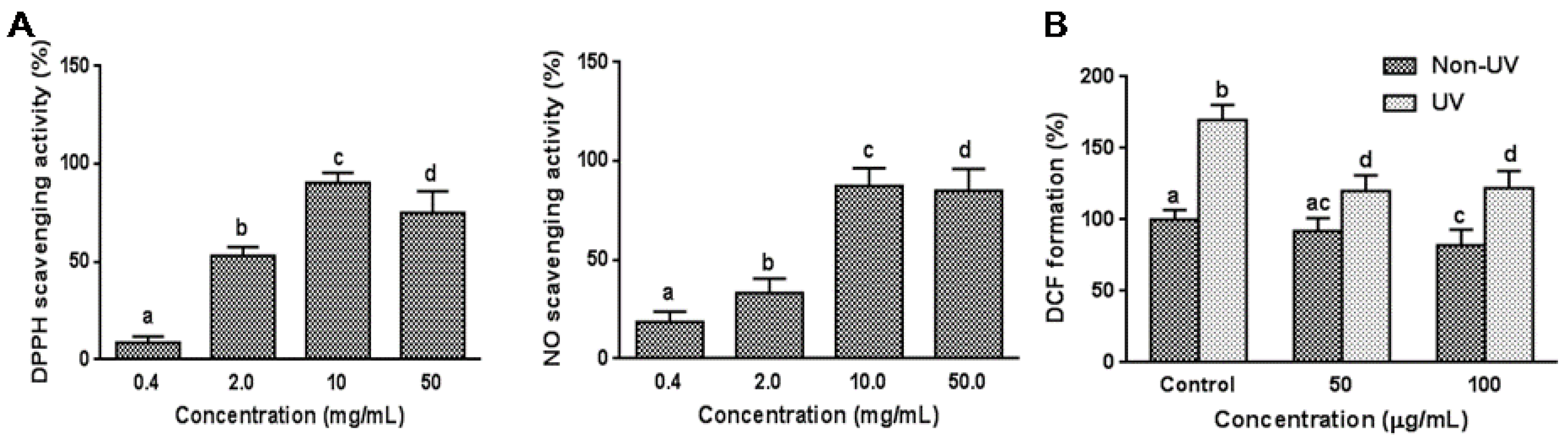
3.1. Effect on Cell Free System Radical Scavenging Activity
3.2. Effect on UVB-Induced ROS Generation in HaCaT Cells
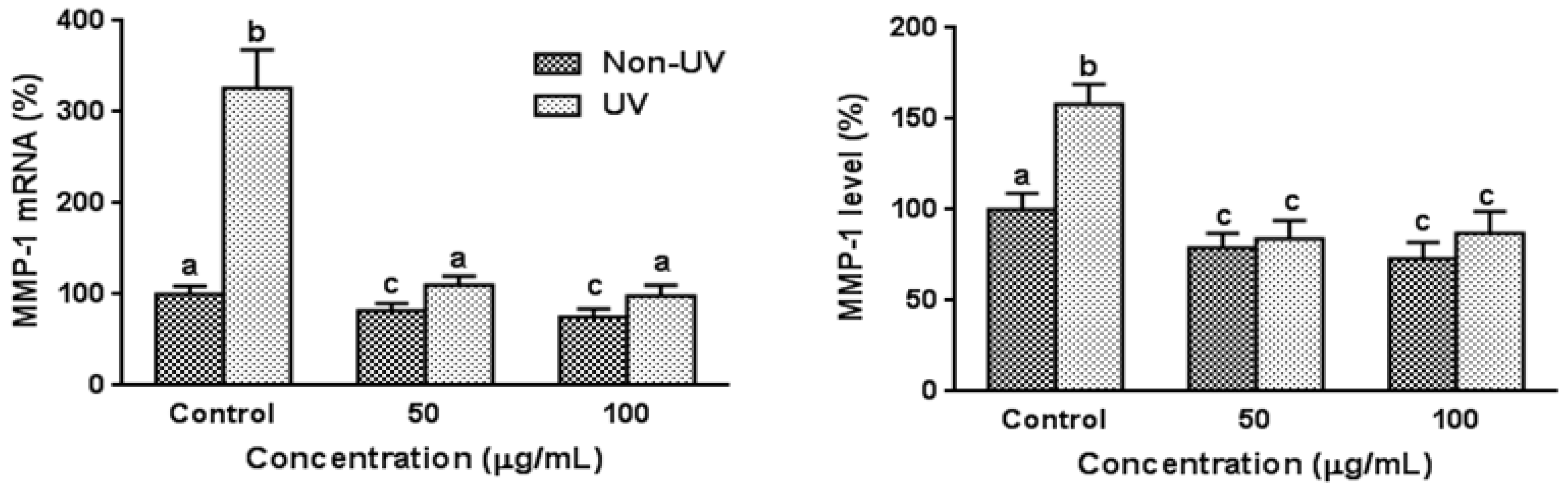
3.3. Effect on UVB-Induced MMP-1 Expression in HaCaT Cells
3.4. Effect on UVB-Induced Pro-Inflammatory Cytokine Production in HaCaT Cells
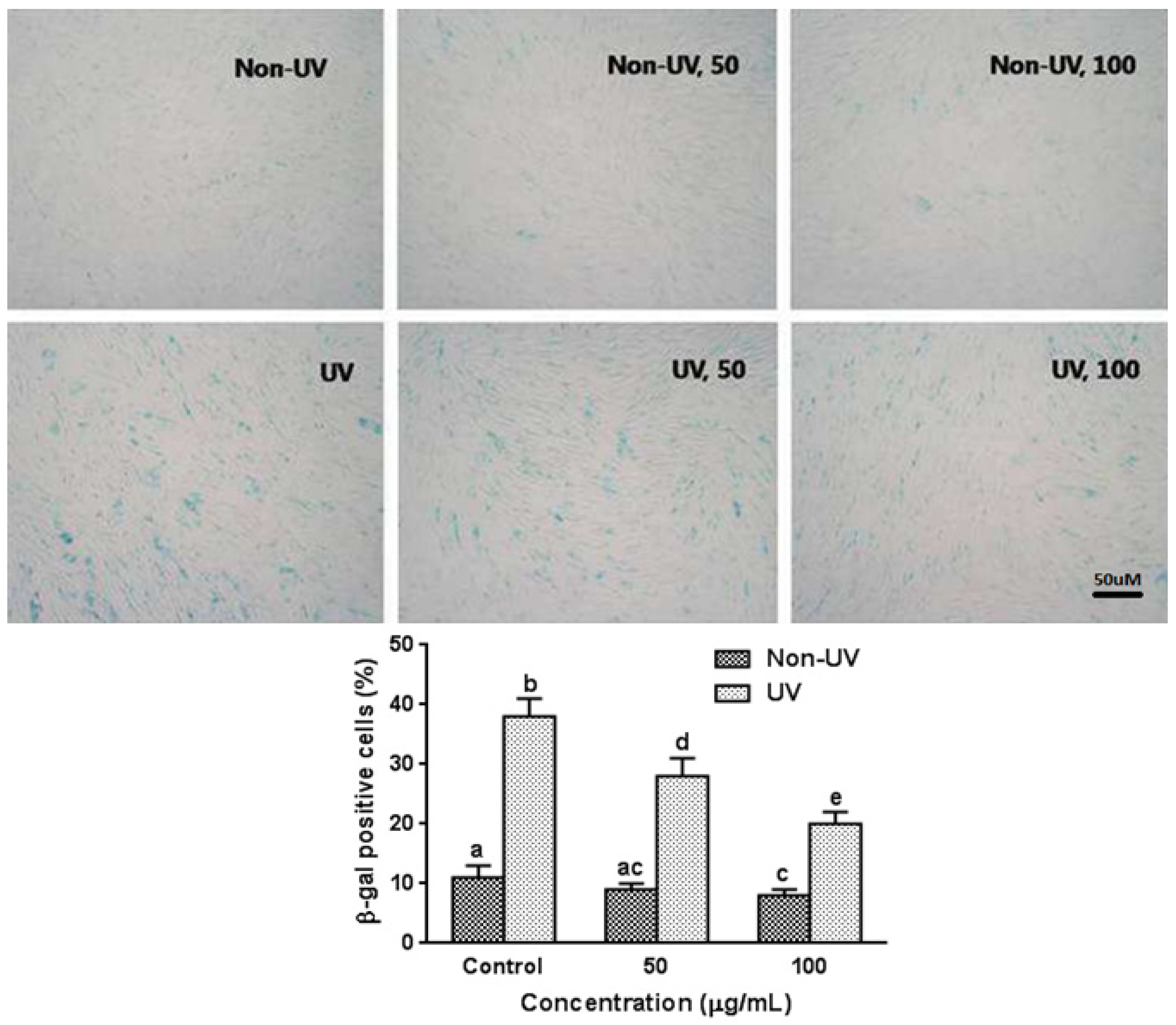
3.5. Effect on UVB-Induced SA-β-gal Activity in HaCaT Cells
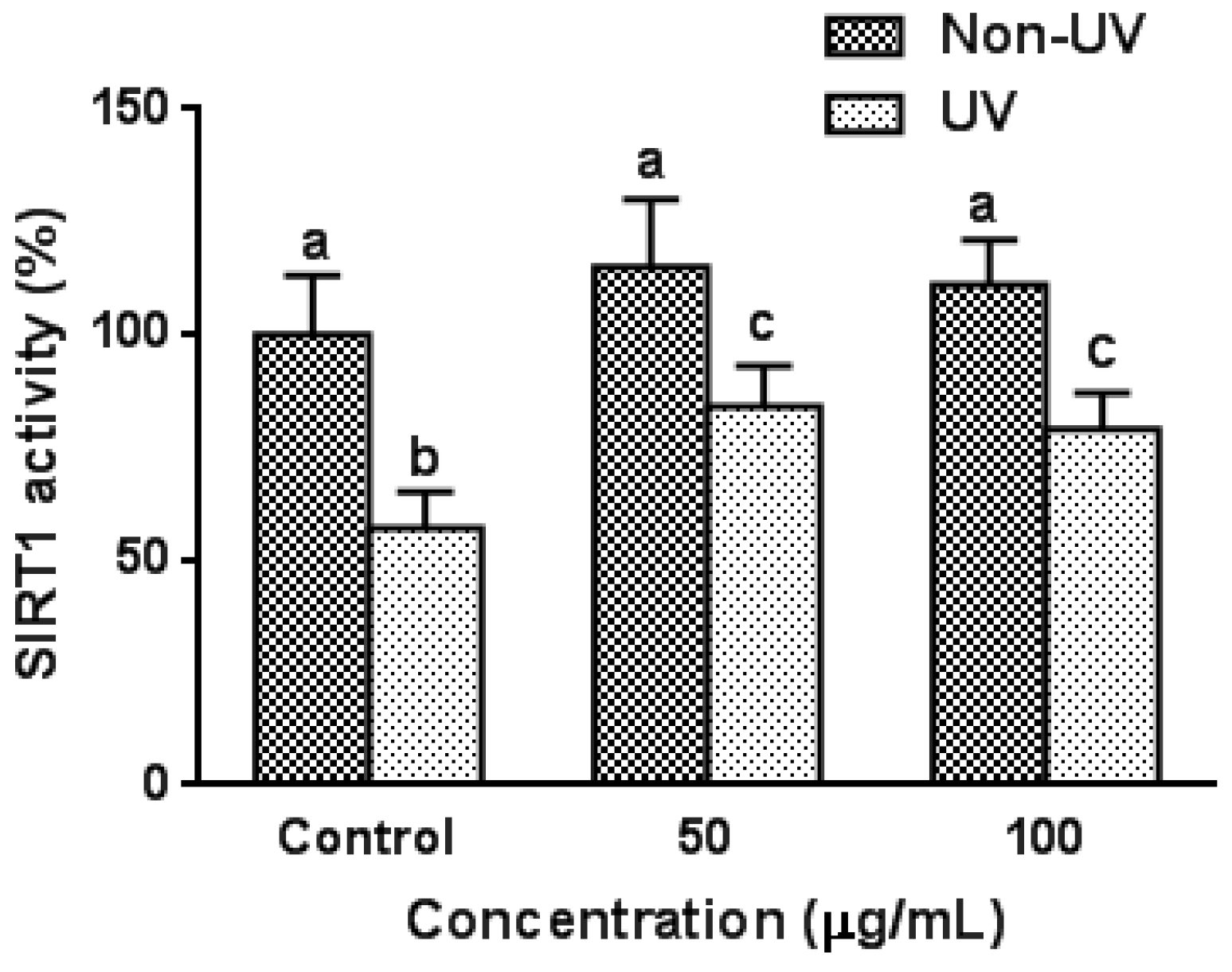
3.6. Effect on UVB-Induced SIRT1 Activity in HaCaT Cells
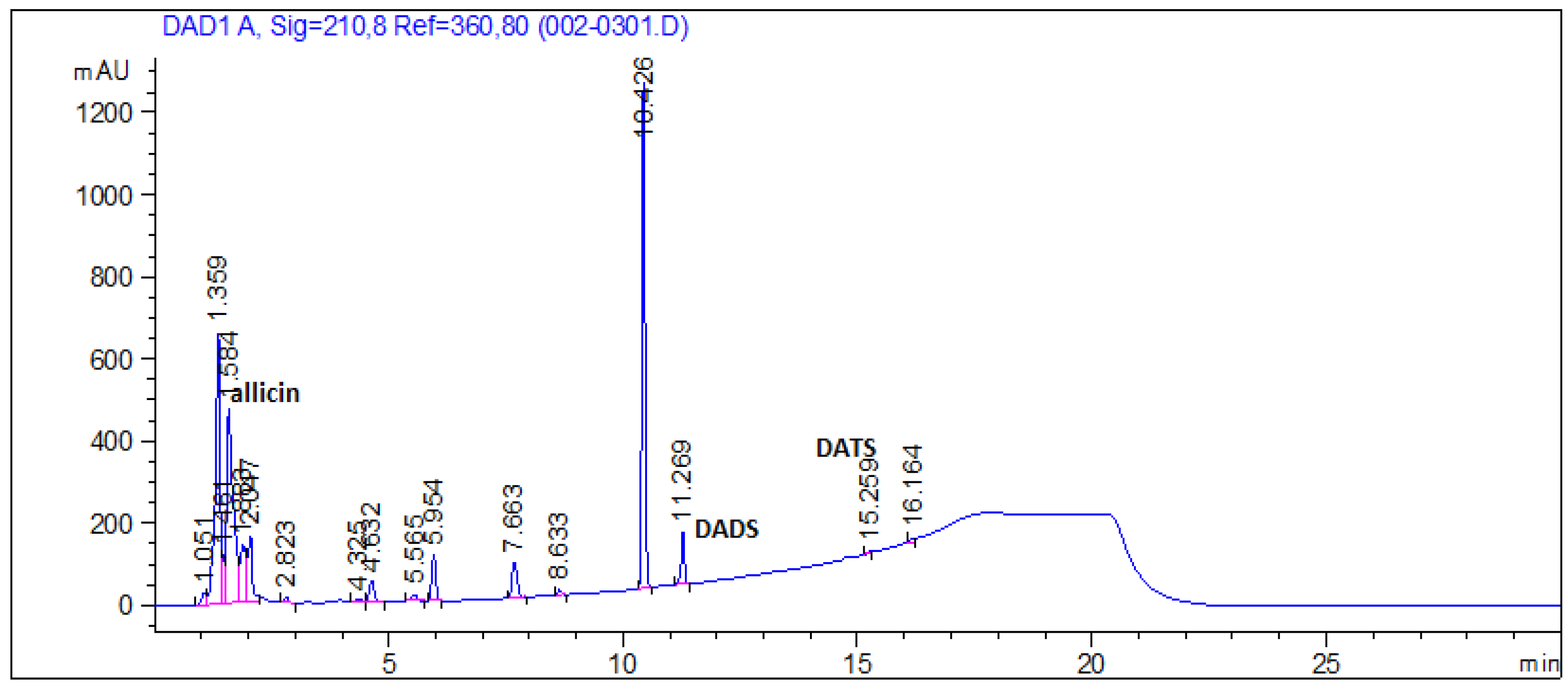
3.7. Organosulfur Compounds in Garlic Extract
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Collado, M.; Blasco, M.A.; Serrano, M. Cellular senescence in cancer and aging. Cell 2007, 130, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Kuilman, T.; Michaloglou, C.; Mooi, W.J.; Peeper, D.S. The essence of senescence. Genes Dev. 2010, 24, 2463–2479. [Google Scholar] [CrossRef] [PubMed]
- Colavitti, R.; Finkel, T. Reactive oxygen species as mediators of cellular senescence. IUBMB Life 2005, 57, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Rane, G.; Dai, X.; Shanmugam, M.K.; Arfuso, F.; Samy, R.P.; Lai, M.K.; Kappei, D.; Kumar, A.P.; Sethi, G. Ageing and the telomere connection: An intimate relationship with inflammation. Ageing Res. Rev. 2016, 25, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Minciullo, P.L.; Catalano, A.; Mandraffino, G.; Casciaro, M.; Crucitti, A.; Maltese, G.; Morabito, N.; Lasco, A.; Gangemi, S.; Basile, G. Inflammaging and Anti-Inflammaging: The Role of Cytokines in Extreme Longevity. Arch. Immunol. Ther. Exp. (Warsz) 2016, 64, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Sung, B.; Jung, K.J.; Zou, Y.; Yu, B.P. The molecular inflammatory process in aging. Antioxid. Redox. Signal. 2006, 8, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation—A review. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Podhaisky, H.P.; Riemschneider, S.; Wohlrab, W. UV light and oxidative damage of the skin. Pharmazie 2002, 57, 30–33. [Google Scholar] [PubMed]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O.; et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef] [PubMed]
- Kaeberlein, M.; McVey, M.; Guarente, L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999, 13, 2570–2580. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.A. Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem. Biophys. Res. Commun. 1999, 260, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Dali-Youcef, N.; Lagouge, M.; Froelich, S.; Koehl, C.; Schoonjans, K.; Auwerx, J. Sirtuins: The ‘magnificent seven’, function, metabolism and longevity. Ann. Med. 2007, 39, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Braunstein, M.; Rose, A.B.; Holmes, S.G.; Allis, C.D.; Broach, J.R. Transcriptional silencing in yeast is associated with reduced nucleosome acetylation. Genes Dev. 1993, 7, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Benavente, C.A.; Schnell, S.A.; Jacobson, E.L. Effects of niacin restriction on sirtuin and PARP responses to photodamage in human skin. PLoS ONE 2012, 7, e42276. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Lu, S.; Kivlin, R.; Wallin, B.; Card, E.; Bagdasarian, A.; Tamakloe, T.; Wang, W.J.; Song, X.; Chu, W.M.; et al. SIRT1 confers protection against UVB- and H2O2-induced cell death via modulation of p53 and JNK in cultured skin keratinocytes. J. Cell Mol. Med. 2009, 13, 3632–3643. [Google Scholar] [CrossRef] [PubMed]
- Cellini, L.; Di Campli, E.; Masull, M.; Di Bartolomeo, S.; Allocati, N. Inhibitions of Helicobacter pylori by garlic extract (Allium sativum). FEMS Immunol. Med. Microbiol. 1996, 13, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Durak, I.; Kavutcu, M.; Aytac, B.; Avc, A.; Devrim, E.; Ozbek, H.; Oztürk, H.S. Effects of garlic extract consumption on blood lipid and oxidant/antioxidant parameters in humans with high blood cholesterol. J. Nutr. Biochem. 2004, 15, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Lau, B.H. Suppression of LDL oxidation by garlic compounds is a possible mechanism of cardiovascular health benefit. J. Nutr. 2006, 136 (Suppl. S3), 765S–768S. [Google Scholar] [PubMed]
- Xiao, H.; Parkin, K.L. Antioxidant functions of selected allium thiosulfinates and S-alk(en)yl-l-cysteine sulfoxides. J. Agric. Food Chem. 2002, 50, 2488–2493. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.Y.; Yeh, S.M. Garlic reduces plasma lipids by inhibiting hepatic cholesterol and triacylglycerol synthesis. Lipids 1994, 29, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.H.; Son, J.K.; Jung, B.; Zheng, M.; Kim, J.R. Epifriedelanol from the root bark of Ulmus davidiana inhibits cellular senescence in human primary cells. Planta Med. 2011, 77, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Rittié, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef]
- Svendsen, L.; Rattan, S.I.; Clark, B.F. Testing garlic for possible anti-ageing effects on long term growth characteristics, morphology and macromolecular synthesis of human fibroblasts in culture. J. Ethnopharm. 1994, 43, 125–133. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, K.S.; Kim, S.H.; Baek, S.H.; Kim, H.Y.; Lee, C.; Kim, J.R. Induction of cellular senescence by secretory phospholipase A2 in human dermal fibroblasts through an ROS mediated p53 pathway. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Deliconstantinos, G.; Villiotou, V.; Stavrides, J.C. Nitric oxide and peroxynitrite released by ultraviolet B-irradiated human endothelial cells are possibly involved in skin erythema and inflammation. Exp. Physiol. 1996, 81, 1021–1033. [Google Scholar] [CrossRef] [PubMed]
- Boora, F.; Chirisa, E.; Mukanganyama, S. Evaluation of Nitrite Radical Scavenging Properties of Selected Zimbabwean Plant Extracts and Their Phytoconstituents. J. Food Process. 2014, 2014, 918018. [Google Scholar] [CrossRef]
- Jagetia, G.C.; Rao, S.K.; Baliga, M.S.; S Babu, K. The evaluation of nitric oxide scavenging activity of certain herbal formulations in vitro: A preliminary study. Phytother. Res. 2004, 18, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, S.A.; Kamble, G.S.; Devare, S.; Deshpande, N.R.; Salvekar, J.P. In vitro evaluation of free radical scavenging potential of Cassia auriculata L. J. Chem. Pharm. Res. 2011, 3, 766–772. [Google Scholar]
- Chung, L.Y. The antioxidant properties of garlic compounds: Allyl cysteine, alliin, allicin, and allyl disulfide. J. Med. Food 2006, 9, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Laxdal, V.A.; Yu, M.; Raney, B.L. Antioxidant activity of allicin, an active principle in garlic. Mol. Cell. Biochem. 1995, 148, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Li, F.M.; Li, T.; Li, W.; Yang, L.D. Changes in antioxidant capacity, levels of soluble sugar, total polyphenol, organosulfur compound and constituents in garlic clove during storage. Ind. Crops Prod. 2015, 69, 137–142. [Google Scholar]
- Kim, S.R.; Jung, Y.R.; An, H.J.; Kim, D.H.; Jang, E.J.; Choi, Y.J.; Moon, K.M.; Park, M.H.; Park, C.H.; Chung, K.W.; et al. Anti-Wrinkle and Anti-Inflammatory Effects of Active Garlic Components and the Inhibition of MMPs via NF-κB Signaling. PLoS ONE 2013, 8, e73877. [Google Scholar] [CrossRef] [PubMed]
- Cherng, J.M.; Tsai, K.D.; Perng, D.S.; Wang, J.S.; Wei, C.C.; Lin, J.C. Diallyl sulfide protects against ultraviolet B-induced skin cancers in SKH-1 hairless mouse: Analysis of early molecular events in carcinogenesis. Photodermatol. Photoimmunol. Photomed. 2011, 27, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Philips, N. Experimental physiology in anti-skin aging. In Skin Anatomy and Physiology Research Development; Columbus, F., Ed.; Nova Science: Hauppauge, NY, USA, 2009; pp. 216–230. [Google Scholar]
- Philips, N.; Samuel, M.; Arena, R.; Chen, Y.J.; Conte, J.; Natarajan, P.; Haas, G.; Gonzalez, S. Direct inhibition of elastase and matrixmetalloproteinases and stimulation of biosynthesis of fibrillar collagens, elastin, and fibrillins by xanthohumol. J. Cosmet. Sci. 2010, 61, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K. Garlic supplementation ameliorate UV-induced Photoaging in Hairless Mice by regulating antioxidative activity and MMPs expression. Molecules 2016, 21, 70. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Harris, T.; Guralnik, J.M.; Tracy, R.P.; Corti, M.C.; Cohen, H.J.; Penninx, B.; Pahor, M.; Wallace, R.; Havlik, R.J. Serum IL-6 level and the development of disability in older persons. J. Am. Geriatr. Soc. 1999, 47, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Bruunsgaard, H.; Ladelund, S.; Pedersen, A.N.; Schroll, M.; Jørgensen, T.; Pedersen, B.K. Predicting death from tumour necrosis factor-alpha and interleukin-6 in 80-year-old people. Clin. Exp. Immunol. 2003, 132, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Adhami, V.M.; Mukhtar, H. Photochemoprevention of ultraviolet B signaling and photocarcinogenesis. Mutat. Res. 2005, 571, 153–173. [Google Scholar] [CrossRef] [PubMed]
- Yoshizumi, M.; Nakamura, T.; Kato, M.; Ishioka, T.; Kozawa, K.; Wakamatsu, K.; Kimura, H. Release of cytokines/chemokines and cell death in UVB-irradiated human keratinocytes, HaCaT. Cell. Biol. Int. 2008, 32, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.Y.; Choi, J.S.; Kang, S.W.; Lee, Y.J.; Park, J.; Kang, Y.H. Dietary compound ellagic acid alleviates skin wrinkle and inflammation induced by UV-B irradiation. Exp. Dermatol. 2010, 19, e182–e190. [Google Scholar] [CrossRef] [PubMed]
- Lang, A.; Lahav, M.; Sakhnini, E.; Barshack, I.; Fidder, H.H.; Avidan, B.; Bardan, E.; Hershkoviz, R.; Bar-Meir, S.; Chowers, Y. Allicin inhibits spontaneous and TNF-alpha induced secretion of proinflammatory cytokines and chemokines from intestinal epithelial cells. Clin. Nutr. 2004, 23, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- You, S.X.; Nakanishi, E.; Kuwata, H.; Chen, J.H.; Nakasone, Y.; He, X.; He, J.H.; Liu, X.X.; Zhang, S.R.; Zhang, B.; et al. Inhibitory effects and molecular mechanisms of garlic organosulfur compounds on the production of inflammatory mediators. Mol. Nutr. Food Res. 2013, 57, 2049–2060. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.C.; Hu, M.L. A fluorimetric method using fluorescein di-β-D-galactopyranoside for quantifying the senescence-associated β-galactosidase activity in human foreskin fibroblast Hs68 cells. Anal. Biochem. 2004, 325, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Saunders, L.R.; Verdin, E. Sirtuins: Critical regulators at the crossroads between cancer and aging. Oncogene 2007, 26, 5489–5504. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Gan, Q.; Han, L.; Li, J.; Zhang, H.; Sun, Y.; Zhang, Z.; Tong, T. SIRT1 overexpression antagonizes cellular senescence with activated ERK/S6k1 signaling in human diploid fibroblasts. PLoS ONE 2008, 3, e1710. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Arai, N.; Kohno, K.; Ushio, S.; Fukuda, S. Anti-oxidative and anti-aging activities of 2-O-α-glucopyranosyl-l-ascorbic acid on human dermal fibroblasts. Eur. J. Pharmacol. 2012, 674, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Ohguchi, K.; Itoh, T.; Akao, Y.; Inoue, H.; Nozawa, Y.; Ito, M. SIRT1 modulates expression of matrix metalloproteinases in human dermal fibroblasts. Br. J. Dermatol. 2010, 163, 689–694. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.K. Protective Effect of Garlic on Cellular Senescence in UVB-Exposed HaCaT Human Keratinocytes. Nutrients 2016, 8, 464. https://doi.org/10.3390/nu8080464
Kim HK. Protective Effect of Garlic on Cellular Senescence in UVB-Exposed HaCaT Human Keratinocytes. Nutrients. 2016; 8(8):464. https://doi.org/10.3390/nu8080464
Chicago/Turabian StyleKim, Hye Kyung. 2016. "Protective Effect of Garlic on Cellular Senescence in UVB-Exposed HaCaT Human Keratinocytes" Nutrients 8, no. 8: 464. https://doi.org/10.3390/nu8080464
APA StyleKim, H. K. (2016). Protective Effect of Garlic on Cellular Senescence in UVB-Exposed HaCaT Human Keratinocytes. Nutrients, 8(8), 464. https://doi.org/10.3390/nu8080464