Temporal Change of the Content of 10 Oligosaccharides in the Milk of Chinese Urban Mothers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Trial Design
2.2. Milk Collection and Storage
2.3. Ethical and Legal Considerations
2.4. Analytical Method
2.4.1. Chemicals and Reagents
2.4.2. Sample Preparation
2.5. Method Validation
2.6. Data Analysis
3. Results
3.1. Study Population
3.2. Analytical Method Performance
3.3. Analysis of Milk Samples
4. Discussion
4.1. Factors Influencing Oligosaccharide Expression
4.2. Fucosylated Oligosaccharides
4.3. Sialylated Oligosaccharides
4.4. LNT and LNnT
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 2′-FL | 2′-fucosyllactose |
| 3-FL | 3-fucosyllactose |
| 3′-SL | 3′-sialyllactose |
| 6′-SL | 6′-sialyllactose |
| A-Tetra | A-tetrasaccharide |
| CE | Capillary electrophoresis |
| Fuc | Fucose |
| FucT2 | Fucosyltransferase-2 |
| Gal | Galactose |
| GDP | Guanosine 5′-diphosphate |
| Glc | Glucose |
| GlcNAc | N-acetylglucosamine |
| HMO | Human milk oligosaccharides |
| HPLC | High performance liquid chromatography |
| LNFP-I | Lacto-N-fucosylpentaose-I |
| LNFP-II | Lacto-N-fucosylpentaose-II |
| LNFP-III | Lacto-N-fucosylpentaose-III |
| LNFP-V | Lacto-N-fucosylpentaose-V |
| LNH | Lacto-N-hexaose |
| LNnFP | Lacto-N-neofucosylpentaose |
| LNnT | Lacto-N-neotetraose |
| LNO | Lacto-N-octaose |
| LNT | Lacto-N-tetraose |
| LoD | Limit of detection |
| LoQ | Limit of quantification |
| MS | Mass spectrometry |
| Neu5Ac | N-acetylneuraminic acid |
| NLO | Non-lactose oligosaccharide |
| NMR | Nuclear magnetic resonance spectroscopy |
| PAD | Pulsed amperometric detection |
| UV | Ultraviolet detection |
References
- Eidelman, A.I.; Schanler, R.J.J.M.; Landers, S.; Noble, L.; Szucs, K.; Viehmann, L.; Feldman-Winter, L.; Lawrence, R.; Kim, S.; Onyema, N. Breastfeeding and the use of human milk. Pediatrics 2012, 129, e827–e841. [Google Scholar]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Brand-Miller, J. The role and potential of sialic acid in human nutrition. Eur. J. Clin. Nutr. 2003, 57, 1351–1369. [Google Scholar] [CrossRef] [PubMed]
- Amitay, E.L.; Keinan-Boker, L. Breastfeeding and childhood leukemia incidence: A meta-analysis and systematic review. JAMA Pediatr. 2015, 169, e151025. [Google Scholar] [CrossRef] [PubMed]
- Urashima, T.; Asakuma, S.; Leo, F.; Fukuda, K.; Messer, M.; Oftedal, O.T. The predominance of type I oligosaccharides is a feature specific to human breast milk. Adv. Nutr. 2012, 3, 473S–482S. [Google Scholar] [CrossRef] [PubMed]
- Kunz, C.; Rodriguez-Palmero, M.; Koletzko, B.; Jensen, R. Nutritional and biochemical properties of human milk, part I: General aspects, proteins, and carbohydrates. Clin. Perinatol. 1999, 26, 307–333. [Google Scholar] [PubMed]
- Coppa, G.V.; Gabrielli, O.; Pierani, P.; Catassi, C.; Carlucci, A.; Giorgi, P.L. Changes in carbohydrate composition in human milk over 4 months of lactation. Pediatrics 1993, 91, 637–641. [Google Scholar] [PubMed]
- Fong, B.; Ma, K.; McJarrow, P.J. Quantification of bovine milk oligosaccharides using liquid chromatography-selected reaction monitoring-mass spectrometry. J. Agric. Food Chem. 2011, 59, 9788–9795. [Google Scholar] [CrossRef] [PubMed]
- Urashima, T.; Taufik, E.; Fukuda, K.; Asakuma, S. Recent advances in studies on milk oligosaccharides of cows and other domestic farm animals. Biosci. Biotechnol. Biochem. 2013, 77, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Urashima, T.; Asakuma, S.; Messer, M. Milk oligosaccharides. In Comprehensive Glycoscience: From Chemistry to Systems Biology; Kamerling, J.P., Boons, G.-J., Lee, Y.C., Suzuki, A., Taniguchi, N., Voragen, A.G.J., Eds.; Elsevier: Oxford, UK, 2007; Volume 4, pp. 695–724. [Google Scholar]
- Urashima, T.; Fukuda, K.; Kitaoka, M.; Ohnishi, M.; Terabayashi, T.; Kobata, A. Milk Oligosaccharides; Nova Science Publishers Inc.: New York, NY, USA, 2011. [Google Scholar]
- Urashima, T.; Asakuma, S.; Kitaoka, M.; Messer, M. Indiginous oligosaccharides in milk. In Encyclopedia of Dairy Sciences, 2th ed.; Fuquay, J.W., Fox, P.F., McSweeney, P.L.H., Eds.; Academic Press: San Diego, CA, USA, 2011; Volume 3, pp. 241–273. [Google Scholar]
- Thurl, S.; Henker, J.; Siegel, M.; Tovar, K.; Sawatzki, G. Detection of four human milk groups with respect to lewis blood group dependent oligosaccharides. Glycoconj. J. 1997, 14, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Prieto, P.A. Profiles of human milk oligosaccharides and production of some human milk oligosaccharides in transgenic animals. Adv. Nutr. 2012, 3, 456S–464S. [Google Scholar] [CrossRef] [PubMed]
- Schenkel-Brunner, H. Blood group antigens. In Comprehensive Glycoscience; Kamerling, J.P., Boons, G.J., Lee, C.Y., Suzuki, A., Taniguchi, N., Voragen, A.G.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 3, pp. 343–372. [Google Scholar]
- Kunz, C.; Rudloff, S.; Baier, W.; Klein, N.; Strobel, S. Oligosaccharides in human milk: Structural, functional, and metabolic aspects. Ann. Rev. Nutr. 2000, 20, 699–722. [Google Scholar] [CrossRef] [PubMed]
- Jantscher-Krenn, E.; Bode, L. Human milk oligosaccharides and their potential benefits for the breast-fed neonate. Minerva Pediatr. 2012, 64, 83–99. [Google Scholar] [PubMed]
- Newburg, D.S.; He, Y. Neonatal gut microbiota and human milk glycans cooperate to attenuate infection and inflammation. Clin. Obstet. Gynecol. 2015, 58, 814–826. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, S.; Schols, H.A.; van den Heuvel, E.G.; Voragen, A.G.; Gruppen, H. Ce-lif-msn profiling of oligosaccharides in human milk and feces of breast-fed babies. Electrophoresis 2010, 31, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Coppa, G.V.; Pierani, P.; Zampini, L.; Bruni, S.; Carloni, I.; Gabrielli, O. Characterization of oligosaccharides in milk and feces of breast-fed infants by high-performance anion-exchange chromatography. Adv. Exp. Med. Biol. 2001, 501, 307–314. [Google Scholar] [PubMed]
- Asakuma, S.; Hatakeyama, E.; Urashima, T.; Yoshida, E.; Katayama, T.; Yamamoto, K.; Kumagai, H.; Ashida, H.; Hirose, J.; Kitaoka, M. Physiology of consumption of human milk oligosaccharides by infant gut-associated bifidobacteria. J. Biol. Chem. 2011, 286, 34583–34592. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Lim, J.Y.; Kim, B.S.; Cho, S.J.; Kim, N.Y.; Kim, O.B.; Kim, Y. Comparison of the gut microbiota profile in breast-fed and formula-fed Korean infants using pyrosequencing. Nutr. Res. Pract. 2015, 9, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Fallani, M.; Young, D.; Scott, J.; Norin, E.; Amarri, S.; Adam, R.; Aguilera, M.; Khanna, S.; Gil, A.; Edwards, C.A.; et al. Intestinal microbiota of 6-week-old infants across europe: Geographic influence beyond delivery mode, breast-feeding, and antibiotics. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Huo, G.; Li, X.; Yang, L.; Duan, C. Impact of diet in shaping gut microbiota revealed by a comparative study in infants during the six months of life. J. Microbiol. Biotechnol. 2014, 24, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, M.; Wu, S.; Lebrilla, C.B.; Chapkin, R.S.; Ivanov, I.; Donovan, S.M. Fecal microbiota composition of breast-fed infants is correlated with human milk oligosaccharides consumed. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Sharon, N.; Ofek, I. Safe as mother’s milk: Carbohydrates as future anti-adhesion drugs for bacterial diseases. Glycoconj. J. 2000, 17, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Gonia, S.; Tuepker, M.; Heisel, T.; Autran, C.; Bode, L.; Gale, C.A. Human milk oligosaccharides inhibit candida albicans invasion of human premature intestinal epithelial cells. J. Nutr. 2015, 145, 1992–1998. [Google Scholar] [CrossRef] [PubMed]
- Hakkarainen, J.; Toivanen, M.; Leinonen, A.; Frangsmyr, L.; Stromberg, N.; Lapinjoki, S.; Nassif, X.; Tikkanen-Kaukanen, C. Human and bovine milk oligosaccharides inhibit neisseria meningitidis pili attachment in vitro. J. Nutr. 2005, 135, 2445–2448. [Google Scholar] [PubMed]
- Hester, S.N.; Chen, X.; Li, M.; Monaco, M.H.; Comstock, S.S.; Kuhlenschmidt, T.B.; Kuhlenschmidt, M.S.; Donovan, S.M. Human milk oligosaccharides inhibit rotavirus infectivity in vitro and in acutely infected piglets. Br. J. Nutr. 2013, 110, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Jantscher-Krenn, E.; Lauwaet, T.; Bliss, L.A.; Reed, S.L.; Gillin, F.D.; Bode, L. Human milk oligosaccharides reduce entamoeba histolytica attachment and cytotoxicity in vitro. Br. J. Nutr. 2012, 108, 1839–1846. [Google Scholar] [CrossRef] [PubMed]
- Marotta, M.; Ryan, J.T.; Hickey, R.M. The predominant milk oligosaccharide 6′-sialyllactose reduces the internalisation of pseudomonas aeruginosa in human pneumocytes. J. Funct. Foods 2014, 6, 367–373. [Google Scholar] [CrossRef]
- Martin-Sosa, S.; Martin, M.J.; Hueso, P. The sialylated fraction of milk oligosaccharides is partially responsible for binding to enterotoxigenic and uropathogenic escherichia coli human strains. J. Nutr. 2002, 132, 3067–3072. [Google Scholar] [PubMed]
- Weichert, S.; Jennewein, S.; Hufner, E.; Weiss, C.; Borkowski, J.; Putze, J.; Schroten, H. Bioengineered 2′-fucosyllactose and 3-fucosyllactose inhibit the adhesion of pseudomonas aeruginosa and enteric pathogens to human intestinal and respiratory cell lines. Nutr. Res. 2013, 33, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, S.; Ridet, J.L.; Kusy, N.; Gao, H.; Crevoisier, F.; Guinchard, S.; Kochhar, S.; Sigrist, H.; Sprenger, N. Glycoprofiling with micro-arrays of glycoconjugates and lectins. Glycobiology 2005, 15, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Goehring, K.C.; Kennedy, A.D.; Prieto, P.A.; Buck, R.H. Direct evidence for the presence of human milk oligosaccharides in the circulation of breastfed infants. PLoS ONE 2014, 9, e101692. [Google Scholar] [CrossRef] [PubMed]
- Ruhaak, L.R.; Stroble, C.; Underwood, M.A.; Lebrilla, C.B. Detection of milk oligosaccharides in plasma of infants. Anal. Bioanal. Chem. 2014, 406, 5775–5784. [Google Scholar] [CrossRef] [PubMed]
- Klein, N.; Schwertmann, A.; Peters, M.; Kunz, C.; Strobel, S. Immunomodulatory effects of breast milk oligosaccharides. Adv. Exp. Med. Biol. 2000, 478, 251–259. [Google Scholar] [PubMed]
- Newburg, D.S. Neonatal protection by an innate immune system of human milk consisting of oligosaccharides and glycans. J. Anim. Sci. 2009, 87, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, N.; Duncan, P.I. Sialic acid utlization. Adv. Nutr. 2012, 3, 392S–397S. [Google Scholar] [CrossRef] [PubMed]
- Ten Bruggencate, S.J.; Bovee-Oudenhoven, I.M.; Feitsma, A.L.; van, H.E.; Schoterman, M.H. Functional role and mechanisms of sialyllactose and other sialylated milk oligosaccharides. Nutr. Rev. 2014, 72, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Brand-Miller, J.; McVeagh, P.; Petocz, P. Concentration and distribution of sialic acid in human milk and infant formulas. Am. J. Clin. Nutr. 2001, 74, 510–515. [Google Scholar] [PubMed]
- Wang, B.; Yu, B.; Karim, M.; Hu, H.; Sun, Y.; McGreevy, P.; Petocz, P.; Held, S.; Brand-Miller, J. Dietary sialic acid supplementation improves learning and memory in piglets. Am. J. Clin. Nutr. 2007, 85, 561–569. [Google Scholar] [PubMed]
- Erney, R.M.; Malone, W.T.; Skelding, M.B.; Marcon, A.A.; Kleman-Leyer, K.M.; O’Ryan, M.L.; Ruiz-Palacios, G.; Hilty, M.D.; Pickering, L.K.; Prieto, P.A. Variability of human milk neutral oligosaccharides in a diverse population. J. Pediatr. Gastroenterol. Nutr. 2000, 30, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Coppa, G.V.; Pierani, P.; Zampini, L.; Carloni, I.; Carlucci, A.; Gabrielli, O. Oligosaccharides in human milk during different phases of lactation. Acta Paediatr. Suppl. 1999, 88, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Nakhla, T.; Daotian, F.; Zopf, D.; Brodsky, N.L.; Hurt, H. Neutral oligosaccharide content of preterm human milk. Br. J. Nutr. 1999, 82, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Thurl, S.; Muller, W.B.; Sawatzki, G. Quantification of individual oligosaccharide compounds from human milk using high-ph anion-exchange chromatography. Anal. Biochem. 1996, 235, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, O.; Zampini, L.; Galeazzi, T.; Padella, L.; Santoro, L.; Peila, C.; Giuliani, F.; Bertino, E.; Fabris, C.; Coppa, G.V. Preterm milk oligosaccharides during the first month of lactation. Pediatrics 2011, 128, e1520–e1531. [Google Scholar] [CrossRef] [PubMed]
- Thurl, S.; Munzert, M.; Henker, J.; Boehm, G.; Muller-Werner, B.; Jelinek, J.; Stahl, B. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. Br. J. Nutr. 2010, 104, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, M.; Simpore, J.; D’Agata, A.; Sotgiu, S.; Musumeci, S. Oligosaccharides in colostrum of Italian and Burkinabe women. J. Pediatr. Gastroenterol. Nutr. 2006, 43, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Erney, R.; Hilty, M.; Pickering, L.; Ruiz-Palacios, G.; Prieto, P. Human milk oligosaccharides: A novel method provides insight into human genetics. Adv. Exp. Med. Biol. 2001, 501, 285–297. [Google Scholar] [PubMed]
- Leo, F.; Asakuma, S.; Nakamura, T.; Fukuda, K.; Senda, A.; Urashima, T. Improved determination of milk oligosaccharides using a single derivatization with anthranilic acid and separation by reversed-phase high-performance liquid chromatography. J. Chromatogr. A 2009, 1216, 1520–1523. [Google Scholar] [CrossRef] [PubMed]
- Asakuma, S.; Urashima, T.; Akahori, M.; Obayashi, H.; Nakamura, T.; Kimura, K.; Watanabe, Y.; Arai, I.; Sanai, Y. Variation of major neutral oligosaccharides levels in human colostrum. Eur. J. Clin. Nutr. 2007, 62, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, P.; Warren, C.D.; Ruiz Palacios, G.M.; Pickering, L.K.; Newburg, D.S. Milk oligosaccharide profiles by reversed-phase hplc of their perbenzoylated derivatives. Anal. Biochem. 1997, 251, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Zhu, L.; Newburg, D.S. Simultaneous quantification of sialyloligosaccharides from human milk by capillary electrophoresis. Anal. Biochem. 2007, 370, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Sumiyoshi, W.; Urashima, T.; Nakamura, T.; Arai, I.; Saito, T.; Tsumura, N.; Wang, B.; Brand-Miller, J.; Watanabe, Y.; Kimura, K. Determination of each neutral oligosaccharide in the milk of Japanese women during the course of lactation. Br. J. Nutr. 2003, 89, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Warren, C.D.; Chaturvedi, P.; Newburg, A.R.; Oftedal, O.T.; Tilden, C.D.; Newburg, D.S. Comparison of oligosaccharides in milk specimens from humans and twelve other species. Adv. Exp. Med. Biol. 2001, 501, 325–332. [Google Scholar] [PubMed]
- Chaturvedi, P.; Warren, C.D.; Altaye, M.; Morrow, A.L.; Ruiz-Palacios, G.; Pickering, L.K.; Newburg, D.S. Fucosylated human milk oligosaccharides vary between individuals and over the course of lactation. Glycobiology 2001, 11, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Sjogren, Y.M.; Duchen, K.; Lindh, F.; Bjorksten, B.; Sverremark-Ekstrom, E. Neutral oligosaccharides in colostrum in relation to maternal allergy and allergy development in children up to 18 months of age. Pediatr. Allergy Immunol. 2007, 18, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Chen, C.; Newburg, D.S. Quantification of neutral human milk oligosaccharides by graphitic carbon high-performance liquid chromatography with tandem mass spectrometry. Anal. Biochem. 2013, 433, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Hayama, T.; Yoshida, H.; Itoyama, M.; Todoroki, K.; Yamaguchi, M.; Nohta, H. Liquid chromatography/tandem mass spectrometry with fluorous derivatization method for selective analysis of sialyl oligosaccharides. Rapid Commun. Mass Spectrom. 2014, 28, 2481–2489. [Google Scholar] [CrossRef] [PubMed]
- Galeotti, F.; Coppa, G.V.; Zampini, L.; Maccari, F.; Galeazzi, T.; Padella, L.; Santoro, L.; Gabrielli, O.; Volpi, N. Capillary electrophoresis separation of human milk neutral and acidic oligosaccharides derivatized with 2-aminoacridone. Electrophoresis 2014, 35, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Song, J.F.; Weng, M.Q.; Wu, S.M.; Xia, Q.C. Analysis of neutral saccharides in human milk derivatized with 2-aminoacridone by capillary electrophoresis with laser-induced fluorescence detection. Anal. Biochem. 2002, 304, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Radzanowski, G.G.; Garrett, P.N.; Li, X.; Burgher, M.A.; Contractor, N.; Pramuk, K. Short-chain milk oligosaccharide levels in human milk and infant plasma. In Proceedings of the Joint Annual Meeting of the ASPET/BPS at Experimental Biology (EB), Boston, MA, USA, 20–24 April 2013; Federation Amer Soc Exp Bio: Bethesda, MD, USA.
- Smilowitz, J.T.; O’Sullivan, A.; Barile, D.; German, J.B.; Lonnerdal, B.; Slupsky, C.M. The human milk metabolome reveals diverse oligosaccharide profiles. J. Nutr. 2013, 143, 1709–1718. [Google Scholar] [CrossRef] [PubMed]
- Galeotti, F.; Coppa, G.V.; Zampini, L.; Maccari, F.; Galeazzi, T.; Padella, L.; Santoro, L.; Gabrielli, O.; Volpi, N. On-line high-performance liquid chromatography-fluorescence detection-electrospray ionization-mass spectrometry profiling of human milk oligosaccharides derivatized with 2-aminoacridone. Anal. Biochem. 2012, 430, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Hellerstein, S.; Feldman, S.; Duan, T. China’s 50% caesarean delivery rate: Is it too high? BJOG 2015, 122, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zhang, Y.; Ning, Y.; You, L.; Ma, D.; Zheng, Y.; Yang, X.; Li, W.; Wang, J.; Wang, P. Breast milk macronutrient composition and the associated factors in urban Chinese mothers. Chin. Med. J. 2014, 127, 1721–1725. [Google Scholar] [PubMed]
- Bénet, T.; Austin, S. On-line cleanup for 2-aminobenzamide-labeled oligosaccharides. Anal. Biochem. 2011, 414, 166–168. [Google Scholar] [CrossRef] [PubMed]
- Newburg, D.S.; Ruiz-Palacios, G.M.; Altaye, M.; Chaturvedi, P.; Meinzen-Derr, J.; Guerrero, M.D.; Morrow, A.L. Innate protection conferred by fucosylated oligosaccharides of human milk against diarrhea in breastfed infants. Glycobiology 2004, 14, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Morrow, A.L.; Ruiz-Palacios, G.M.; Altaye, M.; Jiang, X.; Guerrero, M.L.; Meinzen-Derr, J.K.; Farkas, T.; Chaturvedi, P.; Pickering, L.K.; Newburg, D.S. Human milk oligosaccharides are associated with protection against diarrhea in breast-fed infants. J. Pediatr. 2004, 145, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Kobata, A. Structures and application of oligosaccharides in human milk. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010, 86, 731–747. [Google Scholar] [CrossRef] [PubMed]
- Combs, M.R. Lewis blood group system review. Immunohematology 2009, 25, 112–118. [Google Scholar] [PubMed]
- Bloodbook. Racial & Ethnic Distribution of Abo Blood Types Bloodbook.Com. Available online: http://www.bloodbook.com/world-abo.html (accessed on 29 January 2016).
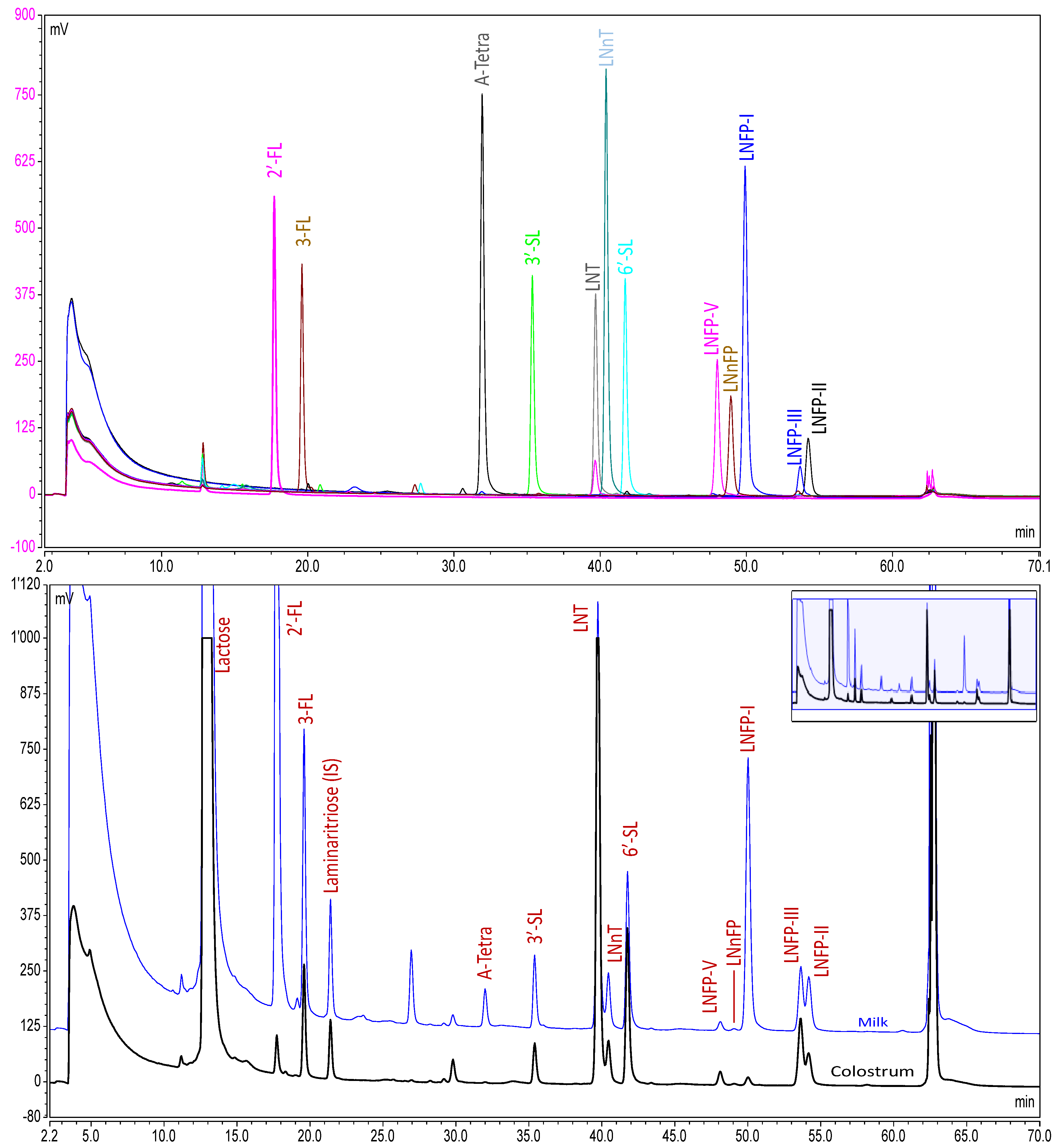
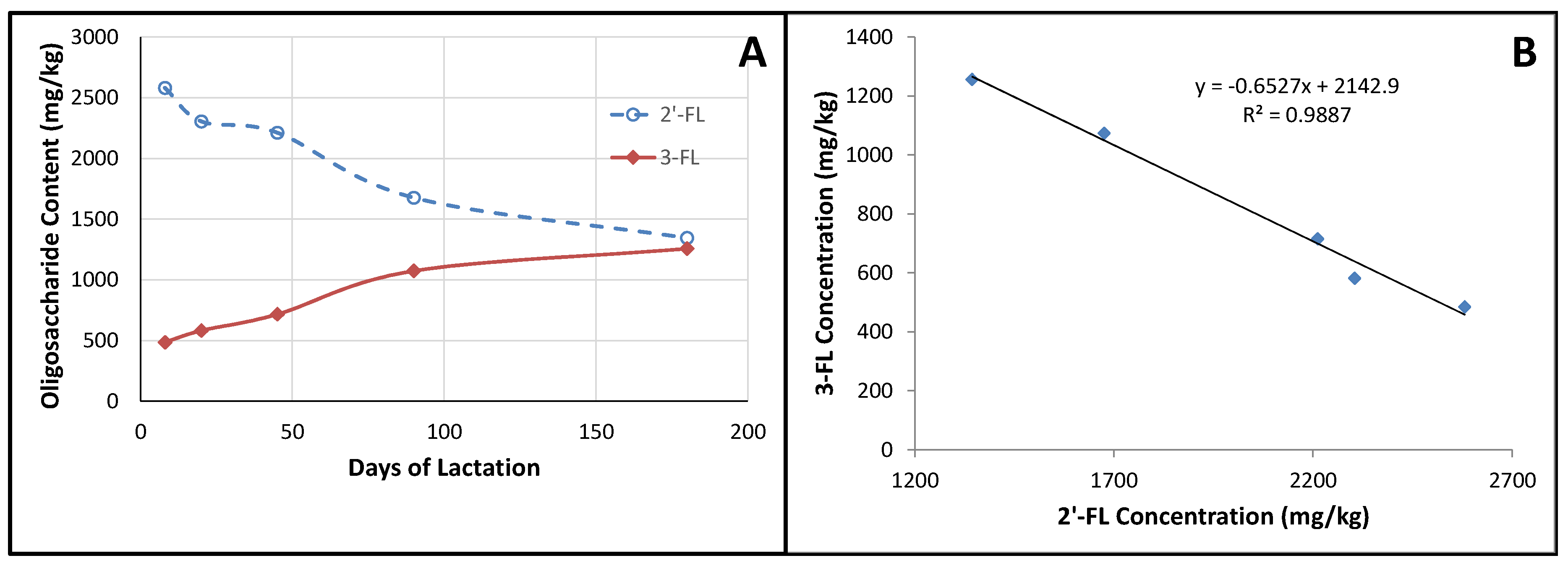
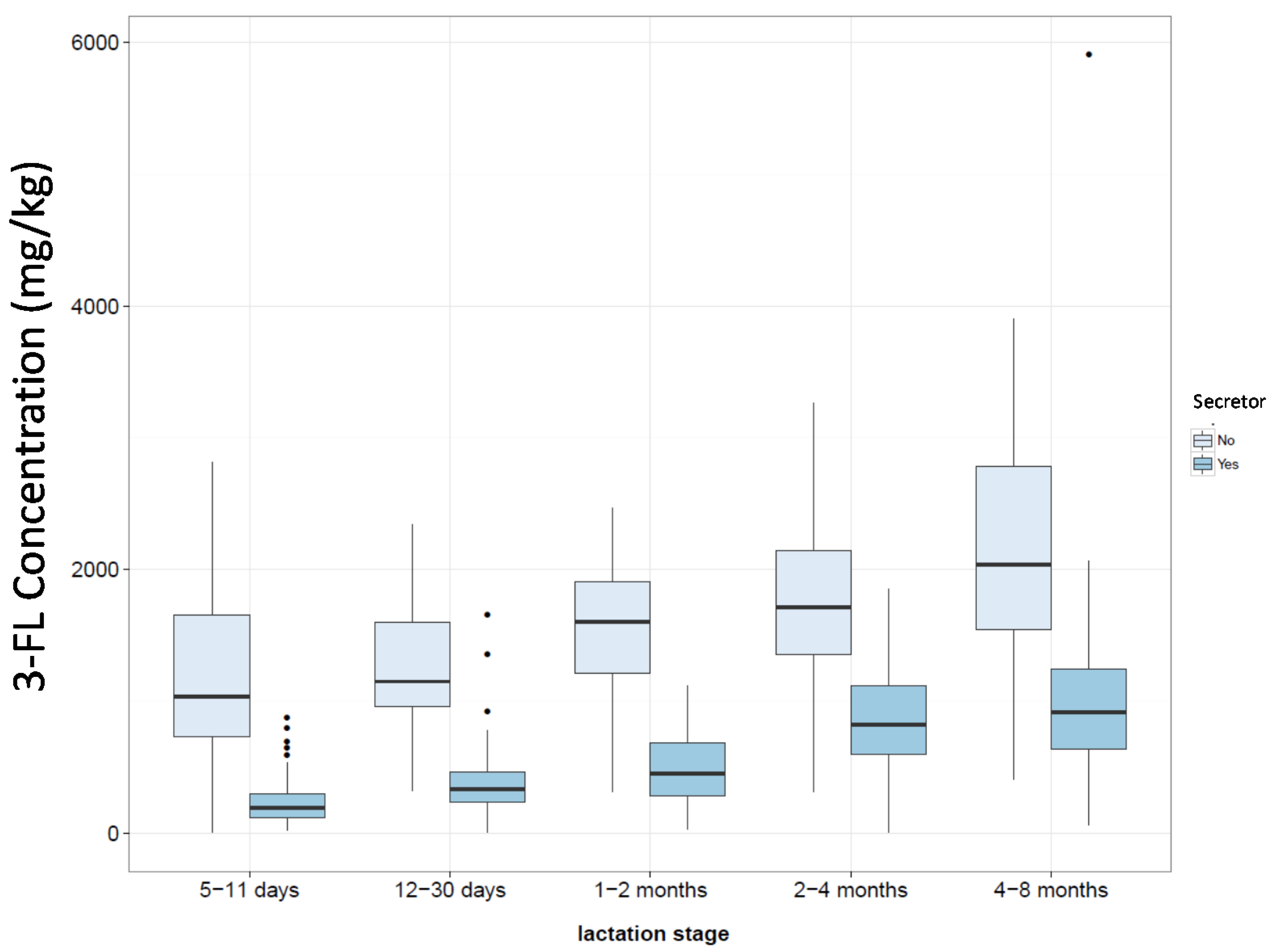
| n | 5–11 Days | 12–30 Days | 1–2 Months | 2–4 Months | 4–8 Months |
|---|---|---|---|---|---|
| 90 | 90 | 90 | 90 | 90 | |
| Mothers | |||||
| Age (years), mean (SD) | 27 (4) | 27 (3) | 28 (4) | 27 (4) | 26 (4) |
| Height (m), mean (SD) | 160 (4) | 160 (5) | 161 (5) | 161 (5) | 159 (5) |
| Weight (kg), mean (SD) | 60.7 (8.7) | 60.8 (7.9) | 61.9 (8.9) | 58.4 (8.3) | 56.2 (8.1) |
| BMI (kg/m2), mean | 23.7 (3.2) | 23.7 (3.0) | 23.9 (3.1) | 22.5 (2.9) | 22.2 (3.1) |
| Gestational weight gain (kg), mean (SD) | 16.7 (7.4) | 16.2 (6.0) | 15.9 (5.7) | 15.9 (5.9) | 14.9 (7.6) |
| Postpartum weight loss (kg), mean (SD) | 9.1 (6.1) | 8.6 (5.3) | 9.8 (4.0) | 10.0 (6.2) | 10.6 (5.9) |
| Non-smokers (%) | 100 | 99 | 100 | 98 | 100 |
| Cesarean delivery (%) | 42 | 48 | 59 | 39 | 38 |
| Household income (RMB/Month) | |||||
| <2000 | 22 | 19 | 27 | 29 | 34 |
| 2000–4000 | 41 | 50 | 46 | 44 | 46 |
| >4000 | 33 | 24 | 26 | 24 | 20 |
| Unknown | 1 | 7 | 2 | 0 | 0 |
| Infant | |||||
| Males (%) | 57 | 53 | 53 | 60 | 48 |
| Gestational age at birth (weeks), mean (SD) | 39.3 (1.2) | 39.2 (1.3) | 39.2 (1.6) | 39.4 (1.3) | 39.5 (1.5) |
| HMO | Linear Range | LoD | LoQ | RSD(r) a | RSD(iR) a | Native Content | High Spike | Medium Spike | Low Spike | Spike Recovery c (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg/kg) | (mg/kg) | (mg/kg) | (%) | (%) | (mg/kg) | (mg/kg) | (mg/kg) | (mg/kg) | High | Med | Low | |
| 2′-FL | 52.76–5276 | 4.4 | 53 | 1.8 | 3.2 | 1100 | 586 | 293 | 58.6 | 100 | 100 | 105 |
| 3-FL | 43.33–4333 | 3.6 | 43 | 4.4 | 4.7 | 785 | 481 | 241 | 48.1 | 99.7 | 99.6 | 101 |
| 3′-SL | 23.01–2301 | 1.9 | 23 | 2.7 | 3.6 | 79.5 | 256 | 128 | 25.6 | 101 | 99.5 | 101 |
| 6′-SL | 22.21–2221 | 1.9 | 22 | 1.8 b | 2.6 b | 20.0 | 247 | 123 | 24.7 | 101 | 99.9 | 97.4 |
| A-Tetra | 19.58–1958 | 4.9 | 20 | 2.4 b | 2.8 b | nd d | 218 | 109 | 21.8 | 99.9 | 98.7 | 100 |
| LNT | 14.00–5600 | 4.7 | 14 | 1.7 | 3.8 | 382 | 622 | 311 | 62.2 | 100 | 98.8 | 104 |
| LNnT | 19.96–1996 | 5.0 | 20 | 1.6 | 4.7 | 93.3 | 222 | 111 | 22.2 | 100 | 100 | 101 |
| LNFP-I | 14.95–5979 | 5.0 | 15 | 2.1 | 3.6 | 167 | 664 | 332 | 66.4 | 100 | 99.0 | 103 |
| LNFP-V | 13.36–1336 | 3.3 | 13 | 8.1 | 8.1 | 20.1 | 148 | 74.2 | 14.8 | 101 | 102 | 105 |
| LNnFP | 12.42–1241 | 3.1 | 12 | 2.1 b | 2.8 b | 8.85 | 138 | 69.0 | 13.8 | 100 | 99.6 | 101 |
| Oligosaccharide | Lactation Stage | n > LoD | n < LoD | Oligosaccharide Concentration (mg/kg) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | Median | SD | |||||
| 2′-FL | α-l-Fuc-(1→2)-β-d-Gal-(1→4)-d-Glc | 5–11 days | 88 | 0 | 26 | 4900 | 2000 | 2100 | 1400 |
| 12–30 days | 88 | 0 | 26 | 4000 | 1900 | 1900 | 1200 | ||
| 1–2 months | 90 | 0 | 26 | 4400 | 1700 | 1800 | 1100 | ||
| 2–4 months | 90 | 0 | 26 | 3800 | 1300 | 1300 | 900 | ||
| 4–8 months | 90 | 0 | 26 | 3000 | 1100 | 1200 | 710 | ||
| 3-FL | β-d-Gal-(1→4)-[α-l-Fuc-(1→3)-]-d-Glc | 5–11 days | 88 | 0 | 22 | 2800 | 490 | 230 | 600 |
| 12–30 days | 88 | 0 | 22 | 2400 | 570 a,b | 400 | 480 | ||
| 1–2 months | 90 | 0 | 22 | 2500 | 720 b | 620 | 550 | ||
| 2–4 months | 90 | 0 | 22 | 3300 | 1100 a,b | 980 | 610 | ||
| 4–8 months | 90 | 0 | 63 | 5900 | 1300 b | 1100 | 900 | ||
| 3′-SL | α-d-Neu5Ac-(2→3)-β-d-Gal-(1→4)-d-Glc | 5–11 days | 88 | 0 | 60 | 230 | 110 | 110 | 35 |
| 12–30 days | 88 | 0 | 53 | 180 | 94 a,b | 87 | 25 | ||
| 1–2 months | 90 | 0 | 49 | 160 | 80 a,b | 77 | 22 | ||
| 2–4 months | 90 | 0 | 43 | 140 | 79 b | 75 | 20 | ||
| 4–8 months | 90 | 0 | 46 | 260 | 83 b | 77 | 28 | ||
| 6′-SL | α-d-Neu5Ac-(2→6)-β-d-Gal-(1→4)-d-Glc | 5–11 days | 88 | 0 | 11 | 690 | 330 | 340 | 140 |
| 12–30 days | 88 | 0 | 68 | 600 | 250 a,b | 250 | 93 | ||
| 1–2 months | 90 | 0 | 32 | 420 | 140 a,b | 120 | 81 | ||
| 2–4 months | 90 | 0 | 25 | 210 | 78 a,b | 70 | 40 | ||
| 4–8 months | 90 | 0 | 11 | 99 | 39 a,b | 35 | 21 | ||
| A-Tetra | α-d-GalNAc-(1→3)-[α-l-Fuc-(1→2)]-β-d-Gal-(1→4)-d-Glc | 5–11 days | 18 | 70 | 9.8 | 47 | 13 | 9.8 | 9.5 |
| 12–30 days | 13 | 75 | 9.8 | 160 | 27 | 9.8 | 42 | ||
| 1–2 months | 12 | 78 | 9.8 | 140 | 25 | 9.8 | 36 | ||
| 2–4 months | 13 | 77 | 9.8 | 57 | 18 | 9.8 | 15 | ||
| 4–8 months | 9 | 81 | 9.8 | 68 | 22 | 9.8 | 21 | ||
| LNT | β-d-Gal-(1→3)-β-d-GlcNAc-(1→3)-β-d-Gal-(1→4)-d-Glc | 5–11 days | 88 | 0 | 97 | 3000 | 880 | 790 | 530 |
| 12–30 days | 88 | 0 | 130 | 2200 | 620 a,b | 550 | 340 | ||
| 1–2 months | 90 | 0 | 95 | 1200 | 370 a,b | 290 | 220 | ||
| 2–4 months | 90 | 0 | 41 | 960 | 290 a,b | 250 | 170 | ||
| 4–8 months | 90 | 0 | 21 | 750 | 250 b | 190 | 160 | ||
| LNnT | β-d-Gal-(1→4)-β-d-GlcNAc-(1→3)-β-d-Gal-(1→4)-d-Glc | 5–11 days | 88 | 0 | 10 | 390 | 180 | 170 | 85 |
| 12–30 days | 88 | 0 | 23 | 370 | 120 a,b | 110 | 67 | ||
| 1–2 months | 90 | 0 | 10 | 240 | 83 a,b | 81 | 43 | ||
| 2–4 months | 90 | 0 | 10 | 170 | 65 a,b | 54 | 39 | ||
| 4–8 months | 90 | 0 | 10 | 200 | 50 a,b | 43 | 36 | ||
| LNFP-I | α-l-Fuc-(1→2)-β-d-Gal-(1→3)-β-d-GlcNAc-(1→3)-β-d-Gal-(1→4)-d-Glc | 5–11 days | 81 | 7 | 7.5 | 4000 | 910 | 880 | 740 |
| 12–30 days | 80 | 8 | 7.5 | 1700 | 540 | 490 | 400 | ||
| 1–2 months | 74 | 16 | 7.5 | 1400 | 340 b | 290 | 240 | ||
| 2–4 months | 73 | 17 | 7.5 | 660 | 180 a,b | 140 | 140 | ||
| 4–8 months | 74 | 16 | 7.5 | 860 | 160 b | 110 | 150 | ||
| LNFP-V | β-d-Gal-(1→3)-β-d-GlcNAc-(1→3)-β-d-Gal-(1→4)-[α-l-Fuc-(1→3)-]-d-Glc | 5–11 days | 86 | 2 | 6.7 | 250 | 41 | 22 | 49 |
| 12–30 days | 84 | 4 | 6.7 | 240 | 39 | 24 | 41 | ||
| 1–2 months | 86 | 4 | 6.7 | 110 | 26 a,b | 16 | 25 | ||
| 2–4 months | 87 | 3 | 6.7 | 130 | 25 | 19 | 24 | ||
| 4–8 months | 88 | 2 | 6.7 | 75 | 20 b | 18 | 15 | ||
| LNnFP | β-d-Gal-(1→4)-β-d-GlcNAc-(1→3)-β-d-Gal-(1→4)-[α-l-Fuc-(1→3)-]-d-Glc | 5–11 days | 80 | 8 | 6.2 | 51 | 11 | 6.2 | 9.0 |
| 12–30 days | 83 | 5 | 6.2 | 31 | 8.1 a,b | 6.2 | 4.7 | ||
| 1–2 months | 81 | 9 | 6.2 | 22 | 7.9 b | 6.2 | 4.1 | ||
| 2–4 months | 79 | 11 | 6.2 | 21 | 8.2 b | 6.2 | 4.1 | ||
| 4–8 months | 75 | 15 | 6.2 | 23 | 7.5 b | 6.2 | 3.7 | ||
| Oligosaccharide | Lactation Stage | n > LoQ | n < LoQ | Oligosaccharide Concentration (mg/kg) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | Median | SD | |||||
| 2′-FL | α-l-Fuc-(1→2)-β-d-Gal-(1→4)-d-Glc | 5–11 days | 67 | 21 | 56 | 4900 | 2600 | 2500 | 970 |
| 12–30 days | 70 | 18 | 290 | 4000 | 2300 | 2300 | 800 | ||
| 1–2 months | 71 | 19 | 690 | 4400 | 2200 | 2100 | 730 | ||
| 2–4 months | 71 | 19 | 84 | 3800 | 1800 a,b | 1600 | 670 | ||
| 4–8 months | 71 | 19 | 290 | 3000 | 1300 a,b | 1400 | 510 | ||
| 3-FL | β-d-Gal-(1→4)-[α-l-Fuc-(1→3)-]-d-Glc | 5–11 days | 84 | 4 | 47 | 2800 | 510 | 250 | 600 |
| 12–30 days | 86 | 2 | 50 | 2300 | 580 a,b | 400 | 480 | ||
| 1–2 months | 88 | 2 | 63 | 2500 | 730 b | 620 | 550 | ||
| 2–4 months | 88 | 2 | 72 | 3300 | 1100 a,b | 990 | 600 | ||
| 4–8 months | 90 | 0 | 63 | 5900 | 1300 b | 1100 | 900 | ||
| 3′-SL | α-d-Neu5Ac-(2→3)-β-d-Gal-(1→4)-d-Glc | 5–11 days | 88 | 0 | 60 | 230 | 110 | 110 | 350 |
| 12–30 days | 88 | 0 | 53 | 180 | 94 a,b | 87 | 25 | ||
| 1–2 months | 90 | 0 | 49 | 160 | 80 a,b | 77 | 22 | ||
| 2–4 months | 90 | 0 | 43 | 140 | 79 b | 75 | 20 | ||
| 4–8 months | 90 | 0 | 46 | 260 | 83 b | 77 | 28 | ||
| 6′-SL | α-d-Neu5Ac-(2→6)-β-d-Gal-(1→4)-d-Glc | 5–11 days | 85 | 3 | 33 | 690 | 340 | 350 | 120 |
| 12–30 days | 88 | 0 | 68 | 600 | 250 a,b | 250 | 93 | ||
| 1–2 months | 90 | 0 | 32 | 420 | 140 a,b | 120 | 81 | ||
| 2–4 months | 90 | 0 | 25 | 210 | 78 a,b | 70 | 40 | ||
| 4–8 months | 73 | 17 | 23 | 99 | 45 a,b | 42 | 18 | ||
| A-Tetra | α-d-GalNAc-(1→3)-[α-l-Fuc-(1→2)]-β-d-Gal-(1→4)-d-Glc | 5–11 days | 2 | 86 | 28 | 47 | 38 | 380 | 13 |
| 12–30 days | 3 | 85 | 31 | 160 | 86 | 71 | 64 | ||
| 1–2 months | 4 | 86 | 22 | 140 | 56 | 32 | 54 | ||
| 2–4 months | 4 | 86 | 20 | 57 | 36 | 33 | 17 | ||
| 4–8 months | 3 | 87 | 34 | 68 | 46 | 37 | 19 | ||
| LNT | β-d-Gal-(1→3)-β-d-GlcNAc-(1→3)-β-d-Gal-(1→4)-d-Glc | 5–11 days | 88 | 0 | 97 | 3000 | 880 | 790 | 530 |
| 12–30 days | 88 | 0 | 130 | 2200 | 620 a,b | 550 | 340 | ||
| 1–2 months | 90 | 0 | 95 | 1200 | 370 a,b | 290 | 220 | ||
| 2–4 months | 90 | 0 | 41 | 960 | 290 a,b | 250 | 170 | ||
| 4–8 months | 90 | 0 | 21 | 750 | 250 b | 190 | 160 | ||
| LNnT | β-d-Gal-(1→4)-β-d-GlcNAc-(1→3)-β-d-Gal-(1→4)-d-Glc | 5–11 days | 85 | 3 | 26 | 390 | 180 | 170 | 81 |
| 12–30 days | 83 | 5 | 23 | 370 | 120 a,b | 110 | 67 | ||
| 1–2 months | 87 | 3 | 23 | 240 | 85 a,b | 82 | 42 | ||
| 2–4 months | 83 | 7 | 20 | 170 | 69 a,b | 59 | 38 | ||
| 4–8 months | 74 | 16 | 21 | 200 | 59 a,b | 49 | 34 | ||
| LNFP-I | α-l-Fuc-(1→2)-β-d-Gal-(1→3)-β-d-GlcNAc-(1→3)-β-d-Gal-(1→4)-d-Glc | 5–11 days | 68 | 20 | 15 | 4000 | 1100 | 1000 | 690 |
| 12–30 days | 71 | 17 | 39 | 1700 | 600 | 530 | 370 | ||
| 1–2 months | 73 | 17 | 20 | 1400 | 340 | 290 | 240 | ||
| 2–4 months | 70 | 20 | 21 | 660 | 190 a,b | 150 | 140 | ||
| 4–8 months | 69 | 21 | 21 | 860 | 170 b | 120 | 150 | ||
| LNFP-V | β-d-Gal-(1→3)-β-d-GlcNAc-(1→3)-β-d-Gal-(1→4)-[α-l-Fuc-(1→3)-]-d-Glc | 5–11 days | 58 | 30 | 14 | 250 | 57 | 30 | 53 |
| 12–30 days | 64 | 24 | 13 | 240 | 49 | 34 | 42 | ||
| 1–2 months | 52 | 38 | 14 | 110 | 38 a,b | 26 | 25 | ||
| 2–4 months | 57 | 33 | 14 | 130 | 35 | 26 | 24 | ||
| 4–8 months | 54 | 36 | 14 | 75 | 29 b | 24 | 13 | ||
| LNnFP | β-d-Gal-(1→4)-β-d-GlcNAc-(1→3)-β-d-Gal-(1→4)-[α-l-Fuc-(1→3)-]-d-Glc | 5–11 days | 24 | 62 | 13 | 51 | 22 | 18 | 9.9 |
| 12–30 days | 14 | 74 | 13 | 31 | 17 a,b | 16 | 5.3 | ||
| 1–2 months | 13 | 77 | 13 | 22 | 17 | 17 | 3.4 | ||
| 2–4 months | 17 | 73 | 13 | 21 | 16 | 14 | 3.1 | ||
| 4–8 months | 9 | 81 | 13 | 23 | 17 b | 17 | 3.4 | ||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Austin, S.; De Castro, C.A.; Bénet, T.; Hou, Y.; Sun, H.; Thakkar, S.K.; Vinyes-Pares, G.; Zhang, Y.; Wang, P. Temporal Change of the Content of 10 Oligosaccharides in the Milk of Chinese Urban Mothers. Nutrients 2016, 8, 346. https://doi.org/10.3390/nu8060346
Austin S, De Castro CA, Bénet T, Hou Y, Sun H, Thakkar SK, Vinyes-Pares G, Zhang Y, Wang P. Temporal Change of the Content of 10 Oligosaccharides in the Milk of Chinese Urban Mothers. Nutrients. 2016; 8(6):346. https://doi.org/10.3390/nu8060346
Chicago/Turabian StyleAustin, Sean, Carlos A. De Castro, Thierry Bénet, Yangfeng Hou, Henan Sun, Sagar K. Thakkar, Gerard Vinyes-Pares, Yumei Zhang, and Peiyu Wang. 2016. "Temporal Change of the Content of 10 Oligosaccharides in the Milk of Chinese Urban Mothers" Nutrients 8, no. 6: 346. https://doi.org/10.3390/nu8060346
APA StyleAustin, S., De Castro, C. A., Bénet, T., Hou, Y., Sun, H., Thakkar, S. K., Vinyes-Pares, G., Zhang, Y., & Wang, P. (2016). Temporal Change of the Content of 10 Oligosaccharides in the Milk of Chinese Urban Mothers. Nutrients, 8(6), 346. https://doi.org/10.3390/nu8060346






