The Distribution of Obesity Phenotypes in HIV-Infected African Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Sampling Procedure
2.2. Data Collection
2.2.1. Interviews
2.2.2. Physical Examination
2.2.3. Laboratory Measurements
2.3. Definitions
2.3.1. Socio-Demographic Characteristics
2.3.2. Body Size Phenotype
2.4. Statistical Analysis
3. Results
3.1. Socio-Demographic Characteristics
3.2. Profile of HIV Infection
3.3. Profile of Cardio-Metabolic Abnormalities
3.4. Distribution of Body Size Phenotypes
3.5. Distribution of Metabolic Phenotypes within and Across BMI Categories
3.6. Prediction of Body Mass Index Categories by the Continuous Metabolic Traits
4. Discussion
Limitations and Strengths
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| ART | antiretroviral therapy |
| AIDS | acquired immunodeficiency syndrome |
| BMI | body mass index |
| B × M | interaction between BMI and metabolic status |
| BP | blood pressure |
| CVD | cardiovascular disease |
| DBP | diastolic blood pressure |
| FPG | fasting plasma glucose |
| HDL-C | high density lipoprotein cholesterol |
| HIV | human immunodeficiency virus |
| HOMA-IR | homeostatic model assessment of insulin resistance |
| Hs-CRP | high sensitivity-C reactive protein |
| LDL-C | low density lipoprotein cholesterol |
| M × G | interaction between metabolic status and gender |
| NWMH | normal-weight metabolically healthy |
| NWMA | normal weight metabolically abnormal |
| OvMH | overweight metabolically healthy |
| OvMA | overweight metabolically abnormal |
| OMH | obese metabolically healthy |
| OMA | obese metabolically abnormal |
| PDA | personal digital assistant |
| SBP | systolic blood pressure |
| SD | standard deviation |
| T2DM | type 2 diabetes mellitus |
| WC | waist circumference |
References
- Murray, C.J.; Ortblad, K.F.; Guinovart, C.; Lim, S.S.; Wolock, T.M.; Roberts, D.A.; Dansereau, E.A.; Graetz, N.; Barber, R.M.; Brown, J.C.; et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: A systematic analysis for the global burden of disease study 2013. Lancet 2014, 384, 1005–1070. [Google Scholar] [CrossRef]
- World Health Organisation. Millennium Development Goals (mdgs) 6: Combat HIV/AIDS, Malaria and Other Diseases. Available online: http://www.who.int/topics/millennium_development_goals/diseases/en/ (accessed on 21 March 2016).
- Samji, H.; Cescon, A.; Hogg, R.S.; Modur, S.P.; Althoff, K.N.; Buchacz, K.; Burchell, A.N.; Cohen, M.; Gebo, K.A.; Gill, M.J.; et al. Closing the gap: Increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS ONE 2013, 8, e81355. [Google Scholar] [CrossRef] [PubMed]
- Boodram, B.; Plankey, M.W.; Cox, C.; Tien, P.C.; Cohen, M.H.; Anastos, K.; Karim, R.; Hyman, C.; Hershow, R.C. Prevalence and correlates of elevated body mass index among HIV-positive and HIV-negative women in the women‘s interagency HIV study. AIDS Patient Care STDS 2009, 23, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Crum-Cianflone, N.; Roediger, M.P.; Eberly, L.; Headd, M.; Marconi, V.; Ganesan, A.; Weintrob, A.; Barthel, R.V.; Fraser, S.; Agan, B.K. Increasing rates of obesity among HIV-infected persons during the HIV epidemic. PLoS ONE 2010, 5, e10106. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.; Ruppik, M.; Rickenbach, M.; Spoerri, A.; Furrer, H.; Battegay, M.; Cavassini, M.; Calmy, A.; Bernasconi, E.; Schmid, P.; et al. Decreasing mortality and changing patterns of causes of death in the swiss HIV cohort study. HIV Med. 2013, 14, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Palella, F.J., Jr.; Baker, R.K.; Moorman, A.C.; Chmiel, J.S.; Wood, K.C.; Brooks, J.T.; Holmberg, S.D. Mortality in the highly active antiretroviral therapy era: Changing causes of death and disease in the HIV outpatient study. J. Acquir. Immune Defic. Syndr. 2006, 43, 27–34. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Global Status Report on Noncommunicable Diseases 2014; WHO: Geneva, Switzerland, 2014; p. 298. [Google Scholar]
- Cornier, M.A. The metabolic syndrome. Endocr. Rev. 2008, 29, 777–822. [Google Scholar] [CrossRef] [PubMed]
- Wildman, R.P.; Muntner, P.; Reynolds, K.; McGinn, A.P.; Rajpathak, S.; Wylie-Rosett, J.; Sowers, M.R. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: Prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004). Arch. Intern. Med. 2008, 168, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Van Wijk, J.P.H.; Cabezas, M.C. Hypertriglyceridemia, metabolic syndrome, and cardiovascular disease in HIV-infected patients: Effects of antiretroviral therapy and adipose tissue distribution. Int. J. Vasc. Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Giralt, M.; Domingo, P.; Guallar, J.P.; de la Concepcion, M.L.R.; Alegre, M.; Domingo, J.C.; Villarroya, F. HIV-1 infection alters gene expression in adipose tissue, which contributes to HIV-1/haart-associated lipodystrophy. Antivir. Ther. 2006, 11, 729–740. [Google Scholar] [PubMed]
- Grinspoon, S.; Carr, A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N. Engl. J. Med. 2005, 352, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Anuurad, E.; Bremer, A.; Berglund, L. HIV protease inhibitors and obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Worm, S.W.; Lundgren, J.D. The metabolic syndrome in HIV. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Stanley, T.L.; Grinspoon, S.K. Body composition and metabolic changes in HIV-infected patients. J. Infect. Dis. 2012, 205, S383–S390. [Google Scholar] [CrossRef] [PubMed]
- Thompson-Paul, A.M.; Wei, S.C.; Mattson, C.L.; Robertson, M.; Hernandez-Romieu, A.C.; Bell, T.K.; Skarbinski, J. Obesity among HIV-infected adults receiving medical care in the United States: Data from the cross-sectional medical monitoring project and national health and nutrition examination survey. Medicine 2015, 94. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- South African National Department of Health. National Consolidated Guidelines for the Prevention of Mother-to-Child Transmission of HIV (Pmtct) and the Management of HIV in Children, Adolescents and Adults; National Department of Health of Republic of South Africa: Pretoria, South Africa, 2015.
- Rey-Lopez, J.P.; de Rezende, L.F.; Pastor-Valero, M.; Tess, B.H. The prevalence of metabolically healthy obesity: A systematic review and critical evaluation of the definitions used. Obes. Rev. 2014, 15, 781–790. [Google Scholar] [CrossRef] [PubMed]
- McFadden, D.L. Conditional logit analysis of qualitative choice behavior. In Frontiers in Econometrics; Zarembka, P., Ed.; Academic Press: New York, NY, USA, 1973; pp. 105–142. [Google Scholar]
- Shisana, O.; Labadarios, D.; Rehle, T.; Simbayi, L. South African National Health and Nutrition Examination Survey (Sanhanes-1); Human Science Research Council (HSRC) Press: Cape Town, South Africa, 2013. [Google Scholar]
- Mbanya, V.N.; Echouffo-Tcheugui, J.B.; Akhtar, H.; Mbanya, J.C.; Kengne, A.P. Obesity phenotypes in urban and rural cameroonians: A cross-sectional study. Diabetol. Metab. Syndr. 2015, 7, 21. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matsha, T.E.; Hartnick, M.D.; Kisten, Y.; Eramus, R.T.; Kengne, A.P. Obesity phenotypes and subclinical cardiovascular diseases in a mixed-ancestry South African population: A cross-sectional study. J. Diabetes 2014, 6, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Beraldo, R.A.; Meliscki, G.C.; Silva, B.R.; Navarro, A.M.; Bollela, V.R.; Schmidt, A.; Foss-Freitas, M.C. Comparing the ability of anthropometric indicators in identifying metabolic syndrome in HIV patients. PLoS ONE 2016, 11, e0149905. [Google Scholar] [CrossRef] [PubMed]
- Velho, S.; Paccaud, F.; Waeber, G.; Vollenweider, P.; Marques-Vidal, P. Metabolically healthy obesity: Different prevalences using different criteria. Eur. J. Clin. Nutr. 2010, 64, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.M.; Dillon, C.; Harrington, J.M.; McCarthy, V.J.; Kearney, P.M.; Fitzgerald, A.P.; Perry, I.J. Defining metabolically healthy obesity: Role of dietary and lifestyle factors. PLoS ONE 2013, 8, e76188. [Google Scholar] [CrossRef] [PubMed]
- Jennings, C.L.; Lambert, E.V.; Collins, M.; Joffe, Y.; Levitt, N.S.; Goedecke, J.H. Determinants of insulin-resistant phenotypes in normal-weight and obese black african women. Obesity 2008, 16, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.A.; Peer, N.; Mills, E.J.; Kengne, A.P. A meta-analysis of the metabolic syndrome prevalence in the global HIV-infected population. PLoS ONE 2016, 11, e0150970. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Garcia, E.; Guallar-Castillon, P.; Leon-Muñoz, L.; Rodriguez-Artalejo, F. Prevalence and determinants of metabolically healthy obesity in Spain. Atherosclerosis 2013, 231, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Lee, K. Metabolically obese but normal weight (MONW) and metabolically healthy but obese (MHO) phenotypes in Koreans: Characteristics and health behaviors. Asia Pac. J. Clin. Nutr. 2009, 18, 280–284. [Google Scholar] [PubMed]
- Hirigo, A.T.; Tesfaye, D.Y. Influences of gender in metabolic syndrome and its components among people living with HIV virus using antiretroviral treatment in Hawassa, Southern Ethiopia. BMC Res. Notes 2016, 9, 145. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhuang, R.; Luo, X.; Yin, L.; Pang, C.; Feng, T.; You, H.; Zhai, Y.; Ren, Y.; Zhang, L.; et al. Prevalence of metabolically healthy obese and metabolically obese but normal weight in adults worldwide: A meta-analysis. Horm. Metab. Res. 2015, 47, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Kimokoti, R.W.; Judd, S.E.; Shikany, J.M.; Newby, P.K. Metabolically healthy obesity is not associated with food intake in white or black men. J. Nutr. 2015, 145, 2551–2561. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Overall, (n = 748) | Men, (n = 157) | Women, (n = 591) | p |
|---|---|---|---|---|
| Age, year | 38 (32–44) | 41 (35–47) | 37 (31–43) | <0.001 |
| Education level, n (%) | <0.001 | |||
| Primary | 113/746 (15.1) | 38/157 (24.2) | 75/589 (12.7) | |
| Secondary and above | 633/746 (84.9) | 119/157 (75.8) | 514/589 (87.3) | |
| Employed, n (%) | 408/747 (54.6) | 78/157 (49.7) | 330/590 (55.9) | 0.162 |
| Smoking habit, n (%) | <0.001 | |||
| Never smoke | 461/718 (64.7) | 34/156 (22.2) | 427/562 (76.4) | |
| Current smoker | 187/718 (25.3) | 93/156 (58.8) | 90/562 (16.1) | |
| Past smoker | 70/718 (13.3) | 29/156 (45.3) | 42/562 (9.0) | |
| Heavy drinker, n (%) | 64/187 (34.2) | 22/64 (34.4) | 42/123 (34.1) | 0.975 |
| HIV duration, years | 5 (2–9) | 4 (2–7) | 5 (2.5–9) | <0.001 |
| CD4, cells/mm3 | 392(240–604) | 272(193–448) | 410(253–627) | 0.001 |
| ART treatment, n (%) | 0.005 | |||
| Non-ART | 46/699 (6.6) | 7/149 (4.7) | 39/550 (7.1) | |
| first line | 426/699 (60.9) | 78/149 (52.3) | 348/550 (63.3) | |
| second line | 79/699 (11.3) | 17/149 (11.4) | 62/550 (11.3) | |
| Others | 148/699 (21.2) | 47/149 (31.5) | 101/550 (18.3) | |
| Body mass index (kg/m2) | ||||
| Median (P25–P75) | 26.3 (22.1–32) | 21.4 (19.8–22.4) | 28.3 (23.8–28.9) | <0.001 |
| <25, n (%) | 325 (43.4) | 126 (80.3) | 199 (33.7) | |
| 25.0–29.9, n (%) | 171 (22.9) | 21 (13.4) | 150 (25.4) | |
| ≥30, n (%) | 252 (33.7) | 10 (6.4) | 242 (40.9) | |
| Waist circumference, cm | 88 (77.5–98) | 78.9 (73.9–88.3) | 90 (79.5–100.8) | <0.001 |
| Systolic BP, mmHg | 117 (107–129.5) | 123.5 (114.5–140) | 115 (105.8–127) | <0.001 |
| Diastolic BP, mmHg | 82 (75–90.5) | 83 (76–94) | 81.5 (74.8–89.8) | 0.129 |
| Total cholesterol, mmol/L | 4.3 (3.7–5.1) | 4.2 (3.5–5.0) | 4.4 (3.8–5.1) | 0.009 |
| HDL-cholesterol, mmol/L | 1.27 (1.03–1.5) | 1.2 (1.0–1.5) | 1.29 (1.08–1.52) | 0.010 |
| LDL-cholesterol, mmol/L | 2.5 (2.0–3.1) | 2.3 (1.7–3.0) | 2.5 (2.0–3.1) | 0.012 |
| Triglycerides, mmol/L | 1.0 (0.74–1.34) | 1.12 (0.75–1.27) | 0.97 (0.74–1.28) | 0.023 |
| Fasting glucose, mmol/L | 5.0 (4.6–5.4) | 5.1 (4.8–5.5) | 4.9 (4.6–5.4) | 0.010 |
| HOMA-IR | 1.36 (0.84–2.24) | 0.94 (0.53–1.64) | 1.49 (0.93–2.37) | <0.001 |
| C-reactive protein, mg/L | 5.6 (2.4–12) | 5.0 (2.1–16.2) | 5.6 (2.4–14.2) | 0.728 |
| Treated hypertension, n (%) | 110 (14.7) | 11 (7) | 99 (16.8) | 0.002 |
| Treated diabetes, n (%) | 28 (3.7) | 8 (5.1) | 20 (3.4) | 0.432 |
| BMI Categories | Normal Weight (n = 325) | Overweight (n = 171) | Obese (n = 252) | p-Trend | p-Interaction | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabolic Status | Healthy | Abnormal | p | Healthy | Abnormal | p | Healthy | Abnormal | p | Overall | Healthy | Abnormal | B × M | M × G |
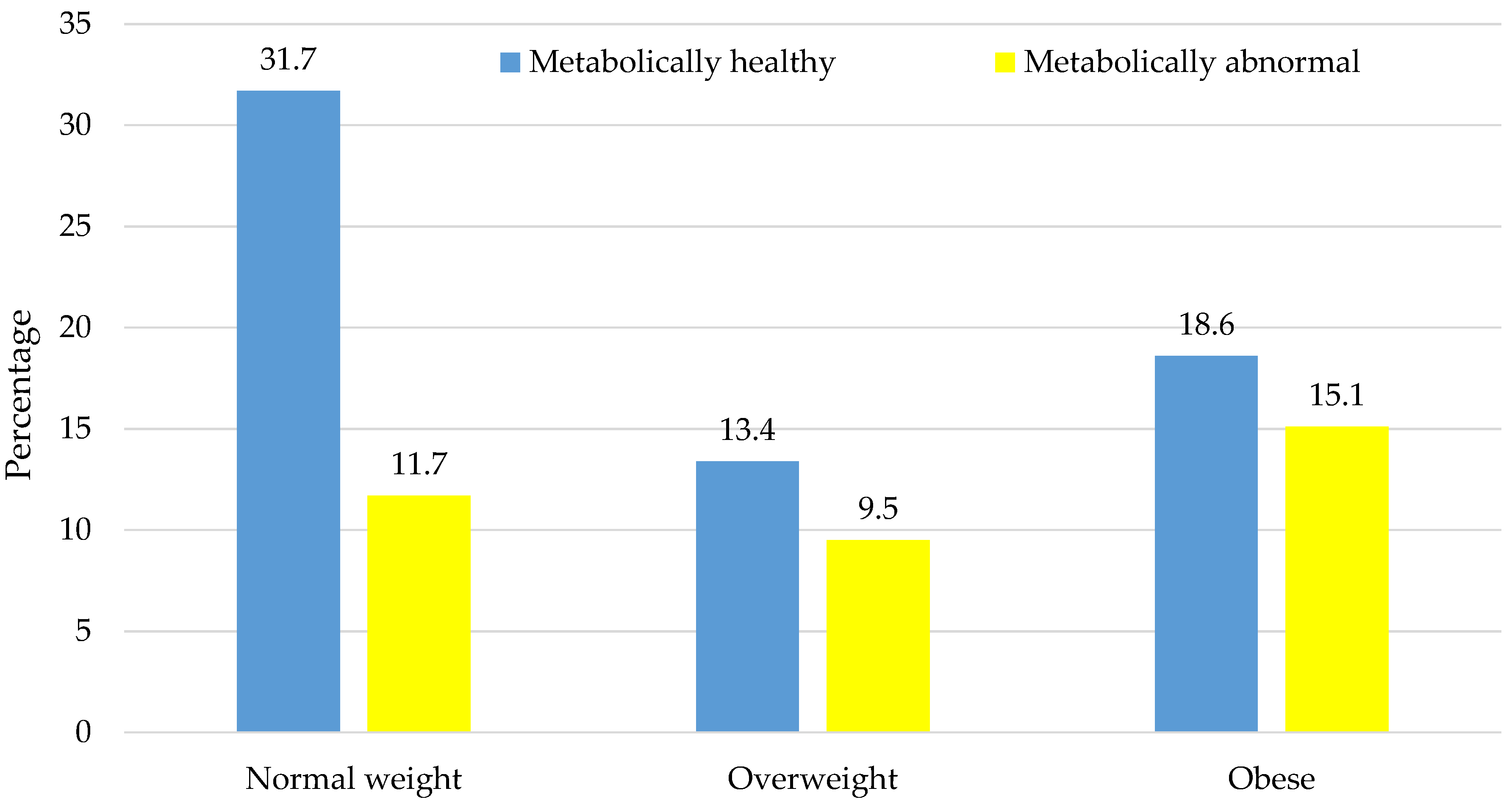
| Prevalence, n (%) | 237 (31.7) | 88 (11.7) | 100 (13.4) | 71 (9.5) | 139 (18.6) | 113 (15.1) | <0.001 | - | - | - | - | |||
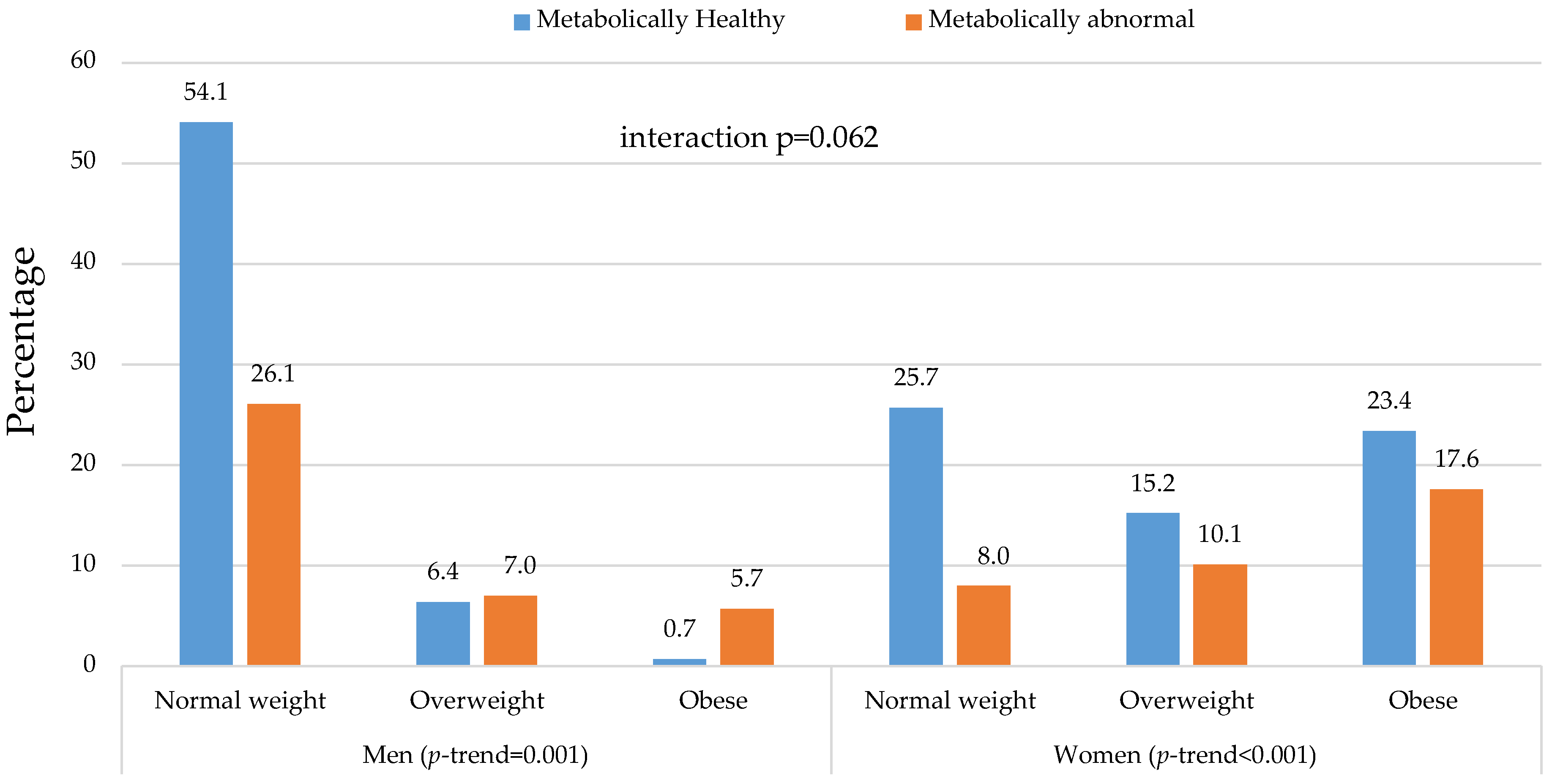
| Men, n (%) | 85 (35.9) | 41 (46.6) | 0.078 | 10 (10.0) | 11 (15.5) | 0.281 | 1 (0.7) | 9 (8.0) | 0.006 | <0.001 | <0.001 | <0.001 | 0.759 | - |
| Age, years | 36 (30–44) | 42 (34-49) | <0.001 | 36 (31–42) | 43 (36–47.5) | <0.001 | 37 (31.5–41) | 39 (34–47) | 0.001 | 0.002 | <0.001 | 0.396 | 0.065 | 0.34 |
| ≥7 school-years, n (%) | 190/236 (80.5) | 71/88 (80.7) | 0.972 | 89/100 (89.0) | 58/70 (82.9) | 0.249 | 128/139 (92.1) | 97/113 (85.8) | 0.111 | 0.012 | 0.005 | 0.617 | 0.532 | 0.067 |
| Unemployed, n (%) | 84/236 (35.6) | 48/88 (54.5) | 0.002 | 38/100 (38.0) | 47/71 (66.2) | <0.001 | 64/139 (46.0) | 58/113 (51.3) | 0.404 | 0.081 | 0.131 | 0.132 | 0.056 | 0.061 |
| Smoking habit, n (%) | 0.523 | 0.327 | 0.144 | <0.001 | <0.001 | <0.001 | 0.925 | 0.238 | ||||||
| Never | 105/230 (45.7) | 33/85 (38.8) | 78/96 (81.3) | 49/68 (72.0) | 111/132 (84.1) | 85/107 (79.4) | ||||||||
| Current smoker | 99/230 (43.0) | 40/85 (47.1) | 12/96 (12.5) | 11/68 (16.2) | 15/132 (11.4) | 10/107 (9.3) | ||||||||
| Past smokers | 26/230 (11.3) | 12/85 (14.1) | 6/96 (6.2) | 8/68 (11.8) | 6/132 (4.5) | 12/107 (11.2) | ||||||||
| Heavy drinkers, n (%) | 26/74 (35.1) | 8/27 (29.6) | 0.643 | 7/25 (28.0) | 8/19 (42.1) | 0.356 | 7/22 (31.8) | 8/20 (40.0) | 0.748 | 0.973 | 0.801 | 0.638 | 0.518 | 0.766 |
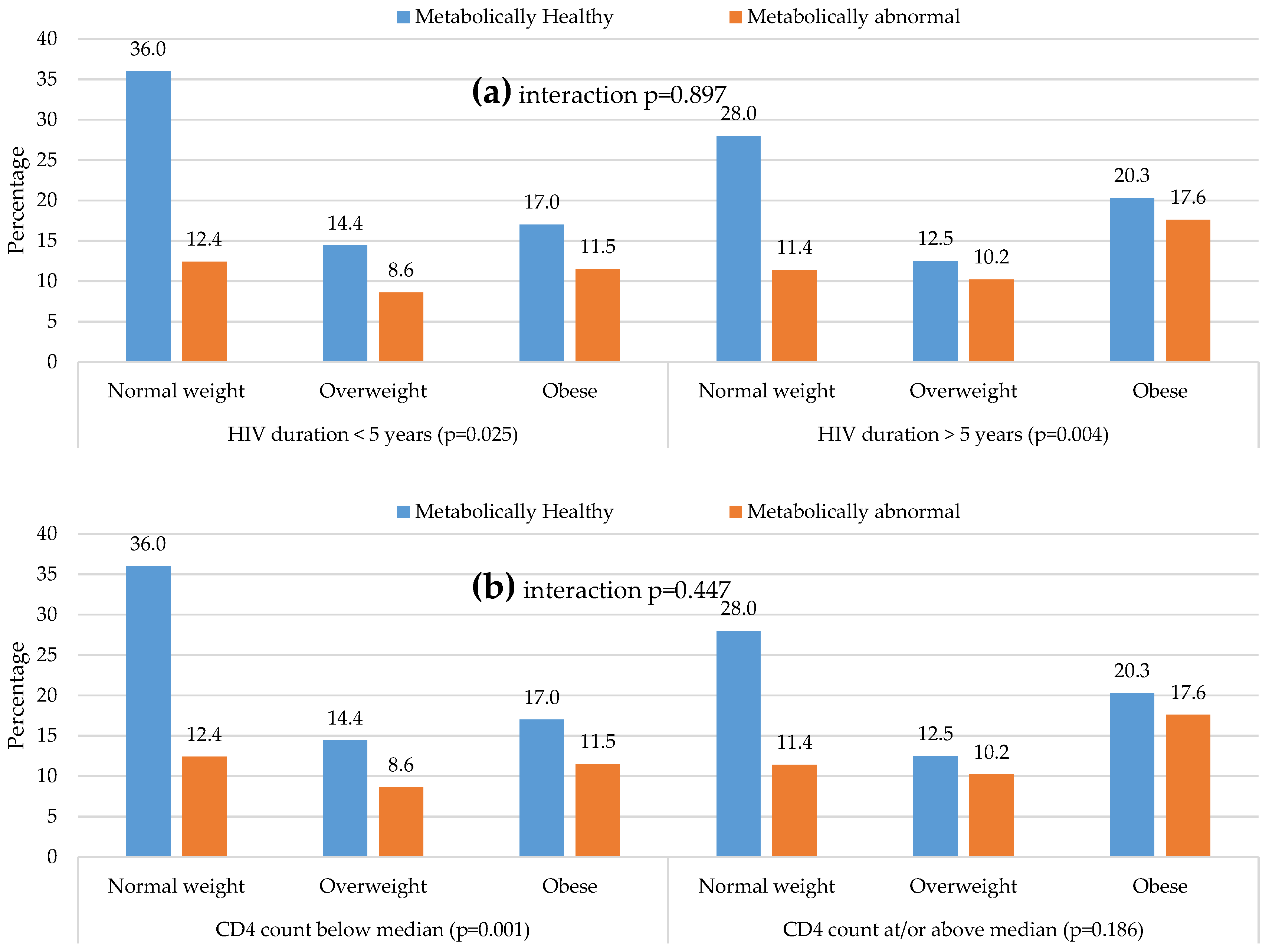
| HIV diagnosed duration, years | 4 (2–7.8) | 5 (2–8) | 0.577 | 4.3 (2–8) | 6 (2–9) | 0.149 | 5 (3–10) | 6 (4–10) | 0.334 | 0.413 | 0.435 | 0.436 | 0.820 | >0.999 |
| Median CD4 count, /mm3 | 311 (172–473) | 350 (232–544) | 0.288 | 433 (187–630) | 395 (252–626) | 0.494 | 452 (297–677) | 434 (267–699) | 0.627 | 0.335 | 0.213 | 0.627 | 0.77 | 0.430 |
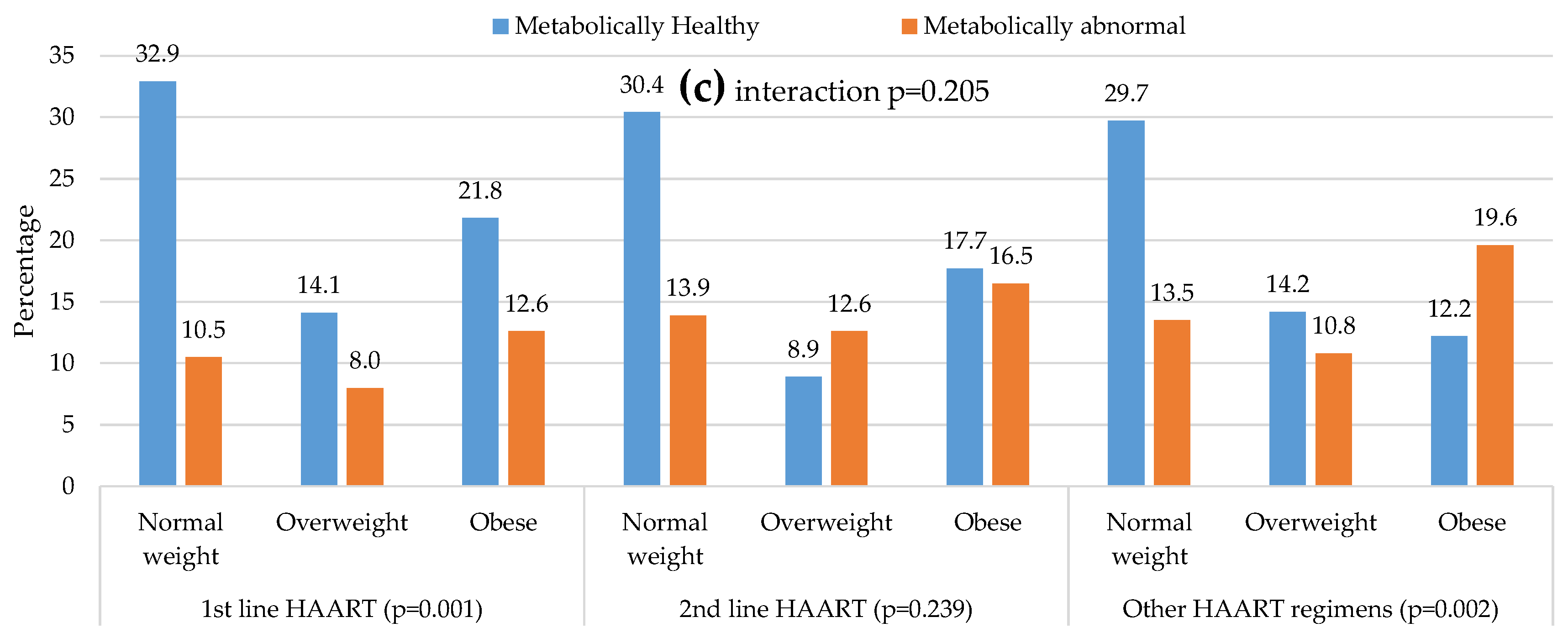
| Antiretroviral regimens, n (%) | 0.448 | 0.201 | 0.009 | 0.947 | 0.386 | 0.963 | 0.363 | 0.179 | ||||||
| First line | 140/208 (67.3) | 45/76 (59.2) | 60/88 (68.3) | 34/60 (56.6) | 93/125 (74.4) | 54/96 (56.2) | ||||||||
| Second line | 24/208 (11.5) | 11/76 (14.5) | 7/88 (8.0) | 10/60 (16.7) | 14/125 (11.2) | 13/96 (13.5) | ||||||||
| Others | 44/208 (21.2) | 20/76 (26.3) | 21/88 (23.7) | 16/60 (26.7) | 18/125 (14.4) | 29/96 (30.2) | ||||||||
| Waist circumference, cm | 77 (72–80) | 78 (72–86) | 0.016 | 89 (85–92) | 93 (86–95) | 0.005 | 101 (95–108) | 104 (99–111) | 0.005 | <0.001 | <0.001 | 0.016 | 0.560 | 0.780 |
| Systolic blood pressure, mmHg | 114 (105–125) | 128 (116–145) | <0.001 | 112 (104–124) | 125 (117–140) | <0.001 | 113 (106–119) | 124 (114–138) | <0.001 | 0.230 | 0.136 | 0.560 | 0.620 | 0.610 |
| Diastolic blood pressure, mmHg | 78 (72–85) | 88 (81–92) | <0.001 | 81 (73–85) | 88 (82–97) | <0.001 | 81 (75–85) | 88 (80–96) | <0.001 | 0.954 | 0.771 | 0.819 | 0.230 | 0.510 |
| Fasting glucose, mmol/L | 4.9 (4.6–5.2) | 5.3 (4.8–6.3) | <0.001 | 4.9 (4.6–5.2) | 5.2 (4.7–5.7) | 0.001 | 4.9 (4.6–5.2) | 5.6 (5.0–6.4) | <0.001 | 0.010 | 0.583 | 0.049 | 0.044 | 0.510 |
| Median HOMA-IR | 0.85 (0.57–1.27) | 1.16 (0.82–1.79) | <0.001 | 1.31 (0.931.81) | 1.76 (1.05–2.49) | 0.003 | 1.9 (1.33–2.44) | 2.52 (1.54–4.67) | <0.001 | <0.001 | 0.001 | 0.006 | 0.630 | 0.290 |
| Diabetes a, n (%) | 2/227 (0.9) | 21/87 (24.1) | <0.001 | 1/96 (1.0) | 10/68 (14.7) | 0.001 | 2/128 (1.6) | 27/110 (24.6) | <0.001 | 0.077 | 0.839 | 0.252 | 0.148 | 0.752 |
| Hypertension b, n (%) | 52 (21.9) | 50 (56.8) | <0.001 | 20 (20.0) | 43 (60.6) | <0.001 | 35 (25.2) | 70 (62.0) | <0.001 | 0.038 | 0.615 | 0.757 | 0.841 | 0.107 |
| Triglycerides, mmol/L | 0.9 (0.7–1.2) | 1.2 (1.0–1.9) | <0.001 | 0.9 (0.7–1.2) | 1.2 (1.0–1.9) | <0.001 | 0.9 (0.7–1.2) | 1.4 (1.0–1.9) | <0.001 | 0.033 | 0.647 | 0.472 | 0.720 | 0.002 |
| HDL-cholesterol, mmol/L | 1.4 (1.1–1.7) | 1.2 (0.9–1.3) | <0.001 | 1.4 (1.2–1.7) | 1.1 (1.0–1.2) | <0.001 | 1.4 (1.2–1.6) | 1.1 (1.0–1.2) | <0.001 | 0.001 | 0.021 | 0.024 | 0.790 | 0.640 |
| LDL-cholesterol, mmol/L | 2.2 (1.8–2.9) | 2.5 (1.9–3.1) | 0.181 | 2.4 (2.0–3.0) | 2.5 (2.0–3.3) | 0.423 | 2.6 (2.2–3.1) | 2.8 (2.3–3.4) | 0.019 | 0.867 | 0.769 | 0.713 | 0.910 | 0.330 |
| Total cholesterol, mmol/l | 4.2 (3.6–5.0) | 4.2 (3.5–4.9) | 0.483 | 4.3 (3.7–5.1) | 4.2 (3.6–4.9) | 0.665 | 4.5 (3.9–5.1) | 4.5 (4.0–5.2) | 0.208 | 0.126 | 0.350 | 0.438 | >0.999 | 0.710 |
| C-reactive protein, mg/L | 4.2 (1.5–12.1) | 5.2 (2.5–16.1) | 0.102 | 4.4 (2.3–10.4) | 4.4 (2.0–8.5) | 0.959 | 7.8 (3.5–15.8) | 8.0 (3.8–16.6) | 0.590 | 0.803 | 0.523 | 0.236 | 0.770 | 0.130 |
| Predictors | Normal Weight | Overweight | Obese | R2 | ||
|---|---|---|---|---|---|---|
| Reference | OR (95% CI) | p-Value | OR (95% CI) | p-Value | ||
| Age, per year | 1.00 | 1.02 (1.00–1.04) | 0.093 | 1.01 (0.99–1.04) | 0.195 | 0.081 |
| Sex, men | 1.00 | 0.19 (0.11–0.32) | <0.001 | 0.06 (0.03–0.11) | <0.001 | |
| Systolic blood pressure, per mmHg | 1.00 | 1.01 (1.00–1.02) | 0.204 | 1.01 (1.00–1.02) | 0.167 | 0.083 |
| Diastolic blood pressure, per mmHg | 1.00 | 1.02 (1.00–1.04) | 0.012 | 1.02 (1.01–1.04) | 0.005 | 0.087 |
| Triglycerides, per mmol/L | 1.00 | 2.06 (1.41–3.02) | <0.001 | 2.70 (1.86–3.92) | <0.001 | 0.102 |
| HDL-Cholesterol, per mmol/L | 1.00 | 0.40 (0.24–0.66) | <0.001 | 0.27 (0.16–0.44) | <0.001 | 0.102 |
| LDL-cholesterol, per mmol/L | 1.00 | 1.15 (0.92–1.45) | <0.001 | 1.56 (1.26–1.93) | <0.001 | 0.092 |
| Fasting plasma glucose, per mmol/L | 1.00 | 0.93 (0.79–1.10) | 0.417 | 1.18 (1.04–1.32) | 0.007 | 0.090 |
| HOMA-IR | 1.00 | 1.42 (1.19–1.70) | <0.001 | 1.77 (1.49–2.10) | <0.001 | 0.183 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, K.A.; Peer, N.; De Villiers, A.; Mukasa, B.; Matsha, T.E.; Mills, E.J.; Kengne, A.P. The Distribution of Obesity Phenotypes in HIV-Infected African Population. Nutrients 2016, 8, 299. https://doi.org/10.3390/nu8060299
Nguyen KA, Peer N, De Villiers A, Mukasa B, Matsha TE, Mills EJ, Kengne AP. The Distribution of Obesity Phenotypes in HIV-Infected African Population. Nutrients. 2016; 8(6):299. https://doi.org/10.3390/nu8060299
Chicago/Turabian StyleNguyen, Kim Anh, Nasheeta Peer, Anniza De Villiers, Barbara Mukasa, Tandi E. Matsha, Edward J. Mills, and Andre Pascal Kengne. 2016. "The Distribution of Obesity Phenotypes in HIV-Infected African Population" Nutrients 8, no. 6: 299. https://doi.org/10.3390/nu8060299
APA StyleNguyen, K. A., Peer, N., De Villiers, A., Mukasa, B., Matsha, T. E., Mills, E. J., & Kengne, A. P. (2016). The Distribution of Obesity Phenotypes in HIV-Infected African Population. Nutrients, 8(6), 299. https://doi.org/10.3390/nu8060299





