Dietary Patterns in Pregnancy in New Zealand—Influence of Maternal Socio-Demographic, Health and Lifestyle Factors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Assessments
2.2.1. Dietary Intake and Dietary Patterns
2.2.2. Covariates
2.3. Statistical Analysis
3. Results
3.1. Sample Demographics
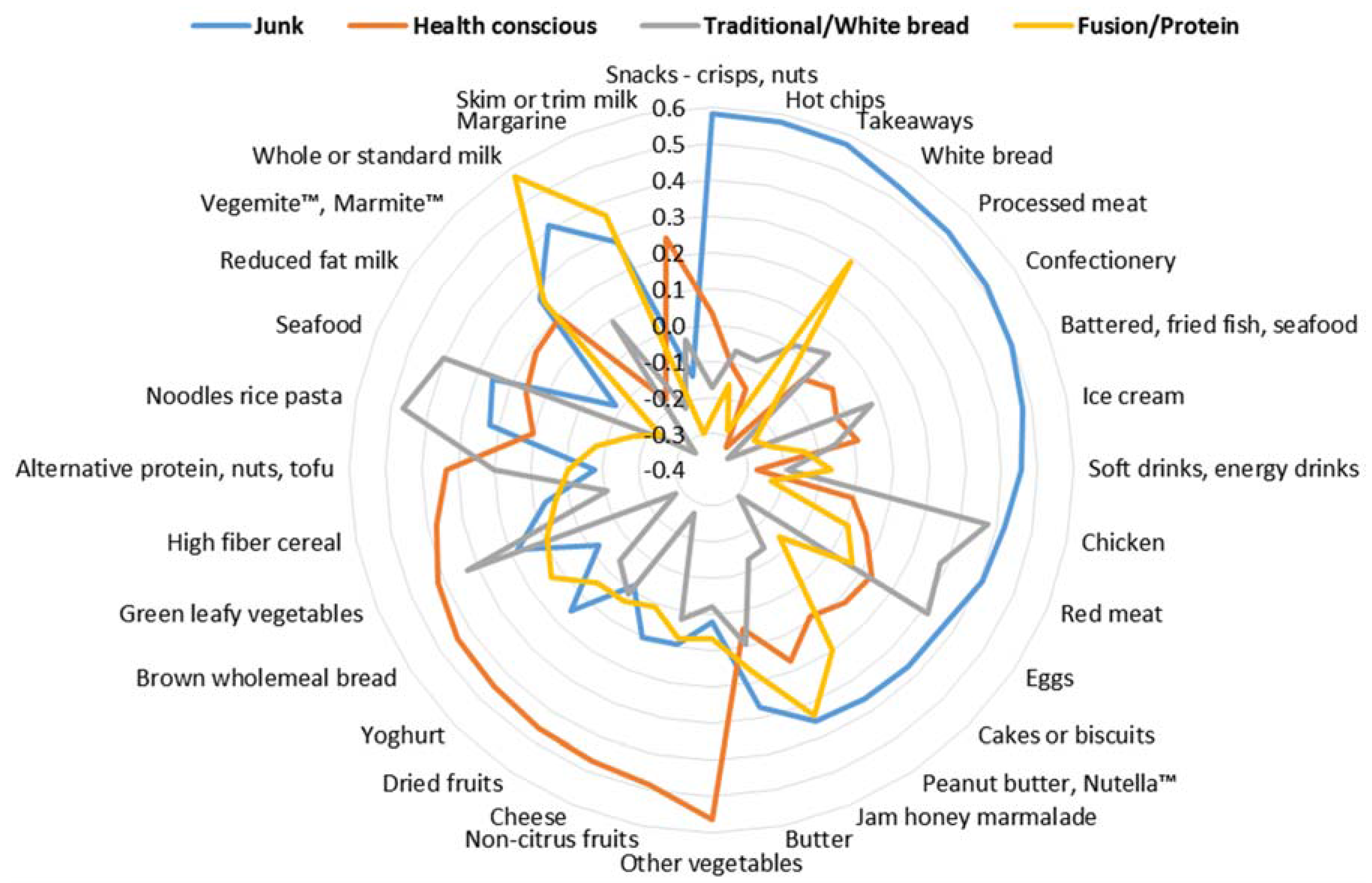
3.2. Dietary Components Identified
3.3. Associations with Maternal Socio-Demographic, Health and Lifestyle Factors
3.4. Associations with Adherence to Dietary Guideline Recommendations
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ALSPAC | Avon Longitudinal Study of Parents and Children |
| BMI | body mass index |
| DNBC | Danish National Birth Cohort |
| DIPP | Finnish Type 1 Diabetes Prediction and Prevention |
| FFQ | food frequency questionnaire |
| LMC | lead maternity carer |
| NMC | Norwegian Mother and Child |
| NZ | New Zealand |
| PCA | principal components analysis |
References
- Procter, S.B.; Campbell, C.G. Position of the academy of nutrition and dietetics: Nutrition and lifestyle for a healthy pregnancy outcome. J. Acad. Nutr. Diet. 2014, 114, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Morton, S.M.; Grant, C.C.; Wall, C.R.; Carr, P.E.; Bandara, D.K.; Schmidt, J.M.; Ivory, V.; Inskip, H.M.; Camargo, C.A. Adherence to nutritional guidelines in pregnancy: Evidence from the Growing Up in New Zealand birth cohort study. Public Health Nutr. 2014, 17, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J.K.; Evers, S.E.; Campbell, M.K. Inadequate dietary intakes among pregnant women. Can. J. Diet. Pract. Res. 2012, 73, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, R. Social determinants of food choice. Proc. Nutr. Soc. 1999, 58, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Cespedes, E.M.; Hu, F.B. Dietary patterns: From nutritional epidemiologic analysis to national guidelines. Am. J. Clin. Nutr. 2015, 101, 899–900. [Google Scholar] [CrossRef] [PubMed]
- USDA. Scientific Report of the 2015 Dietary Guidelines Advisory; United States Department of Agriculture: Washington, DC, USA, 2015.
- Rasmussen, M.A.; Maslova, E.; Halldorsson, T.I.; Olsen, S.F. Characterization of dietary patterns in the Danish national birth cohort in relation to preterm birth. PLoS ONE 2014, 9, e93644. [Google Scholar] [CrossRef] [PubMed]
- Arkkola, T.; Uusitalo, U.; Kronberg-Kippila, C.; Mannisto, S.; Virtanen, M.; Kenward, M.G.; Veijola, R.; Knip, M.; Ovaskainen, M.L.; Virtanen, S.M. Seven distinct dietary patterns identified among pregnant finnish women-associations with nutrient intake and sociodemographic factors. Public Health Nutr. 2008, 11, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Cuco, G.; Fernandez-Ballart, J.; Sala, J.; Viladrich, C.; Iranzo, R.; Vila, J.; Arija, V. Dietary patterns and associated lifestyles in preconception, pregnancy and postpartum. Eur. J. Clin. Nutr. 2006, 60, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Northstone, K.; Emmett, P.; Rogers, I. Dietary patterns in pregnancy and associations with socio-demographic and lifestyle factors. Eur. J. Clin. Nutr. 2008, 62, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Brantsaeter, A.L.; Haugen, M.; Samuelsen, S.O.; Torjusen, H.; Trogstad, L.; Alexander, J.; Magnus, P.; Meltzer, H.M. A dietary pattern characterized by high intake of vegetables, fruits, and vegetable oils is associated with reduced risk of preeclampsia in nulliparous pregnant Norwegian women. J. Nutr. 2009, 139, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Englund-Ogge, L.; Brantsaeter, A.L.; Sengpiel, V.; Haugen, M.; Birgisdottir, B.E.; Myhre, R.; Meltzer, H.M.; Jacobsson, B. Maternal dietary patterns and preterm delivery: Results from large prospective cohort study. BMJ 2014, 348, g1446. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, V.K.; Orozova-Bekkevold, I.M.; Mikkelsen, T.B.; Wolff, S.; Olsen, S.F. Major dietary patterns in pregnancy and fetal growth. Eur. J. Clin. Nutr. 2008, 62, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Northstone, K.; Ness, A.R.; Emmett, P.M.; Rogers, I.S. Adjusting for energy intake in dietary pattern investigations using principal components analysis. Eur. J. Clin. Nutr. 2008, 62, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.M.; Wall, C.; Becroft, D.M.; Robinson, E.; Wild, C.J.; Mitchell, E.A. Maternal dietary patterns in pregnancy and the association with small-for-gestational-age infants. Br. J. Nutr. 2010, 103, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Wolff, C.B.; Wolff, H.K. Maternal eating patterns and birth weight of mexican american infants. Nutr. Health 1995, 10, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Özden, C.; Parsons, C.; Schiff, M.; Walmsley, T. Where on earth is everybody? World Bank Econ. Rev. 2011, 25, 12–56. [Google Scholar]
- Morton, S.M.; Atatoa Carr, P.E.; Grant, C.C.; Robinson, E.M.; Bandara, D.K.; Bird, A.; Ivory, V.C.; Kingi, T.K.; Liang, R.; Marks, E.J.; et al. Cohort profile: Growing Up in New Zealand. Int. J. Epidemiol. 2013, 42, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Morton, S.M.; Ramke, J.; Kinloch, J.; Grant, C.C.; Carr, P.A.; Leeson, H.; Lee, A.C.; Robinson, E. Growing Up in New Zealand cohort alignment with all New Zealand births. Aust. N. Z. J. Public Health 2015, 39, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health. Food and Nutrition Guidelines for Healthy Pregnant and Breastfeeding Women: A Background Paper; Ministry of Health: Wellington, New Zealand, 2006.
- Morton, S.M.B.; Atatoa Carr, P.E.; Bandara, D.K.; Grant, C.C.; Ivory, V.C.; Kingi, T.R.; Liang, R.; Perese, L.M.; Peterson, E.; Pryor, J.E.; et al. Growing up in New Zealand: A Longitudinal Study of New Zealand Children and their Families; Report 1: Before we are born; University of Auckland: Auckland, New Zealand, 2010. [Google Scholar]
- Statistics New Zealand. Report of the Review of the Measurement of Ethnicity June 2004; Statistics New Zealand: Wellington, New Zealand, 2004.
- Salmond, C.; Crampton, P.; Atkinson, J. Nzdep2006 Index of Deprivation; Department of Public Health, University of Otago: Wellington, New Zealand, 2007. [Google Scholar]
- McAdams, M.A.; Van Dam, R.M.; Hu, F.B. Comparison of self-reported and measured BMI as correlates of disease markers in us adults. Obesity 2007, 15, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.L.; Holden, J.M.; Sagovsky, R. Detection of postnatal depression. Development of the 10-item Edinburgh postnatal depression scale. Br. J. Psychiatry 1987, 150, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjostrom, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Newby, P.K.; Tucker, K.L. Empirically derived eating patterns using factor or cluster analysis: A review. Nutr. Rev. 2004, 62, 177–203. [Google Scholar] [CrossRef] [PubMed]
- Cattell, R.B. The scree test for the number of factors. Multivar. Behav. Res. 1966, 1, 245–276. [Google Scholar] [CrossRef] [PubMed]
- Kline, P.K. An Easy Guide to Factor Analysis; Routledge: London, UK, 1994. [Google Scholar]
- Northstone, K.; Emmett, P.M.; Rogers, I. Dietary patterns in pregnancy and associations with nutrient intakes. Br. J. Nutr. 2008, 99, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Crozier, S.R.; Robinson, S.M.; Borland, S.E.; Inskip, H.M.; Group, S.W.S.S. Dietary patterns in the Southampton women’s survey. Eur. J. Clin. Nutr. 2006, 60, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Statistics New Zealand. 2013 Census Quickstats about Culture and Identity; Statistics New Zealand: Wellington, New Zealand, 2014.
- Gilbert, P.A.; Khokhar, S. Changing dietary habits of ethnic groups in Europe and implications for health. Nutr. Rev. 2008, 66, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Satia-Abouta, J.; Patterson, R.E.; Neuhouser, M.L.; Elder, J. Dietary acculturation: Applications to nutrition research and dietetics. J. Am. Diet. Assoc. 2002, 102, 1105–1118. [Google Scholar] [CrossRef]
- Lesser, I.A.; Gasevic, D.; Lear, S.A. The association between acculturation and dietary patterns of south asian immigrants. PLoS ONE 2014, 9, e88495. [Google Scholar] [CrossRef] [PubMed]
- Morton, S.M.; Grant, C.C.; Atatoa Carr, P.E. Too many left at risk by current folic acid supplementation use: Evidence from Growing Up in New Zealand. Aust. N. Z. J. Public Health 2013, 37, 190–191. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.S.; Hiles, S.; Bisquera, A.; Hure, A.J.; McEvoy, M.; Attia, J. A systematic review and meta-analysis of dietary patterns and depression in community-dwelling adults. Am. J. Clin. Nutr. 2014, 99, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Leung, B.M.; Kaplan, B.J. Perinatal depression: Prevalence, risks, and the nutrition link—A review of the literature. J. Am. Diet. Assoc. 2009, 109, 1566–1575. [Google Scholar] [CrossRef] [PubMed]
- Crozier, S.R.; Inskip, H.M.; Barker, M.E.; Lawrence, W.T.; Cooper, C.; Robinson, S.M.; Group, S.W.S.S. Development of a 20-item food frequency questionnaire to assess a ‘prudent’ dietary pattern among young women in southampton. Eur. J. Clin. Nutr. 2010, 64, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Moeller, S.M.; Reedy, J.; Millen, A.E.; Dixon, L.B.; Newby, P.K.; Tucker, K.L.; Krebs-Smith, S.M.; Guenther, P.M. Dietary patterns: Challenges and opportunities in dietary patterns research an experimental biology workshop, 1 April 2006. J. Am. Diet. Assoc. 2007, 107, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Emmett, P. Assessing diet in longitudinal birth cohort studies. Paediatr. Perinat. Epidemiol. 2009, 23, 154–173. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | n (%) | Characteristics | n (%) |
|---|---|---|---|
| Maternal socio-demographic characteristics | Season of interview | ||
| Age group (years) | Summer | 922 (16.3) | |
| <20 | 273 (4.8) | Autumn | 1019 (18.0) |
| 20–29 | 2195 (38.8) | Winter | 1686 (29.8) |
| 30–39 | 2992 (52.8) | Spring | 2037 (36.0) |
| 40 and over | 204 (3.6) | Maternal health characteristics | |
| Pre-pregnancy BMI (kg/m2) | Pre-pregnancy maternal self-rating of health | ||
| Median (Range) | 23.8 (13.0–67.0) | Poor/Fair | 556 (9.8) |
| Self-prioritised ethnicity | Good | 1896 (33.5) | |
| European | 3168 (56.0) | Very good | 2056 (36.3) |
| Māori | 747 (13.2) | Excellent | 1151 (20.3) |
| Pacific | 726 (12.8) | Nausea/sickness—after 1st trimester | |
| Asian | 802 (14.2) | No nausea | 3617 (63.9) |
| Other | 213 (3.8) | Mild nausea (nausea only) | 1154 (20.4) |
| Place of birth | Mod. nausea (occasionally sick) | 646 (11.4) | |
| New Zealand | 3703 (65.4) | Severe nausea (regularly sick) | 244 (4.3) |
| Outside New Zealand | 1961 (34.6) | EPDS score 2 | |
| Education | No (<13) | 4992 (88.1) | |
| Primary | 352 (6.2) | Yes (≥13) | 672 (11.9) |
| Secondary | 1326 (23.5) | Maternal lifestyle characteristics | |
| Tertiary | 3975 (70.3) | Folic acid intake | |
| Socioeconomic deprivation | Never taken folic acid | 800 (14.1) | |
| 1 to 2 (least deprived) | 936 (16.5) | Before pregnancy | 2277 (40.2) |
| 3 to 4 | 1080 (19.1) | After pregnancy | 2587 (45.7) |
| 5 to 6 | 1009 (17.8) | Pre/during pregnancy smoking patterns | |
| 7 to 8 | 1172 (20.7) | Continued smoking | 544 (9.6) |
| 9 to 10 (most deprived) | 1465 (25.9) | Stopped smoking | 550 (9.7) |
| Pregnancy planning | Non-smokers | 4555 (80.6) | |
| Planned | 3488 (61.8) | Pre/during pregnancy alcohol consumption | |
| Unplanned | 2156 (38.2) | Any drinking during | 1649 (29.1) |
| Parity | Stopped drinking | 2481 (43.9) | |
| First child | 2396 (42.3) | Non-drinkers | 1528 (27.0) |
| Subsequent child | 3268 (57.7) | Pre/during pregnancy physical activity | |
| LMC type | Mod/vigorous activity during | 156 (2.8) | |
| GP (Family doctor) | 41 (0.7) | No mod/vigorous activity during | 1354 (23.9) |
| Independent midwife | 3649 (66.0) | Mod/vig. activity before & during | 1894 (33.4) |
| Hospital midwife | 818 (14.8) | No mod/vig. activity before & during | 2260 (39.9) |
| Obstetrician | 445 (8.1) | Actively dieting pre-pregnancy | |
| Shared care GP and midwife | 247 (4.5) | Yes | 1460 (25.8) |
| Combination of care | 331 (6.0) | No | 4201 (74.2) |
| Junk | Health Conscious | Traditional/White Bread | Fusion/Protein | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p | PE | 95% CI | p | PE | 95% CI | p | PE | 95% CI | p | PE | 95% CI | |
| Age group (years) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||||||
| <20 | 0.56 | 0.41, 0.71 | −0.38 | −0.54, −0.22 | 0.48 | 0.34, 0.63 | −0.12 | −0.27, 0.03 | ||||
| 20–29 | 0.12 | 0.07, 0.18 | −0.13 | −0.19, −0.07 | 0.19 | 0.13, 0.24 | −0.12 | −0.18, −0.06 | ||||
| 30–39 | Ref | Ref | Ref | Ref | ||||||||
| 40 and over | −0.15 | −0.28, −0.02 | 0.15 | 0.01, −0.28 | 0.10 | −0.03, 0.23 | 0.14 | 0.01, 0.27 | ||||
| Pre-pregnancy BMI (kg/m2) | 0.271 | −0.003 | −0.0078, 0.0022 | 0.002 | −0.008 | −0.0135, −0.0030 | 0.420 | −0.002 | −0.0069, 0.0029 | 0.033 | −0.006 | −0.0105, −0.0004 |
| Self-prioritized ethnicity | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||||||
| European | Ref | Ref | Ref | Ref | ||||||||
| Maori | 0.21 | 0.12, 0.29 | 0.02 | −0.07, 0.11 | 0.40 | 0.31, 0.48 | 0.12 | 0.04, 0.21 | ||||
| Pacific | 0.25 | 0.14, 0.35 | −0.04 | −0.15, 0.07 | 0.56 | 0.46, 0.67 | 0.52 | 0.42, 0.63 | ||||
| Asian | −0.32 | −0.41, −0.23 | −0.60 | −0.70, −0.51 | 0.09 | 0.00, 0.18 | 0.88 | 0.79, 0.98 | ||||
| Other | −0.13 | −0.26, 0.00 | −0.21 | −0.34, −0.07 | 0.02 | −0.11, 0.15 | 0.27 | 0.14, 0.40 | ||||
| Place of birth | 0.006 | 0.001 | 0.948 | <0.0001 | ||||||||
| New Zealand | Ref | Ref | Ref | Ref | ||||||||
| Outside New Zealand | −0.09 | −0.16, −0.03 | −0.12 | −0.19, −0.05 | 0.00 | −0.07, 0.06 | 0.18 | 0.11, 0.25 | ||||
| Education | 0.034 | <0.0001 | 0.005 | 0.339 | ||||||||
| Primary | 0.17 | 0.04, 0.30 | −0.11 | −0.24, 0.03 | 0.21 | 0.08, 0.33 | −0.10 | −0.23, 0.03 | ||||
| Secondary | 0.01 | −0.05, 0.07 | −0.20 | −0.26, −0.13 | 0.04 | −0.02, 0.10 | −0.01 | −0.08, 0.05 | ||||
| Tertiary | Ref | Ref | Ref | Ref | ||||||||
| Socioeconomic deprivation | 0.750 | 0.103 | <0.0001 | 0.336 | ||||||||
| 1 to 2 (least deprived) | −0.05 | −0.14, 0.03 | 0.11 | 0.02, 0.20 | −0.23 | −0.32, −0.15 | −0.07 | −0.16, 0.01 | ||||
| 3 to 4 | −0.04 | −0.12, 0.04 | 0.03 | −0.05, 0.12 | −0.19 | −0.27, −0.11 | −0.07 | −0.15, 0.01 | ||||
| 5 to 6 | −0.05 | −0.13, 0.03 | 0.06 | −0.02, 0.15 | −0.18 | −0.26, −0.10 | −0.02 | −0.10, 0.06 | ||||
| 7 to 8 | −0.03 | −0.11, 0.05 | 0.08 | −0.01, 0.16 | −0.13 | −0.20, −0.05 | −0.05 | −0.13, 0.03 | ||||
| 9 to 10 (most deprived) | Ref | Ref | Ref | Ref | ||||||||
| Pregnancy planning | 0.390 | 0.206 | 0.0002 | 0.034 | ||||||||
| Planned | 0.03 | −0.03, 0.09 | 0.04 | −0.02, 0.11 | −0.12 | −0.18, −0.06 | −0.07 | −0.13, −0.01 | ||||
| Unplanned | Ref | Ref | Ref | Ref | ||||||||
| Parity | 0.061 | 0.763 | <0.0001 | 0.017 | ||||||||
| First child | Ref | Ref | Ref | Ref | ||||||||
| Subsequent child | 0.05 | 0.00, 0.10 | 0.01 | −0.05, 0.06 | 0.24 | 0.19, 0.30 | −0.06 | −0.12, −0.01 | ||||
| LMC type | 0.046 | 0.174 | 0.056 | 0.841 | ||||||||
| GP (Family doctor) | −0.13 | −0.43, 0.17 | −0.11 | −0.42, 0.20 | 0.09 | −0.21, 0.38 | −0.08 | −0.38, 0.23 | ||||
| Independent midwife | Ref | Ref | Ref | Ref | ||||||||
| Hospital midwife | −0.02 | −0.09, 0.05 | −0.05 | −0.12, 0.03 | −0.01 | −0.08, 0.06 | 0.05 | −0.03, 0.12 | ||||
| Obstetrician | 0.11 | 0.02, 0.20 | −0.10 | −0.19, 0.00 | −0.14 | −0.23, −0.06 | 0.00 | −0.09, 0.09 | ||||
| Shared care GP & midwife | 0.03 | −0.10, 0.16 | −0.11 | −0.25, 0.02 | −0.01 | −0.14, 0.11 | 0.01 | −0.12, 0.14 | ||||
| Combination of care | 0.11 | 0.01, 0.22 | 0.00 | −0.10, 0.11 | −0.03 | −0.13, 0.07 | −0.02 | −0.12, 0.09 | ||||
| Season of interview | 0.470 | 0.939 | 0.0006 | 0.624 | ||||||||
| Summer | Ref | Ref | Ref | Ref | ||||||||
| Autumn | 0.05 | −0.04, 0.13 | 0.01 | −0.08, 0.10 | −0.06 | −0.14, 0.02 | 0.02 | −0.06, 0.10 | ||||
| Winter | 0.01 | −0.07, 0.08 | −0.01 | −0.09, 0.07 | 0.08 | 0.01, 0.15 | −0.02 | −0.10, 0.05 | ||||
| Spring | 0.04 | −0.03, 0.12 | −0.01 | −0.09, 0.06 | −0.01 | −0.08, 0.06 | 0.01 | −0.06, 0.08 | ||||
| Maternal self-rating of health | 0.013 | <0.0001 | 0.844 | 0.0005 | ||||||||
| Poor/Fair | 0.13 | 0.03, 0.22 | −0.20 | −0.30, −0.09 | 0.02 | −0.08, 0.11 | −0.14 | −0.24, −0.04 | ||||
| Good | 0.08 | 0.02, 0.14 | −0.10 | −0.17, −0.04 | 0.01 | −0.05, 0.07 | −0.05 | −0.11, 0.01 | ||||
| Very good | Ref | Ref | Ref | Ref | ||||||||
| Excellent | −0.01 | −0.07, 0.06 | 0.04 | −0.03, 0.11 | −0.02 | −0.09, 0.04 | 0.07 | 0.00, 0.13 | ||||
| Nausea/sickness—after 1st trimester | 0.140 | 0.018 | 0.401 | 0.084 | ||||||||
| No nausea | −0.05 | −0.11, 0.01 | −0.06 | −0.12, 0.00 | 0.03 | −0.03, 0.09 | −0.05 | −0.11, 0.01 | ||||
| Mild nausea (Nausea only) | Ref | Ref | Ref | Ref | ||||||||
| Moderate Nausea (occasionally sick) | −0.09 | −0.18, 0.00 | −0.15 | −0.24, −0.06 | 0.02 | −0.07, 0.11 | −0.10 | −0.19, −0.01 | ||||
| Severe nausea (regularly sick, can’t hold meals) | 0.01 | −0.12, 0.14 | −0.03 | −0.17, 0.10 | 0.11 | −0.02, 0.23 | −0.13 | −0.25, 0.00 | ||||
| EDPS 1 | 0.0005 | 0.138 | 0.284 | 0.102 | ||||||||
| No (<13) | Ref | Ref | Ref | Ref | ||||||||
| Yes (≥13) | 0.14 | 0.06, 0.23 | −0.06 | −0.15, 0.02 | −0.04 | −0.12, 0.04 | −0.07 | −0.15, 0.01 | ||||
| Folic acid intake | 0.0002 | 0.006 | <0.0001 | 0.0002 | ||||||||
| Never taken folic acid | Ref | Ref | Ref | Ref | ||||||||
| Before pregnancy | −0.20 | −0.30, −0.10 | 0.17 | 0.07, 0.28 | −0.25 | −0.35, −0.15 | −0.12 | −0.22, −0.02 | ||||
| After pregnancy | −0.18 | −0.27,−0.09 | 0.12 | 0.03, 0.22 | −0.13 | −0.22, −0.04 | −0.18 | −0.27, −0.09 | ||||
| Pre/during pregnancy smoking patterns | 0.0001 | <0.0001 | <0.0001 | 0.007 | ||||||||
| Continued smoking | 0.21 | 0.11, 0.31 | −0.34 | −0.45, −0.23 | 0.53 | 0.43, 0.63 | −0.11 | −0.21, −0.01 | ||||
| Stopped smoking | 0.07 | −0.02, 0.16 | −0.07 | −0.17, 0.02 | 0.14 | 0.05, 0.23 | −0.13 | −0.22, −0.03 | ||||
| Non-smokers | Ref | Ref | Ref | Ref | ||||||||
| Pre/during pregnancy alcohol consumption | 0.0003 | 0.646 | 0.008 | 0.109 | ||||||||
| Any drinking during | 0.13 | 0.06, 0.20 | −0.01 | −0.08, 0.07 | −0.11 | −0.18, −0.04 | −0.06 | −0.13, 0.02 | ||||
| Stopped drinking | 0.12 | 0.06, 0.19 | 0.02 | −0.05, 0.09 | −0.07 | −0.14, −0.01 | −0.07 | −0.14, 0.00 | ||||
| Non-drinkers | Ref | Ref | Ref | Ref | ||||||||
| Pre/during pregnancy physical activity | 0.414 | <0.0001 | 0.004 | 0.0004 | ||||||||
| Mod/vigorous activity during | 0.03 | −0.12, 0.19 | 0.11 | −0.06, 0.27 | 0.03 | −0.12, 0.19 | 0.10 | −0.06, 0.26 | ||||
| No mod/vigorous activity during | 0.01 | −0.05, 0.07 | 0.13 | 0.06, 0.19 | −0.11 | −0.17, −0.05 | 0.09 | 0.03, 0.16 | ||||
| Mod/vigorous activity before & during | −0.04 | −0.10, 0.02 | 0.16 | 0.10, 0.22 | −0.01 | −0.07, 0.04 | 0.12 | 0.06, 0.18 | ||||
| No mod/vigorous activity before & during | Ref | Ref | Ref | Ref | ||||||||
| Actively dieting pre-pregnancy | <0.0001 | 0.050 | <0.001 | 0.133 | ||||||||
| Yes | 0.14 | 0.08, 0.20 | −0.06 | −0.12, 0.00 | −0.14 | −0.20, −0.08 | −0.05 | −0.11, 0.01 | ||||
| No | Ref | Ref | Ref | Ref | ||||||||
| Junk | Health Conscious | Traditional/White Bread | Fusion/Protein | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | |
| Vegetables and fruits (≥6 serves/day) | <0.0001 | <0.0001 | 0.0005 | <0.0001 | ||||||||
| Yes | 0.56 | 0.48, 0.65 | 6.37 | 5.29, 7.67 | 1.32 | 1.13, 1.54 | 4.79 | 4.05, 5.65 | ||||
| No | Ref | Ref | Ref | Ref | ||||||||
| Breads and Cereals (≥4 serves/day) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||||||
| Yes | 2.56 | 2.21, 2.97 | 3.05 | 2.55, 3.65 | 2.89 | 2.48, 3.38 | 0.56 | 0.46, 0.67 | ||||
| No | Ref | Ref | Ref | Ref | ||||||||
| Milk and milk products (≥3 serves/day) | <0.0001 | <0.0001 | <0.0001 | 0.054 | ||||||||
| Yes | 1.34 | 1.18, 1.53 | 4.60 | 3.97, 5.32 | 2.00 | 1.74, 2.30 | 1.16 | 1.00, 1.35 | ||||
| No | Ref | Ref | Ref | Ref | ||||||||
| Lean meat, meat alternatives and eggs (≥2serves/day) | 0.045 | 0.347 | 0.807 | <0.0001 | ||||||||
| Yes | 1.19 | 1.00, 1.40 | 1.10 | 0.91, 1.33 | 1.02 | 0.86, 1.22 | 14.3 | 11.5, 17.8 | ||||
| No | Ref | Ref | Ref | Ref | ||||||||
| Junk | Health Conscious | Traditional/White Bread | Fusion/Protein | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | |
| No. of food group guidelines | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||||||
| None | Ref | Ref | Ref | Ref | ||||||||
| One guideline | 1.35 | 1.15, 1.59 | 4.81 | 4.00, 5.79 | 2.23 | 1.85, 2.67 | 2.00 | 1.67, 2.41 | ||||
| Two-four guidelines | 1.66 | 1.41, 1.96 | 19.2 | 15.6, 23.6 | 3.99 | 3.32, 4.79 | 5.75 | 4.78, 6.92 | ||||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wall, C.R.; Gammon, C.S.; Bandara, D.K.; Grant, C.C.; Atatoa Carr, P.E.; Morton, S.M.B. Dietary Patterns in Pregnancy in New Zealand—Influence of Maternal Socio-Demographic, Health and Lifestyle Factors. Nutrients 2016, 8, 300. https://doi.org/10.3390/nu8050300
Wall CR, Gammon CS, Bandara DK, Grant CC, Atatoa Carr PE, Morton SMB. Dietary Patterns in Pregnancy in New Zealand—Influence of Maternal Socio-Demographic, Health and Lifestyle Factors. Nutrients. 2016; 8(5):300. https://doi.org/10.3390/nu8050300
Chicago/Turabian StyleWall, Clare R., Cheryl S. Gammon, Dinusha K. Bandara, Cameron C. Grant, Polly E. Atatoa Carr, and Susan M. B. Morton. 2016. "Dietary Patterns in Pregnancy in New Zealand—Influence of Maternal Socio-Demographic, Health and Lifestyle Factors" Nutrients 8, no. 5: 300. https://doi.org/10.3390/nu8050300
APA StyleWall, C. R., Gammon, C. S., Bandara, D. K., Grant, C. C., Atatoa Carr, P. E., & Morton, S. M. B. (2016). Dietary Patterns in Pregnancy in New Zealand—Influence of Maternal Socio-Demographic, Health and Lifestyle Factors. Nutrients, 8(5), 300. https://doi.org/10.3390/nu8050300





