Oral Resveratrol Prevents Osteoarthritis Progression in C57BL/6J Mice Fed a High-Fat Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
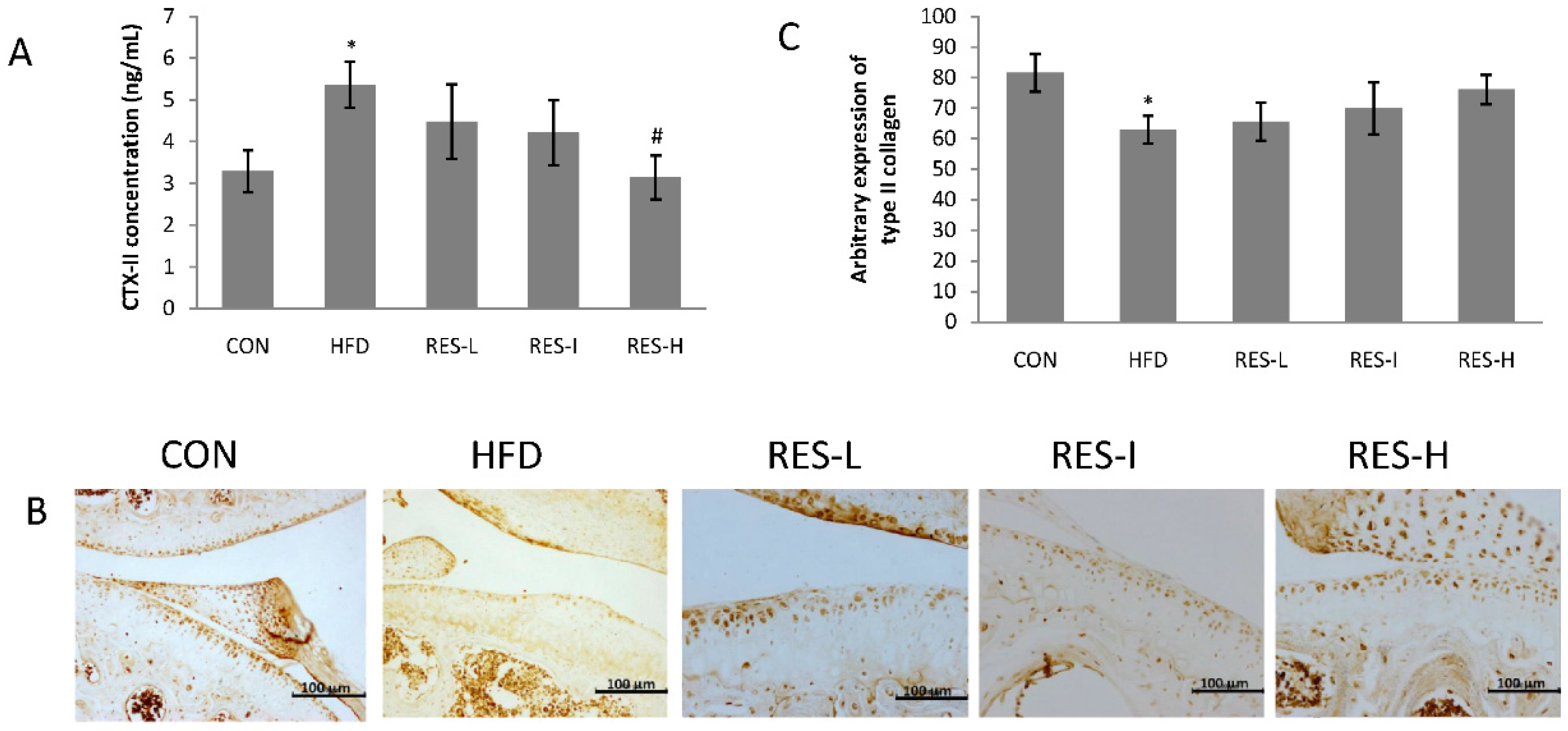
2.2. Serum Triglyceride, Total Cholesterol, and C-Telopeptide of Type II Collagen (CTX-II) Measurements
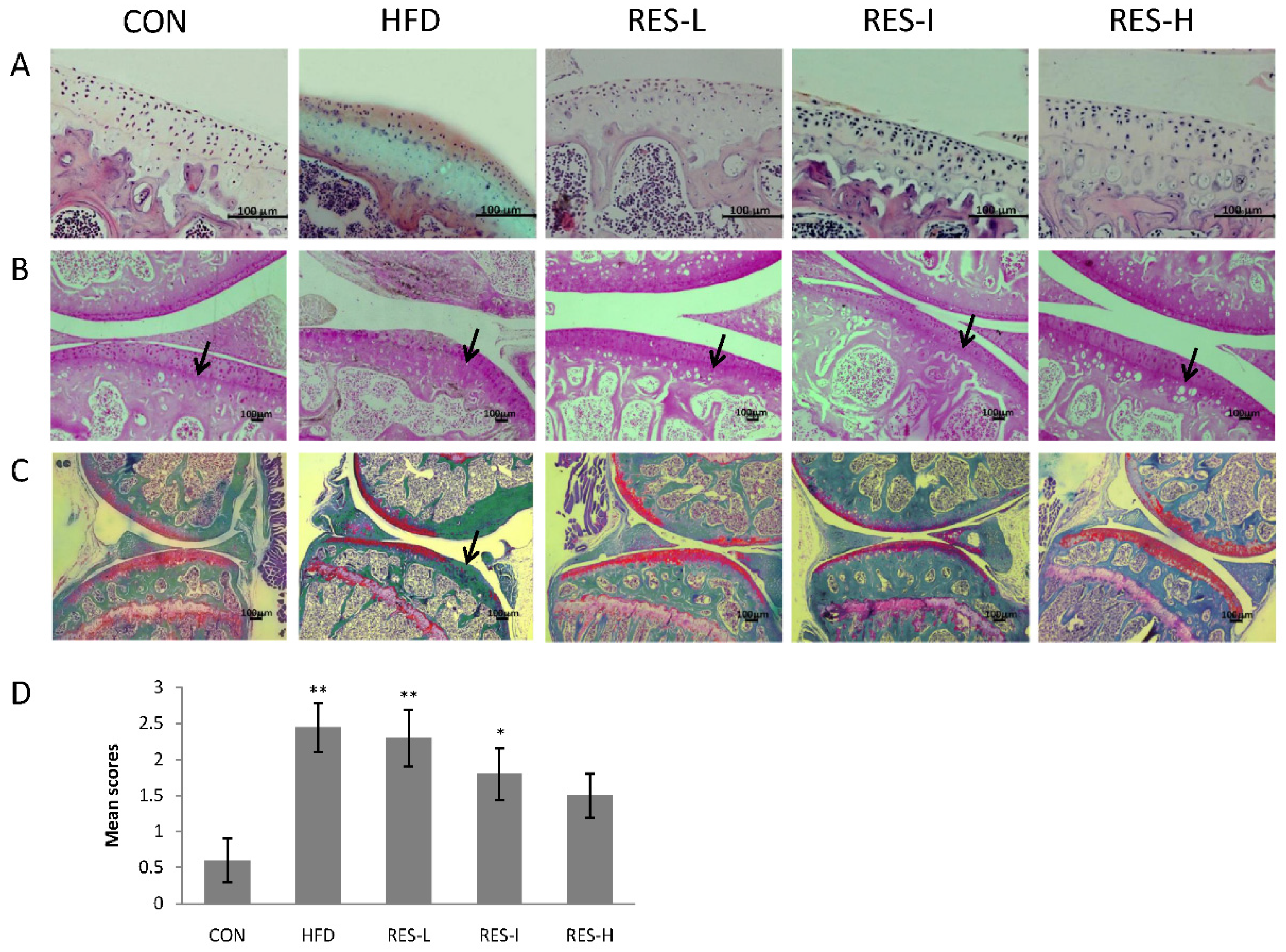
2.3. Assessment of OA
2.4. Immunohistochemistry
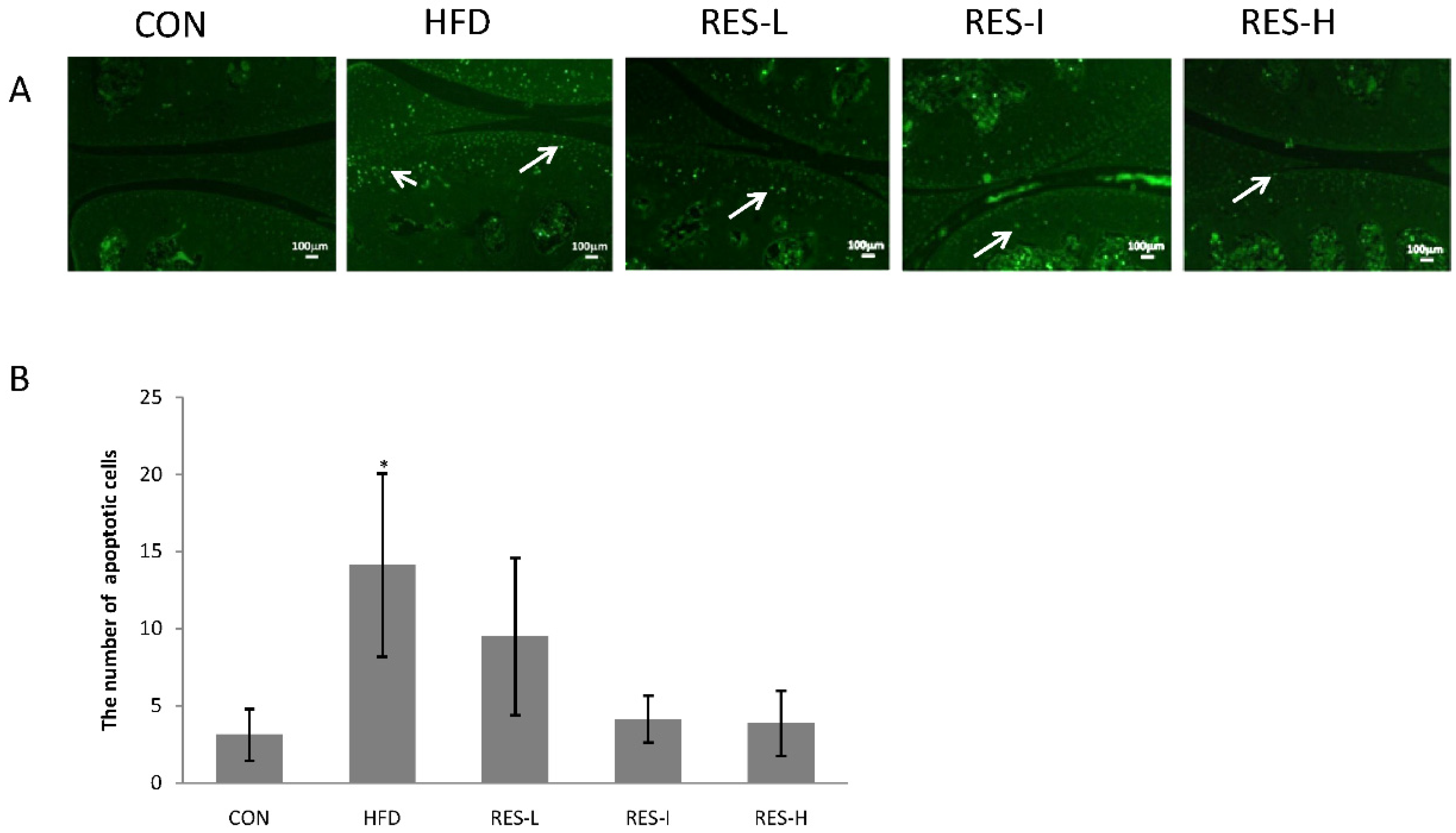
2.5. TUNEL Assay
2.6. Statistical Analysis
3. Results
3.1. Effect of Resveratrol on Body Weight and Serum Lipid Levels
3.2. Histological Assessment of Knee OA in Resveratrol-Treated Mice
3.3. Effect of Resveratrol on the Degradation of Type II Collagen in Knee Articular Cartilage
3.4. Effect of Resveratrol on Chondrocyte Apoptosis
3.5. Associations between Mankin Scores, Serum CTX-II Levels, and Type II Collagen Expression
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cheng, D.S.; Visco, C.J. Pharmaceutical therapy for osteoarthritis. PMR 2012, 4, S82–S88. [Google Scholar] [CrossRef] [PubMed]
- Patrignani, P.; Tacconelli, S.; Bruno, A.; Sostres, C.; Lanas, A. Managing the adverse effects of nonsteroidal anti-inflammatory drugs. Expert Rev. Clin. Pharmacol. 2011, 4, 605–621. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Jain, S.; Bhardwaj, A.; Nagpal, R.; Puniya, M.; Tomar, R.; Singh, V.; Parkash, O.; Prasad, G.B.; Marotta, F.; et al. Biological and medicinal properties of grapes and their bioactive constituents: An update. J. Med. Food 2009, 12, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Dave, M.; Attur, M.; Palmer, G.; Al-Mussawir, H.E.; Kennish, L.; Patel, J.; Abramson, S.B. The antioxidant resveratrol protects against chondrocyte apoptosis via effects on mitochondrial polarization and ATP production. Arthritis Rheum. 2008, 58, 2786–2797. [Google Scholar] [CrossRef] [PubMed]
- Csaki, C.; Keshishzadeh, N.; Fischer, K.; Shakibaei, M. Regulation of inflammation signalling by resveratrol in human chondrocytes in vitro. Biochem. Pharmacol. 2008, 75, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Gu, H.; Liu, H.; Jiao, Y.; Li, K.; Zhao, Y.; An, L.; Yang, J. Protective effect of resveratrol against IL-1β-induced inflammatory response on human osteoarthritic chondrocytes partly via the TLR4/MyD88/NF-κB signaling pathway: An “in vitro study”. Int. J. Mol. Sci. 2014, 15, 6925–6940. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, J.S.; Chen, J.W.; Li, F.; Tian, J. Effect of resveratrol on cartilage protection and apoptosis inhibition in experimental osteoarthritis of rabbit. Rheumatol. Int. 2012, 32, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cai, L.; Zhang, Y.; Cui, L.; Shen, G. Intra-articular resveratrol injection prevents osteoarthritis progression in a mouse model by activating SIRT1 and thereby silencing HIF-2α. J. Orthop. Res. 2015, 33, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Kamekura, S.; Hoshi, K.; Shimoaka, T.; Chung, U.; Chikuda, H.; Yamada, T.; Uchida, M.; Ogata, N.; Seichi, A.; Nakamura, K.; et al. Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthr. Cartil. 2005, 13, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Griffin, T.M.; Fermor, B.; Huebner, J.L.; Kraus, V.B.; Rodriguiz, R.M.; Wetsel, W.C.; Cao, L.; Setton, L.A.; Guilak, F. Diet-induced obesity differentially regulates behavioral, biomechanical, and molecular risk factors for osteoarthritis in mice. Arthritis Res. Ther. 2010, 12, R130. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmi, G.; Faust, R. Suitability of the C57 black mouse as an experimental animal for the study of skeletal changes due to ageing, with special reference to osteo-arthrosis and its response to tribenoside. Pharmacology 1976, 14, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Säämänen, A.K.; Salminen, H.J.; Dean, P.B.; De Crombrugghe, B.; Vuorio, E.I.; Metsäranta, M.P. Osteoarthritis-like lesions in transgenic mice harboring a small deletion mutation in type II collagen gene. Osteoarthr. Cartil. 2000, 8, 248–257. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Walton, M. Patella displacement and osteoarthrosis of the knee joint in mice. J. Pathol. 1979, 127, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Griffin, T.M.; Huebner, J.L.; Kraus, V.B.; Yan, Z.; Guilak, F. Induction of osteoarthritis and metabolic inflammation by a very high-fat diet in mice: Effects of short-term exercise. Arthritis Rheum. 2012, 64, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Gierman, L.M.; van der Ham, F.; Koudijs, A.; Wielinga, P.Y.; Kleemann, R.; Kooistra, T.; Stoop, R.; Kloppenburg, M.; van Osch, G.J.; Stojanovic-Susulic, V.; et al. Metabolic stress-induced inflammation plays a major role in the development of osteoarthritis in mice. Arthritis Rheum. 2012, 64, 1172–1181. [Google Scholar] [CrossRef] [PubMed]
- Van der Kraan, P.M. Osteoarthritis and a high-fat diet: The full “OA syndrome” in a small animal model. Arthritis Res. Ther. 2010, 12, 130. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Louer, C.R.; Furman, B.D.; Huebner, J.L.; Kraus, V.B.; Olson, S.A.; Guilak, F. Diet-induced obesity significantly increases the severity of posttraumatic arthritis in mice. Arthritis Rheum. 2012, 64, 3220–3230. [Google Scholar] [CrossRef] [PubMed]
- Iwata, M.; Ochi, H.; Hara, Y.; Tagawa, M.; Koga, D.; Okawa, A.; Asou, Y. Initial responses of articular tissues in a murine high-fat diet-induced osteoarthritis model: Pivotal role of the IPFP as a cytokine fountain. PLoS ONE 2013, 8, e60706. [Google Scholar] [CrossRef] [PubMed]
- Silberberg, M.; Silberberg, R. Effects of a high fat diet on the joints of aging mice. AMA Arch. Pathol. 1950, 50, 828–846. [Google Scholar] [PubMed]
- Gelber, A.C.; Hochberg, M.C.; Mead, L.A.; Wang, N.Y.; Wigley, F.M.; Klag, M.J. Body mass index in young men and the risk of subsequent knee and hip osteoarthritis. Am. J. Med. 1999, 107, 542–548. [Google Scholar] [CrossRef]
- Griffin, T.M.; Guilak, F. The role of mechanical loading in the onset and progression of osteoarthritis. Exerc. Sport Sci. Rev. 2005, 33, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, T.; Saito, S.; Tani, H.; Tatefuji, T.; Hashimoto, K. Resveratrol derivative-rich melinjo (Gnetum gnemon L.) seed extract improves obesity and survival of C57BL/6 mice fed a high-fat diet. Biosci. Biotechnol. Biochem. 2015, 79, 2044–2049. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lin, S.; Zhang, L.; Li, Y. Resveratrol prevents renal lipotoxicity in high-fat diet-treated mouse model through regulating PPAR-α pathway. Mol. Cell. Biochem. 2016, 411, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.J.; Mao, S.L.; Taylor, K.L.; Kanjanabuch, T.; Guan, Y.; Zhang, Y.; Brown, N.J.; Swift, L.L.; McGuinness, O.P.; Wasserman, D.H.; et al. Prevention of obesity and insulin resistance in mice lacking plasminogen activator inhibitor 1. Diabetes 2004, 53, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Kuroki, H.; Nakagawa, Y.; Mori, K.; Ohba, M.; Suzuki, T.; Mizuno, Y.; Ando, K.; Takenaka, M.; Ikeuchi, K.; Nakamura, T. Acoustic stiffness and change in plug cartilage over time after autologous osteochondral grafting: Correlation between ultrasound signal intensity and histological score in a rabbit model. Arthritis Res. Ther. 2004, 6, R492–R504. [Google Scholar] [CrossRef] [PubMed]
- Mankin, H.J.; Dorfman, H.; Lippiello, L.; Zarins, A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J. Bone Jt. Surg. Am. 1971, 53, 523–537. [Google Scholar]
- Kalaï, E.; Bahlous, A.; Bouzid, K.; Laadhar, L.; Cheour, E.; Sellami, S.; Abdelmoula, J.; Sahli, H.; Chelly, M. The role of type II collagen fragments and X-ray progression of knee osteoarthritis. Ann. Biol. Clin. 2014, 72, 715–721. [Google Scholar]
- Ishijima, M.; Kaneko, H.; Kaneko, K. The evolving role of biomarkers for osteoarthritis. Ther. Adv. Musculoskelet. Dis. 2014, 6, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Silberberg, M.; Silberberg, R. Skeletal growth, aging and osteoarthritis; effects of enriched diets and of ovariectomy. Bull. Hosp. Jt. Dis. 1951, 12, 256–272. [Google Scholar]
- Silberberg, M.; Silberberg, R. Degenerative joint disease in mice fed a high-fat diet at various ages. Exp. Med. Surg. 1952, 10, 76–87. [Google Scholar] [PubMed]
- Ignatowicz, E.; Baer-Dubowska, W. Resveratrol, a natural chemopreventive agent against degenerative diseases. Pol. J. Pharmacol. 2001, 53, 557–569. [Google Scholar] [PubMed]
- Haider, U.G.; Sorescu, D.; Griendling, K.K.; Vollmar, A.M.; Dirsch, V.M. Resveratrol suppresses angiotensin II-induced Akt/protein kinase B and p70 S6 kinase phosphorylation and subsequent hypertrophy in rat aortic smooth muscle cells. Mol. Pharmacol. 2002, 62, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Elmali, N.; Esenkaya, I.; Harma, A.; Ertem, K.; Turkoz, Y.; Mizrak, B. Effect of resveratrol in experimental osteoarthritis in rabbits. Inflamm. Res. 2005, 54, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Reijman, M.; Hazes, J.M.; Bierma-Zeinstra, S.M.; Koes, B.W.; Christgau, S.; Christiansen, C.; Uitterlinden, A.G.; Pols, H.A. A new marker for osteoarthritis: Cross-sectional and longitudinal approach. Arthritis Rheum. 2004, 50, 2471–2478. [Google Scholar] [CrossRef] [PubMed]
- Conrozier, T.; Balblanc, J.C.; Richette, P.; Mulleman, D.; Maillet, B.; Henrotin, Y.; Rannou, F.; Piroth, C.; Hilliquin, P.; Mathieu, P.; et al. Early effect of hyaluronic acid intra-articular injections on serum and urine biomarkers in patients with knee osteoarthritis: An open-label observational prospective study. J. Orthop. Res. 2012, 30, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Christgau, S.; Garnero, P.; Fledelius, C.; Moniz, C.; Ensig, M.; Gineyts, E.; Rosenquist, C.; Qvist, P. Collagen type II C-telopeptide fragments as an index of cartilage degradation. Bone 2001, 29, 209–215. [Google Scholar] [CrossRef]
- Doi, T.; Nishida, K.; Matsuo, M.; Yoshida, A.; Murakami, T.; Inoue, H. Evidence of oncotic cell death and DNA fragmentation in human hypertrophic chondrocytes in chondro-osteophyte. Osteoarthr. Cartil. 2002, 10, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Blanco, F.J.; Guitian, R.; Vázquez-Martul, E.; de Toro, F.J.; Galdo, F. Osteoarthritis chondrocytes die by apoptosis. A possible pathway for osteoarthritis pathology. Arthritis Rheum. 1998, 41, 284–289. [Google Scholar] [CrossRef]
- Csaki, C.; Mobasheri, A.; Shakibaei, M. Synergistic chondroprotective effects of curcumin and resveratrol in human articular chondrocytes: Inhibition of IL-1β-induced NF-κB-mediated inflammation and apoptosis. Arthritis Res. Ther. 2009, 11, R165. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Csaki, C.; Nebrich, S.; Mobasheri, A. Resveratrol suppresses interleukin-1β-induced inflammatory signaling and apoptosis in human articular chondrocytes: Potential for use as a novel nutraceutical for the treatment of osteoarthritis. Biochem. Pharmacol. 2008, 76, 1426–1439. [Google Scholar] [CrossRef] [PubMed]
- Cooley, J.; Broderick, T.; Al-Nakkash, L.; Plochocki, J.H. Effects of resveratrol treatment on bone and cartilage in obese diabetic mice. J. Diabetes Metab. Disord. 2015, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Kondo, A.; Otsuka, T.; Kuroyanagi, G.; Yamamoto, N.; Matsushima-Nishiwaki, R.; Mizutani, J.; Kozawa, O.; Tokuda, H. Resveratrol inhibits BMP-4-stimulated VEGF synthesis in osteoblasts: Suppression of S6 kinase. Int. J. Mol. Med. 2014, 33, 1013–1018. [Google Scholar] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, H.; Li, K.; Li, X.; Yu, X.; Wang, W.; Ding, L.; Liu, L. Oral Resveratrol Prevents Osteoarthritis Progression in C57BL/6J Mice Fed a High-Fat Diet. Nutrients 2016, 8, 233. https://doi.org/10.3390/nu8040233
Gu H, Li K, Li X, Yu X, Wang W, Ding L, Liu L. Oral Resveratrol Prevents Osteoarthritis Progression in C57BL/6J Mice Fed a High-Fat Diet. Nutrients. 2016; 8(4):233. https://doi.org/10.3390/nu8040233
Chicago/Turabian StyleGu, Hailun, Keyu Li, Xingyao Li, Xiaolu Yu, Wei Wang, Lifeng Ding, and Li Liu. 2016. "Oral Resveratrol Prevents Osteoarthritis Progression in C57BL/6J Mice Fed a High-Fat Diet" Nutrients 8, no. 4: 233. https://doi.org/10.3390/nu8040233
APA StyleGu, H., Li, K., Li, X., Yu, X., Wang, W., Ding, L., & Liu, L. (2016). Oral Resveratrol Prevents Osteoarthritis Progression in C57BL/6J Mice Fed a High-Fat Diet. Nutrients, 8(4), 233. https://doi.org/10.3390/nu8040233



