No Effect of Exercise Intensity on Appetite in Highly-Trained Endurance Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Recruitment
2.2. Baseline Testing
2.3. Experimental Protocol
2.4. Exercise Trials, Gut Hormones and Appetite Assessments
2.5. Subjective Appetite Assessment
2.6. Hormone Analysis
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Moderate and High Intensity Exercise Trials
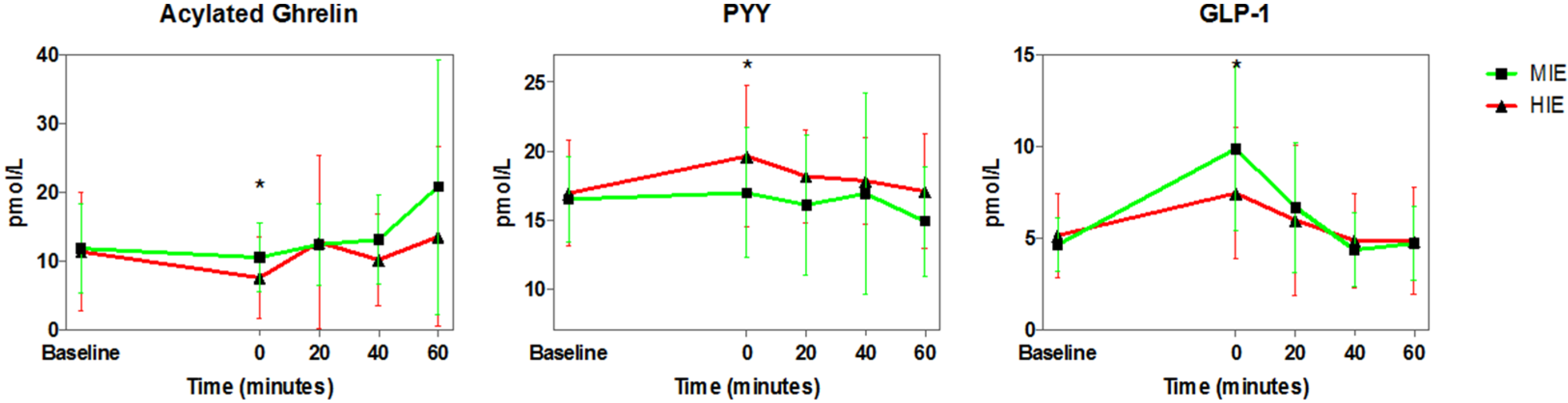
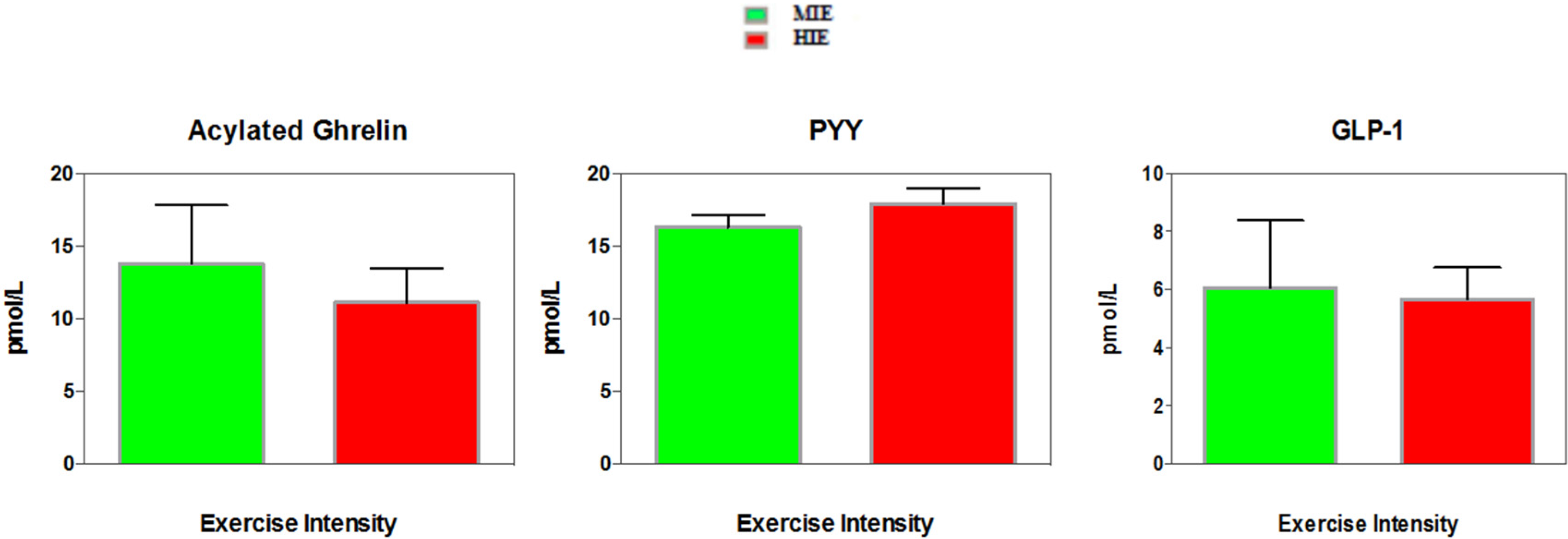
3.3. Appetite Hormones
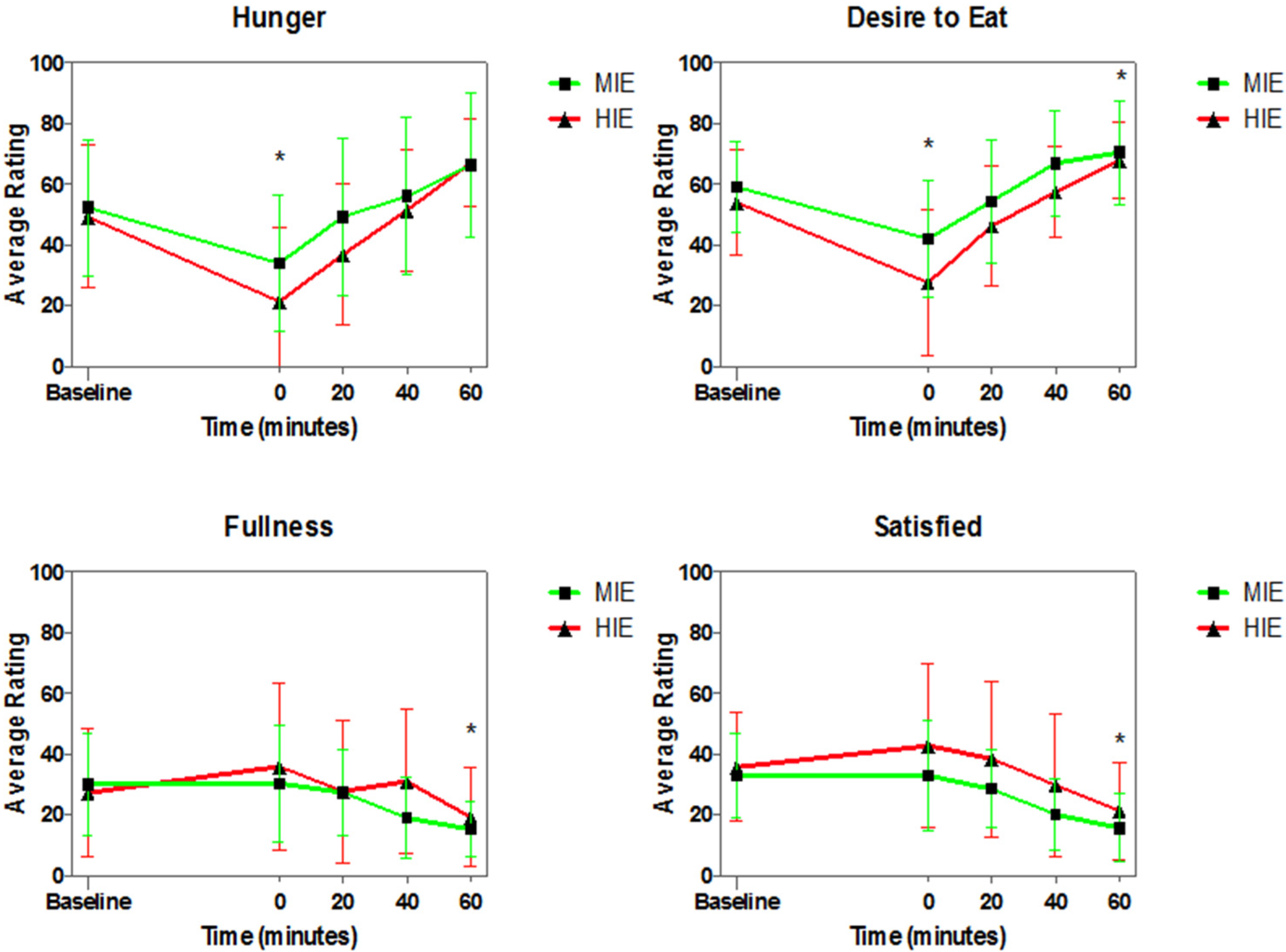
3.4. Appetite Ratings
4. Discussion
4.1. Sex Differences and Exercise Intensity
4.2. Exercise and Appetite-Regulating Hormones
4.3. Appetite Ratings, VAS and Appetite Hormones
4.4. Strengths and Limitations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| TLA | Three letter acronym |
| LD | linear dichroism |
References
- Physical Activity Guidelines for Americans. Available online: http://health.gov/paguidelines/guidelines/ (accessed on 30 March 2016).
- Haskell, W.L.; Lee, I.M.; Pate, R.R.; Powell, K.E.; Blair, S.N.; Franklin, B.A.; Macera, C.A.; Heath, G.W.; Thompson, P.D.; Bauman, A. Physical activity and public health: Updated recommendation for adults from the american college of sports medicine and the american heart association. Circulation 2007, 116, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- National Academy of Sciences; Institute of Medicine; Food and Nutrition Board. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; The National Academies Press: Washington, DC, USA, 2005. [Google Scholar]
- Coll, A.P.; Farooqi, I.S.; O’Rahilly, S. The hormonal control of food intake. Cell 2007, 129, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Morgan, L.M.; Bloom, S.R.; Robertson, M.D. Effects of exercise on gut peptides, energy intake and appetite. J. Endocrinol. 2007, 193, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Stensel, D. Exercise, appetite and appetite-regulating hormones: Implications for food intake and weight control. Ann. Nutr. Metab. 2010, 2, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Broom, D.R.; Stensel, D.J.; Bishop, N.C.; Burns, S.F.; Miyashita, M. Exercise-induced suppression of acylated ghrelin in humans. J. Appl. Physiol. 2007, 102, 2165–2171. [Google Scholar] [CrossRef] [PubMed]
- Broom, D.R.; Batterham, R.L.; King, J.A.; Stensel, D.J. Influence of resistance and aerobic exercise on hunger, circulating levels of acylated ghrelin, and peptide YY in healthy males. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R29–R35. [Google Scholar] [CrossRef] [PubMed]
- King, J.A.; Miyashita, M.; Wasse, L.K.; Stensel, D.J. Influence of prolonged treadmill running on appetite, energy intake and circulating concentrations of acylated ghrelin. Appetite 2010, 54, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.Y.; Yoshikawa, T.; Katsura, Y.; Usui, T.; Fujimoto, S. Comparable effects of moderate intensity exercise on changes in anorectic gut hormone levels and energy intake to high intensity exercise. J. Endocrinol. 2009, 203, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Vatansever-Ozen, S.; Tiryaki-Sonmez, G.; Bugdayci, G.; Ozen, G. The effects of exercise on food intake and hunger: Relationship with acylated ghrelin and leptin. J. Sports Sci. Med. 2011, 10, 283–291. [Google Scholar] [PubMed]
- Larson-Meyer, D.E.; Palm, S.; Bansal, A.; Austin, K.J.; Hart, A.M.; Alexander, B.M. Influence of running and walking on hormonal regulators of appetite in women. J. Obes. 2012, 730409, 730409. [Google Scholar] [CrossRef] [PubMed]
- Deighton, K.; Karra, E.; Batterham, R.L.; Stensel, D.J. Appetite, energy intake, and PYY3-36 responses to energy-matched continuous exercise and submaximal high-intensity exercise. Appl. Physiol. Nutr. Metab. 2013, 38, 947–952. [Google Scholar] [CrossRef] [PubMed]
- American College of Sports Medicine. ACSM’S Guidelines for Exercise Testing and Prescription, 9th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2014. [Google Scholar]
- Tanaka, H.; Monahan, K.D.; Seals, D.R. Age-predicted maximal heart rate revisited. J. Am. Coll. Cardiol. 2001, 37, 153–156. [Google Scholar] [CrossRef]
- Flint, A.; Raben, A.; Blundell, J.E.; Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. 2000, 24, 38–48. [Google Scholar] [CrossRef]
- Hagobian, T.A.; Sharoff, C.G.; Stephens, B.R.; Wade, G.N.; Silva, J.E.; Chipkin, S.R.; Braun, B. Effects of exercise on energy-regulating hormones and appetite in men and women. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R233–R242. [Google Scholar] [CrossRef] [PubMed]
- Hagobian, T.A.; Yamashiro, M.; Hinkel-Lipsker, J.; Streder, K.; Evero, N.; Hackney, T. Effects of acute exercise on appetite hormones and ad libitum energy intake in men and women. Appl. Physiol. Nutr. Metab. 2013, 38, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Balaguera-Cortes, L.; Wallman, K.E.; Fairchild, T.J.; Guelfi, K.J. Energy intake and appetite-related hormones following acute aerobic and resistance exercise. Appl. Physiol. Nutr. Metab. 2011, 36, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Becker, G.F.; Macedo, R.C.; Cunha Gdos, S.; Martins, J.B.; Laitano, O.; Reischak-Oliveira, A. Combined effects of aerobic exercise and high-carbohydrate meal on plasma acylated ghrelin and levels of hunger. Appl. Physiol. Nutr. Metab. 2012, 37, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Kawano, H.; Mineta, M.; Asaka, M.; Miyashita, M.; Numao, S.; Gando, Y.; Ando, T.; Sakamoto, S.; Higuchi, M. Effects of different modes of exercise on appetite and appetite-regulating hormones. Appetite 2013, 66, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.J.; Guelfi, K.J.; Wallman, K.E.; Fairchild, T.J. Mild dehydration does not reduce postexercise appetite or energy intake. Med. Sci. Sports Exerc. 2012, 44, 516–524. [Google Scholar] [CrossRef] [PubMed]
- King, J.A.; Wasse, L.K.; Ewens, J.; Crystallis, K.; Emmanuel, J.; Batterham, R.L.; Stensel, D.J. Differential acylated ghrelin, peptide YY3–36, appetite, and food intake responses to equivalent energy deficits created by exercise and food restriction. J. Clin. Endocrinol. Metab. 2011, 96, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Shorten, A.L.; Wallman, K.E.; Guelfi, K.J. Acute effect of environmental temperature during exercise on subsequent energy intake in active men. Am. J. Clin. Nutr. 2009, 90, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Wasse, L.K.; Sunderland, C.; King, J.A.; Batterham, R.L.; Stensel, D.J. Influence of rest and exercise at a simulated altitude of 4000 m on appetite, energy intake, and plasma concentrations of acylated ghrelin and peptide yy. J. Appl. Physiol. 2012, 112, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Wasse, L.K.; Sunderland, C.; King, J.A.; Miyashita, M.; Stensel, D.J. The influence of vigorous running and cycling exercise on hunger perceptions and plasma acylated ghrelin concentrations in lean young men. Appl. Physiol. Nutr. Metab. 2013, 38, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zoladz, J.A.; Konturek, S.J.; Duda, K.; Majerczak, J.; Sliwowski, Z.; Grandys, M.; Bielanski, W. Effect of moderate incremental exercise, performed in fed and fasted state on cardio-respiratory variables and leptin and ghrelin concentrations in young healthy men. J. Physiol. Pharmacol. 2005, 56, 63–85. [Google Scholar]
- Burns, S.F.; Broom, D.R.; Miyashita, M.; Mundy, C.; Stensel, D.J. A single session of treadmill running has no effect on plasma total ghrelin concentrations. J. Sports Sci. 2007, 25, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Russel, R.R.; Willis, K.S.; Ravussin, E.; Larson-Meyer, E.D. Effects of endurance running and dietary fat on circulating ghrelin and peptide yy. J. Sports Sci. Med. 2009, 8, 574–583. [Google Scholar] [PubMed]
- King, J.A.; Wasse, L.K.; Broom, D.R.; Stensel, D.J. Influence of brisk walking on appetite, energy intake, and plasma acylated ghrelin. Med. Sci. Sports Exerc. 2010, 42, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Deighton, K.; Barry, R.; Connon, C.E.; Stensel, D.J. Appetite, gut hormone and energy intake responses to low volume sprint interval and traditional endurance exercise. Eur. J. Appl. Physiol. 2013, 113, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, A.M.; Johnston, C.F.; Buchanan, K.D.; Boreham, C.; Trinick, T.R.; Riddoch, C.J. Circulating gastrointestinal hormone changes in marathon running. Int. J. Sports Med. 1995, 16, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Hazell, T.J.; Islam, H.; Townsend, L.K.; Schmale, M.S.; Copeland, J.L. Effects of exercise intensity on plasma concentrations of appetite-regulating hormones: Potential mechanisms. Appetite 2016, 98, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.M.; Desbrow, B.; Sabapathy, S.; Leveritt, M. Acute exercise and subsequent energy intake. A meta-analysis. Appetite 2013, 63, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.M.; Sabapathy, S.; Leveritt, M.; Desbrow, B. Acute exercise and hormones related to appetite regulation: A meta-analysis. Sports Med. 2014, 44, 387–403. [Google Scholar] [CrossRef] [PubMed]
- Laan, D.J.; Leidy, H.J.; Lim, E.; Campbell, W.W. Effects and reproducibility of aerobic and resistance exercise on appetite and energy intake in young, physically active adults. Appl. Physiol. Nutr. Metab. 2010, 35, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Imbeault, P.; Saint-Pierre, S.; AlméRas, N.; Tremblay, A. Acute effects of exercise on energy intake and feeding behaviour. Br. J. Nutr. 1997, 77, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Pomerleau, M.; Imbeault, P.; Parker, T.; Doucet, E. Effects of exercise intensity on food intake and appetite in women. Am. J. Clin. Nutr. 2004, 80, 1230–1236. [Google Scholar] [PubMed]
- King, N.A.; Burley, V.J.; Blundell, J.E. Exercise-induced suppression of appetite: Effects on food intake and implications for energy balance. Eur. J. Clin. Nutr. 1994, 48, 715–724. [Google Scholar] [PubMed]
- Crabtree, D.R.; Chambers, E.S.; Hardwick, R.M.; Blannin, A.K. The effects of high-intensity exercise on neural responses to images of food. Am. J. Clin. Nutr. 2014, 99, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Dafopoulos, K.; Sourlas, D.; Kallitsaris, A.; Pournaras, S.; Messinis, I.E. Blood ghrelin, resistin, and adiponectin concentrations during the normal menstrual cycle. Fertil. Steril. 2009, 92, 1389–1394. [Google Scholar] [CrossRef] [PubMed]

| Pre-Exercise | Exercise EE = 500 kcals (2093 kJ) | Post-Exercise (Rest) | ||||||
|---|---|---|---|---|---|---|---|---|
| 48-h Pre-Visit | Baseline | HIE or MIE | 0-min | 20-min | 40-min | 60-min | 24-h Post Visit (Before Meals, Pre-and Post-Exercise) | |
| Gut Hormones | • | • | • | • | • | |||
| VAS | • | • | • | • | • | |||
| Food Log | • | • | ||||||
| PA Log | • | • | ||||||
| Age (years) | 31.1 ± 6.7 |
| Height (cm) | 166 ± 7 |
| Weight (kg) | 58.4 ± 6.4 |
| BMI (kg/m2) | 21.1 ± 1.7 |
| Body Fat (%) | 14.6 ± 2.9 |
| VO2MAX (mL/kg/min) | 55.2 ± 4.3 |
| Moderate Intensity Exercise (MIE) (60% VO2MAX) | High Intensity Exercise (HIE) (85% VO2MAX) | |
|---|---|---|
| Duration (min) | 45.7 ± 10.8 | 33.6 + 5.6 |
| Speed (m/min) | 157.1 ± 17.3 | 199.7 ± 21.3 |
| Grade (%) | 0.9 ± 1.1 | 2.6 ± 2.7 |
| VO2 (mL/kg/min) | 36.4 ± 3.0 | 47.8 ± 3.7 |
| Heart Rate (BPM) | 139 ± 11 | 169 ± 8 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Howe, S.M.; Hand, T.M.; Larson-Meyer, D.E.; Austin, K.J.; Alexander, B.M.; Manore, M.M. No Effect of Exercise Intensity on Appetite in Highly-Trained Endurance Women. Nutrients 2016, 8, 223. https://doi.org/10.3390/nu8040223
Howe SM, Hand TM, Larson-Meyer DE, Austin KJ, Alexander BM, Manore MM. No Effect of Exercise Intensity on Appetite in Highly-Trained Endurance Women. Nutrients. 2016; 8(4):223. https://doi.org/10.3390/nu8040223
Chicago/Turabian StyleHowe, Stephanie M., Taryn M. Hand, D. Enette Larson-Meyer, Kathleen J. Austin, Brenda M. Alexander, and Melinda M. Manore. 2016. "No Effect of Exercise Intensity on Appetite in Highly-Trained Endurance Women" Nutrients 8, no. 4: 223. https://doi.org/10.3390/nu8040223
APA StyleHowe, S. M., Hand, T. M., Larson-Meyer, D. E., Austin, K. J., Alexander, B. M., & Manore, M. M. (2016). No Effect of Exercise Intensity on Appetite in Highly-Trained Endurance Women. Nutrients, 8(4), 223. https://doi.org/10.3390/nu8040223





