Fish Oil-Based Fat Emulsion Reduces Acute Kidney Injury and Inflammatory Response in Antibiotic-Treated Polymicrobial Septic Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Plasma Biochemical Markers and Inflammatory Mediators
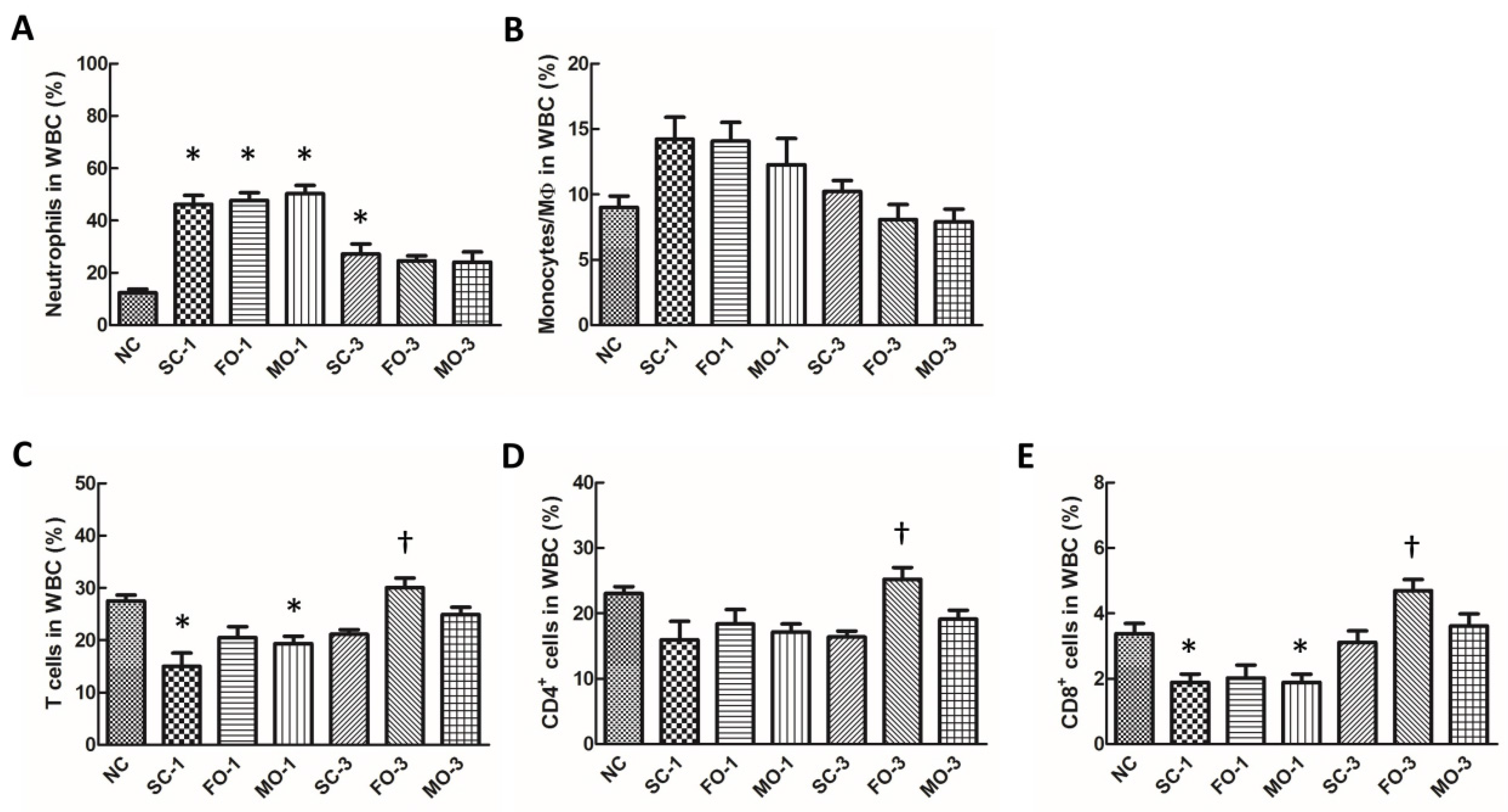
2.4. Blood Leukocyte Distribution
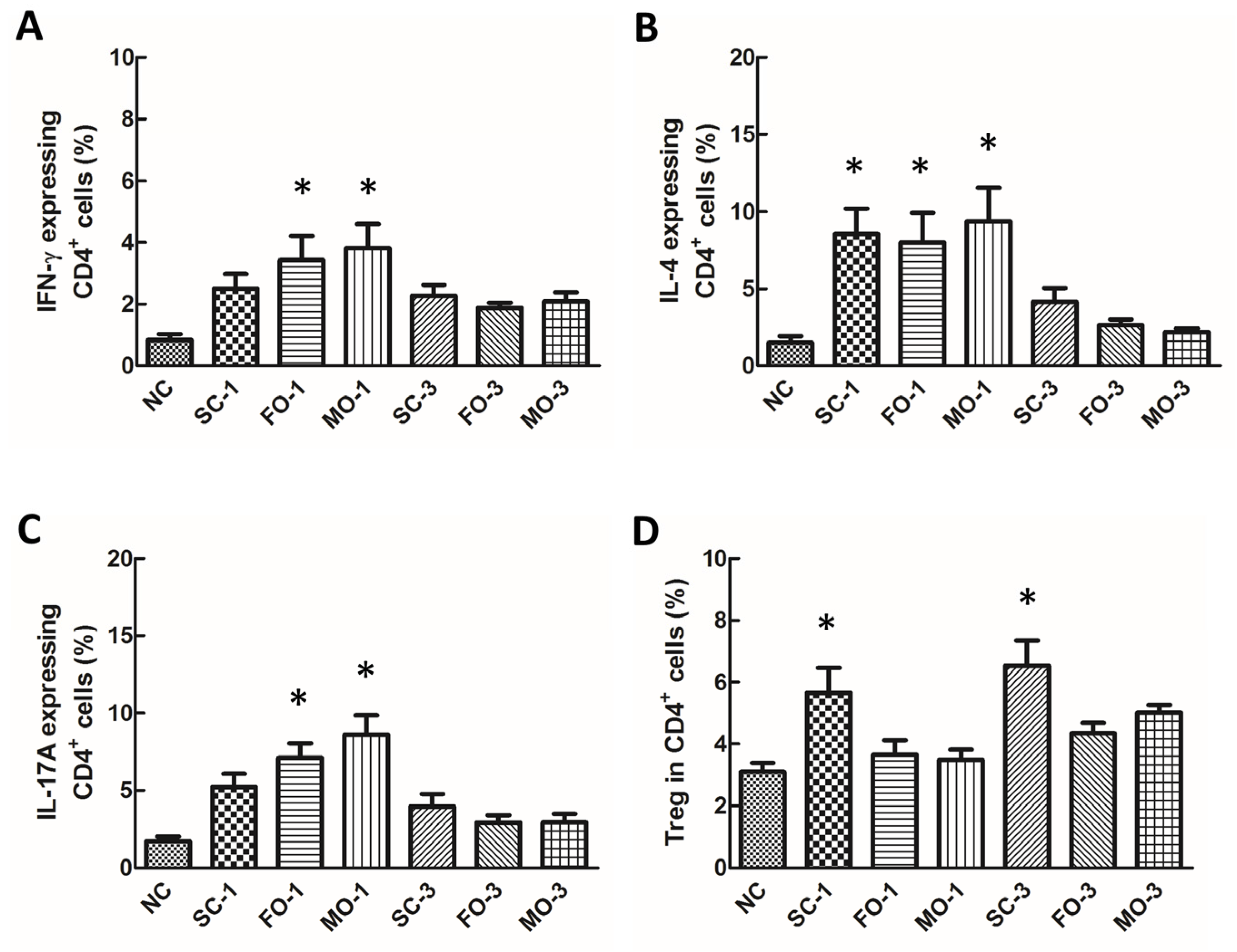
2.5. CD4+ T Cell Subsets in Blood
2.6. Inflammatory Cytokine Concentrations in PLF
2.7. RNA Extraction and Real-Time Polymerase Chain Reaction in Kidney
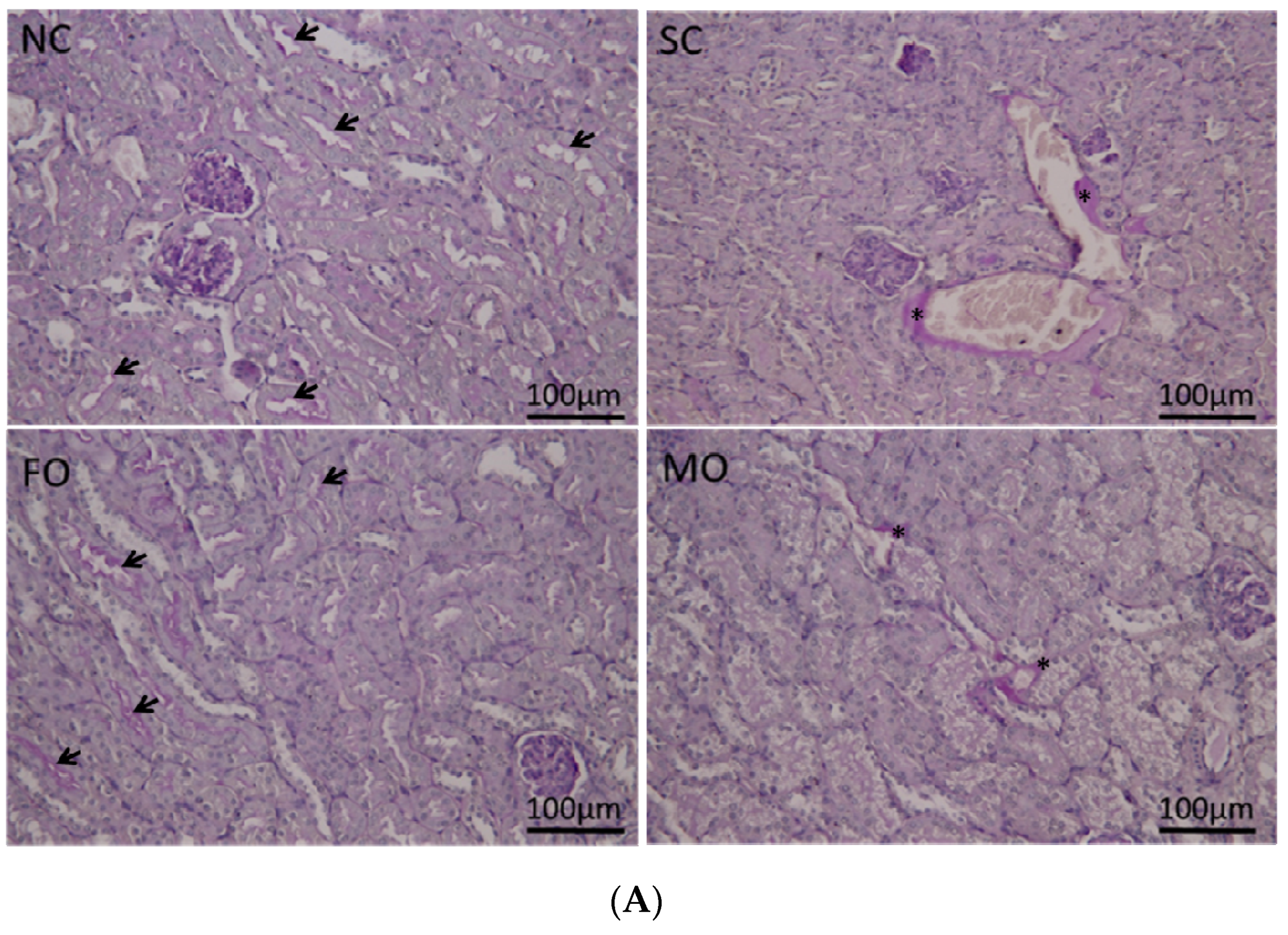
2.8. Renal Histology
2.9. Statistical Analysis
3. Results
3.1. Survival Rates and Body Weights
3.2. Plasma Biochemical Parameters
3.3. Blood Leukocyte Distribution
3.4. Concentrations of Eicosanoids in Plasma and Cytokines in PLF
3.5. Expression of Inflammation-Related Genes in Renal Tissues
3.6. Histological Findings of Renal Tissue
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ostermann, M.; Chang, R.W. Acute kidney injury in the intensive care unit according to RIFLE. Crit. Care Med. 2007, 35, 1837–1843. [Google Scholar] [CrossRef] [PubMed]
- Uchino, S.; Kellum, J.A.; Bellomo, R.; Doig, G.S.; Morimatsu, H.; Morgera, S.; Schetz, M.; Tan, I.; Bouman, C.; Macedo, E.; et al. Acute renal failure in critically ill patients: A multinational, multicenter study. J. Am. Med. Assoc. 2005, 294, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Bagshaw, S.M.; George, C.; Bellomo, R.; Committee, A.D.M. Early acute kidney injury and sepsis: A multicentre evaluation. Crit. Care 2008, 12, R47. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Karl, I.E. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 2003, 348, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Sepsis-induced immunosuppression: From cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 2013, 13, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Chawla, L.S.; Seneff, M.G.; Nelson, D.R.; Williams, M.; Levy, H.; Kimmel, P.L.; Macias, W.L. Elevated plasma concentrations of IL-6 and elevated APACHE II score predict acute kidney injury in patients with severe sepsis. Clin J. Am. Soc. Nephrol. 2007, 2, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505S–1519S. [Google Scholar] [PubMed]
- Lin, M.T.; Hsu, C.S.; Yeh, S.L.; Yeh, C.L.; Chang, K.J.; Lee, P.H.; Chen, W.J. Effects of omega-3 fatty acids on leukocyte Th1/Th2 cytokine and integrin expression in rats with gut-derived sepsis. Nutrition 2007, 23, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Ren, J.; Sun, L.; Gu, G.; Yuan, Y.; Li, J. Fish oil-supplemented parenteral nutrition prolongs survival while beneficially altering phospholipids’ Fatty Acid composition and modulating immune function in rat sepsis. Shock 2011, 36, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Lauretani, F.; Semba, R.D.; Bandinelli, S.; Miller, E.R., 3rd; Ruggiero, C.; Cherubini, A.; Guralnik, J.M.; Ferrucci, L. Plasma polyunsaturated fatty acids and the decline of renal function. Clin. Chem. 2008, 54, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Kielar, M.L.; Jeyarajah, D.R.; Zhou, X.J.; Lu, C.Y. Docosahexaenoic acid ameliorates murine ischemic acute renal failure and prevents increases in mRNA abundance for both TNF-alpha and inducible nitric oxide synthase. J. Am. Soc. Nephrol. 2003, 14, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Meisel, J.A.; Le, H.D.; de Meijer, V.E.; Nose, V.; Gura, K.M.; Mulkern, R.V.C.; Sharif, M.R.A.; Puder, M. Comparison of 5 intravenous lipid emulsions and their effects on hepatic steatosis in a murine model. J. Pediatr. Surg. 2011, 46, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Park, H.J.; Yoon, J.; Hong, S.H.; Oh, C.Y.; Lee, S.K.; Seo, J.M. Reversal of intestinal failure–associated liver disease by switching from a combination lipid emulsion containing fish oil to fish oil monotherapy. J. Parenter. Enter. Nutr. 2016, 40, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.; Fegbeutel, C.; Hattar, K.; Sibelius, U.; Kramer, H.J.; Heuer, K.U.; temmesfeld-Wollbruck, B.; Gokorsch, S.; Grimminger, F.; Seeger, W. Omega-3 vs. omega-6 lipid emulsions exert differential influence on neutrophils in septic shock patients: Impact on plasma fatty acids and lipid mediator generation. Intensive Care Med. 2003, 29, 1472–1481. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, W.; Dhaliwal, R.; Jurewitsch, B.; Stapleton, R.D.; Jeejeebhoy, K.N.; Heyland, D.K. Alternative lipid emulsions in the critically ill: A systematic review of the evidence. Intensive Care Med. 2013, 39, 1683–1694. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Perez, J.; Condotta, S.A.; Badovinac, V.P.; Griffith, T.S. Impact of sepsis on CD4 T cell immunity. J. Leukoc. Biol. 2014, 96, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Doi, K.; Leelahavanichkul, A.; Yuen, P.S.; Star, R.A. Animal models of sepsis and sepsis-induced kidney injury. J. Clin. Investig. 2009, 119, 2868–2878. [Google Scholar] [CrossRef] [PubMed]
- Wichterman, K.A.; Baue, A.E.; Chaudry, I.H. Sepsis and septic shock—A review of laboratory models and a proposal. J. Surg. Res. 1980, 29, 189–201. [Google Scholar] [CrossRef]
- Alwayn, I.P.; Gura, K.; Nosé, V.; Zausche, B.; Javid, P.; Garza, J.; Verbesey, J.; Voss, S.; Ollero, M.; Andersson, C.; et al. Omega-3 fatty acid supplementation prevents hepatic steatosis in a murine model of nonalcoholic fatty liver disease. Pediatr. Res. 2005, 57, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, M.B.; Ott, J.; Mohr, A.; Bi, M.H.; Grosz, A.; Weissmann, N.; Ishii, S.; Grimminger, F.; Seeger, W.; Mayer, K. Immunomodulation by n-3- versus n-6-rich lipid emulsions in murine acute lung injury—Role of platelet-activating factor receptor. Crit. Care Med. 2007, 35, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Heller, A.R.; Rossler, S.; Litz, R.J.; Stehr, S.N.; Heller, S.C.; Koch, R.; Koch, T. Omega-3 fatty acids improve the diagnosis-related clinical outcome. Crit. Care Med. 2006, 34, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Kurus, M.; Ugras, M.; Esrefoglu, M. Effect of resveratrol on tubular damage and interstitial fibrosis in kidneys of rats exposed to cigarette smoke. Toxicol. Ind. Health 2009, 25, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.F.; Hou, Y.C.; Pai, M.H.; Yeh, S.L.; Liu, J.J. Effects of n-3 polyunsaturated fatty acids on the homeostasis of CD4+ T cells and lung injury in mice with polymicrobial sepsis. J. Parenter. Enter. Nutr. 2015. [Google Scholar] [CrossRef] [PubMed]
- Deitch, E.A. Multiple organ failure. Pathophysiology and potential future therapy. Ann. Surg. 1992, 216, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.D.; Coran, A.G.; Drongowski, R.A.; Wesley, J.R. The quantitative transperitoneal absorption of a fat emulsion: Implications for intraperitoneal nutrition. J. Pediatr. Surg. 1983, 18, 724–731. [Google Scholar] [CrossRef]
- Mahedero, G.; Moran, J.M.; Salas, J.; Blanco, M. Absorption of Intralipid and interferences from nutrients infused into the peritoneal cavity of the rat. Am. J. Surg. 1992, 164, 45–50. [Google Scholar] [CrossRef]
- Boomer, J.S.; To, K.; Chang, K.C.; Takasu, O.; Osborne, D.F.; Walton, A.H.; Bricker, T.L.; Jarman, S.D.; Kreisel, D.; Krupnick, A.S.; et al. Immunosuppression in patients who die of sepsis and multiple organ failure. J. Am. Med. Assoc. 2011, 306, 2594–2605. [Google Scholar] [CrossRef] [PubMed]
- Kasten, K.R.; Tschop, J.; Adediran, S.G.; Hildeman, D.A.; Caldwell, C.C. T cells are potent early mediators of the host response to sepsis. Shock 2010, 34, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Leng, F.Y.; Liu, J.L.; Liu, Z.J.; Yin, J.Y.; Qu, H.P. Increased proportion of CD4+CD25+Foxp3+ regulatory T cells during early-stage sepsis in ICU patients. J. Microbiol. Immunol. Infect. 2013, 46, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Venet, F.; Chung, C.S.; Monneret, G.; Huang, X.; Horner, B.; Garber, M.; Ayala, A. Regulatory T cell populations in sepsis and trauma. J. Leukoc. Biol. 2008, 83, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Yessoufou, A.; Plé, A.; Moutairou, K.; Hichami, A.; Khan, N.A. Docosahexaenoic acid reduces suppressive and migratory functions of CD4+CD25+ regulatory T-cells. J. Lipid Res. 2009, 50, 2377–2388. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, R.; Kellum, J.A.; Ronco, C. Defining acute renal failure: Physiological principles. Intensive Care Med. 2004, 30, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Carlson, M.; Raab, Y.; Sevéus, L.; Xu, S.; Hällgren, R.; Venge, P. Human neutrophil lipocalin is a unique marker of neutrophil inflammation in ulcerative colitis and proctitis. Gut 2002, 50, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Zappitelli, M.; Washburn, K.K.; Arikan, A.A.; Loftis, L.; Ma, Q.; Devarajan, P.; Parikh, C.R.; Goldstein, S.L. Urine neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in critically ill children: A prospective cohort study. Crit. Care 2007, 11. [Google Scholar] [CrossRef] [PubMed]
- Mishra, J.; Dent, C.; Tarabishi, R.; Mitsnefes, M.M.; Ma, Q.; Kelly, C.; Ruff, S.M.; Zahedi, K.; Shao, M.; Bean, J.; et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 2005, 365, 1231–1238. [Google Scholar] [CrossRef]
- Erlandsson Harris, H.; Andersson, U. Mini-review: The nuclear protein HMGB1 as a proinflammatory mediator. Eur. J. Immunol. 2004, 34, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Tracey, K.J. Targeting HMGB1 in inflammation. Biochim. Biophy. Acta 2010, 1799, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Lotze, M.T.; Tracey, K.J. High-mobility group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 2005, 5, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Pittet, J.F.; Lee, H.; Morabito, D.; Howard, M.B.; Welch, W.J.; Mackersie, R.C. Serum levels of Hsp 72 measured early after trauma correlate with survival. J. Trauma 2002, 52, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Berquin, I.M.; Owens, R.T.; O’Flaherty, J.T.; Edwards, I.J. Peroxisome proliferator-activated receptor gamma-mediated up-regulation of syndecan-1 by n-3 fatty acids promotes apoptosis of human breast cancer cells. Cancer Res. 2008, 68, 2912–2919. [Google Scholar] [CrossRef] [PubMed]
- Vanden Berghe, W.; Vermeulen, L.; Delerive, P.; De Bosscher, K.; Staels, B.; Haegeman, G. The paradigm for gene regulation: Inflammation, NF-κB and PPAR. Adv. Exp. Med. Biol. 2003, 544, 181–196. [Google Scholar] [PubMed]
- McGuinness, J.; Byrne, J.; Condron, C.; McCarthy, J.; Bouchier-Hayes, D.; Redmond, J.M. Pretreatment with u-3 fatty acid infusion to prevent leukocyte-endothelial injury responses seen in cardiac surgery. J. Thorac. Cardiovasc. Surg. 2008, 136, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Kumlin, M.; Ingelman-Sundberg, M.; Wolk, A. Dietary long-chain n-3 fatty acids for the prevention of cancer: A review of potential mechanisms. Am. J. Clin. Nutr. 2004, 79, 935–945. [Google Scholar] [PubMed]
- Craciun, F.L.; Schuller, E.R.; Remick, D.G. Early enhanced local neutrophil recruitment in peritonitis-induced sepsis improves bacterial clearance and survival. J. Immunol. 2010, 185, 6930–6938. [Google Scholar] [CrossRef] [PubMed]
- Boisrame-Helms, J.; Delabranche, X.; Klymchenko, A.; Drai, J.; Blond, E.; Zobairi, F.; Mely, Y.; Hasselmann, M.; Tofi, F.; Meziani, F. Lipid emulsions differentially affect LPS-induced acute monocytes inflammation: In vitro effects on membrane remodeling and cell viability. Lipids 2014, 49, 1091–1099. [Google Scholar] [CrossRef] [PubMed]


| Fatty Acids | FO (Omegaven®) | MO (SMOFlipid®) |
|---|---|---|
| C8:0 | 16.3 | |
| C10:0 | 11.4 | |
| C14:0 | 4.9 | |
| C16:0 | 10.7 | 9.2 |
| C18:0 | 2.4 | 2.7 |
| C16:1 n-7 | 8.2 | |
| C18:1 n-9 | 12.3 | 27.8 |
| C18:2 n-6 | 3.7 | 18.7 |
| C18:3 n-3 | 1.8 | 2.4 |
| C20:4 n-6 | 2.6 | 0.5 |
| C20:5 n-3 (EPA) | 18.8 | 2.4 |
| C22:5 n-3 | 2.8 | |
| C22:6 n-3 (DHA) | 16.5 | 2.2 |
| Others | 15.3 | 6.4 |
| Gene Name | Accession No. | 5′-3′Primer Sequence |
|---|---|---|
| β-actin | NM_007393.2 | F: AGCCATGTACGTAGCCATCC R: CTCTCAGCTGTGGTGGTGAA |
| HMGB1 | NM_010439.2 | F: TTGCTTTGCCCATTTTGGGTCACA R: CCACAATGGCAGGGCATGTGGA |
| HSP70 | NM_010479.2 | F: GCTGGCTAGGAGACAGATATGTGGC R: AAAGCCCACGTGCAATACACAAAGT |
| MyD88 | NM_010851.2 | F: CATGGTGGTGGTTGTTTCTGAC R: TGGAGACAGGCTGAGTGCAA |
| TLR4 | NM_021297.2 | F: AGAAATTCCTGCAGTGGGTCA R: TCTCTACAGGTGTTGCACATGTCA |
| PPAR-γ | NM_011146.3 | F: GCCCTTTGGTGACTTTATGG R: CAGCAGGTTGTCTTGGATGT |
| TNF-α | NM_013693.2 | F: CCCTCACACTCAGATCATCTTCT R: GCTACGACGTGGGCTACAG |
| NC | SC-1 | FO-1 | MO-1 | SC-3 | FO-3 | MO-3 | |
|---|---|---|---|---|---|---|---|
| BUN (mg/dL) | 24.3 ± 0.98 | 19.2 ± 0.8 | 21.0 ± 1.2 | 20.2 ± 1.2 | 22.3 ± 1.7 | 20.5 ± 1.6 | 22.1 ± 1.5 |
| Cr (mg/mL) | 4.56 ± 1.33 * | 6.01 ± 1.02 | 7.16 ± 1.22 | 7.22 ± 0.96 | 9.83 ± 0.91 † | 6.33 ± 0.95 | 6.57 ± 0.73 |
| ALT (U/L) | 53.3 ± 5.0 * | 142.0 ± 7.7 | 157.4 ± 8.4 | 158.4 ± 10.0 | 71.5 ± 7.9 | 86.4 ± 5.8 | 92.2 ± 5.9 |
| AST (U/L) | 91.2 ± 11.1 * | 419.8 ± 41.2 | 372.8 ± 20.9 | 401.1 ± 15.8 | 193.7 ± 16.8 | 213.0 ± 11.5 | 232.6 ± 16.1 |
| NGAL (ng/mL) | 0.17 ± 0.07 * | 19.66 ± 1.61 | 22.07 ± 0.78 | 19.78 ± 1.28 | 8.56 ± 1.23 | 6.74 ± 0.84 ‡ | 7.58 ± 1.19 |
| NC | SC-1 | FO-1 | MO-1 | SC-3 | FO-3 | MO-3 | |
|---|---|---|---|---|---|---|---|
| Plasma | |||||||
| PGE2 (μg/mL) | 2.17 ± 0.31 | 1.36 ± 0.15 | 0.95 ± 0.11 | 1.33 ± 0.16 | 1.45 ± 0.45 | 1.79 ± 0.67 | 1.49 ± 0.44 |
| Lipoxin A4 (pg/mL) | ND | 9.58 ± 3.95 | 8.52 ± 2.72 | 9.69 ± 4.80 | ND | ND | ND |
| PLF (pg/mL) | |||||||
| IL-1β | ND | 48.0 ± 5.4 | 58.9 ± 7.4 | 53.0 ± 6.5 | 20.3 ± 4.5 | 55.2 ± 7.3 ‡ | 28.4 ± 7.8 |
| TNF-α | ND | 5.5 ± 0.5 | 6.0 ± 0.5 | 6.7 ± 1.6 | 5.3 ± 0.7 | 9.3 ± 2.0 ‡ | 6.2 ± 0.7 |
| IL-6 | ND | 738.3 ± 70.6 | 1288.2 ± 72.8 † | 1052.8 ± 168.3 † | 185.8 ± 43.1 | 239.8 ± 54.7 | 275.2 ± 30.9 |
| IL-10 | 91.8 ± 55.3 * | 19.3 ± 3.5 | 40.0 ± 10.5 ‡ | 25.0 ± 5.5 | 6.5 ± 1.4 | 12.7 ± 2.6 | 11.5 ± 3.2 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shih, J.-M.; Shih, Y.-M.; Pai, M.-H.; Hou, Y.-C.; Yeh, C.-L.; Yeh, S.-L. Fish Oil-Based Fat Emulsion Reduces Acute Kidney Injury and Inflammatory Response in Antibiotic-Treated Polymicrobial Septic Mice. Nutrients 2016, 8, 165. https://doi.org/10.3390/nu8030165
Shih J-M, Shih Y-M, Pai M-H, Hou Y-C, Yeh C-L, Yeh S-L. Fish Oil-Based Fat Emulsion Reduces Acute Kidney Injury and Inflammatory Response in Antibiotic-Treated Polymicrobial Septic Mice. Nutrients. 2016; 8(3):165. https://doi.org/10.3390/nu8030165
Chicago/Turabian StyleShih, Juey-Ming, Yao-Ming Shih, Man-Hui Pai, Yu-Chen Hou, Chiu-Li Yeh, and Sung-Ling Yeh. 2016. "Fish Oil-Based Fat Emulsion Reduces Acute Kidney Injury and Inflammatory Response in Antibiotic-Treated Polymicrobial Septic Mice" Nutrients 8, no. 3: 165. https://doi.org/10.3390/nu8030165
APA StyleShih, J.-M., Shih, Y.-M., Pai, M.-H., Hou, Y.-C., Yeh, C.-L., & Yeh, S.-L. (2016). Fish Oil-Based Fat Emulsion Reduces Acute Kidney Injury and Inflammatory Response in Antibiotic-Treated Polymicrobial Septic Mice. Nutrients, 8(3), 165. https://doi.org/10.3390/nu8030165





