Heat-Killed Enterococcus faecalis EF-2001 Ameliorates Atopic Dermatitis in a Murine Model
Abstract
:1. Introduction
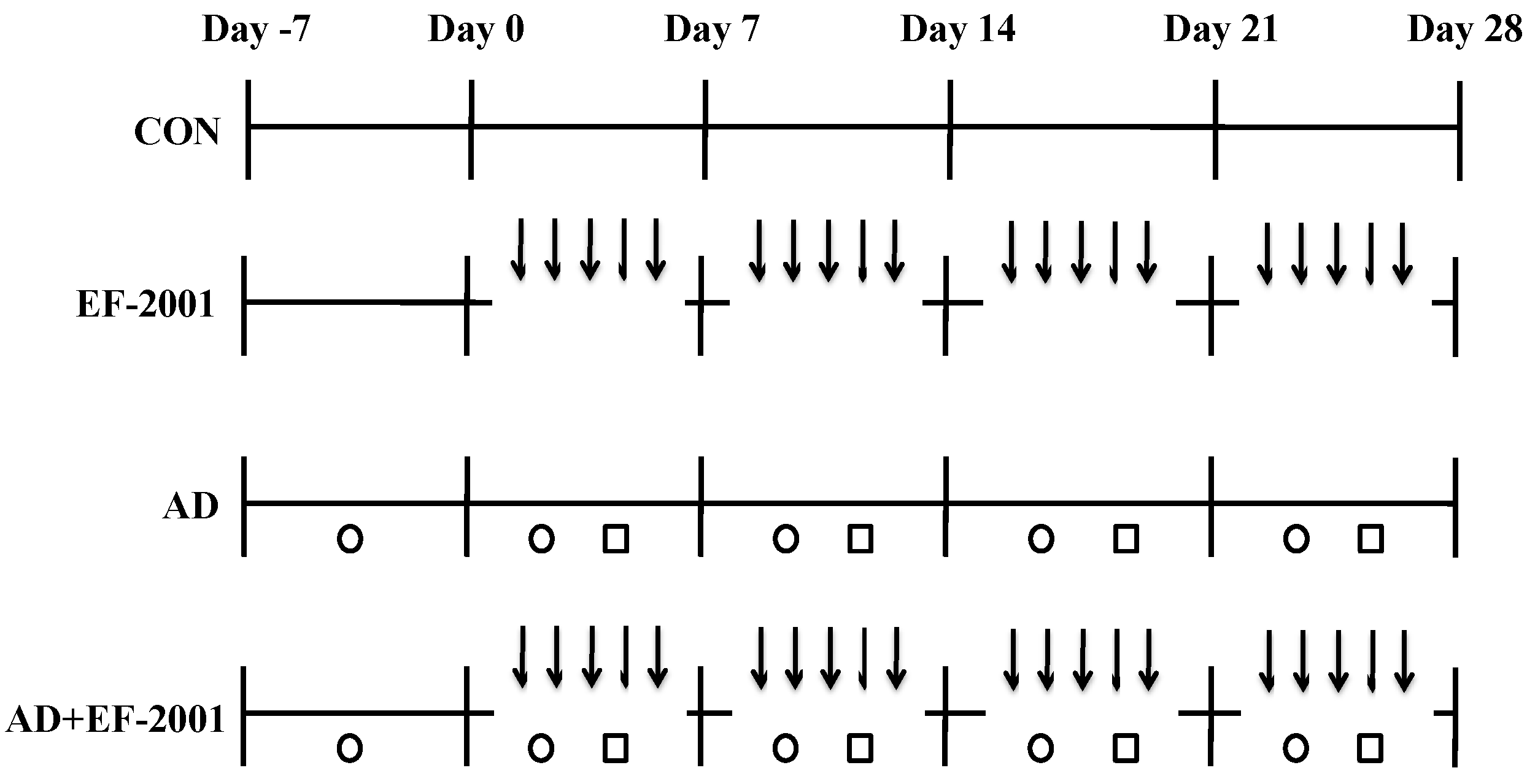
2. Materials and Methods
2.1. Animals
2.2. EF-2001
2.3. Induction of AD Lesions in the Ear
2.4. Histological Observations
2.5. Preparation of Splenocytes
2.6. Real-Time Polymerase Chain Reaction (PCR)
2.7. Statistical Analysis
3. Results
3.1. Effect of EF-2001 on the Ear Thickness and Histopathological Observation
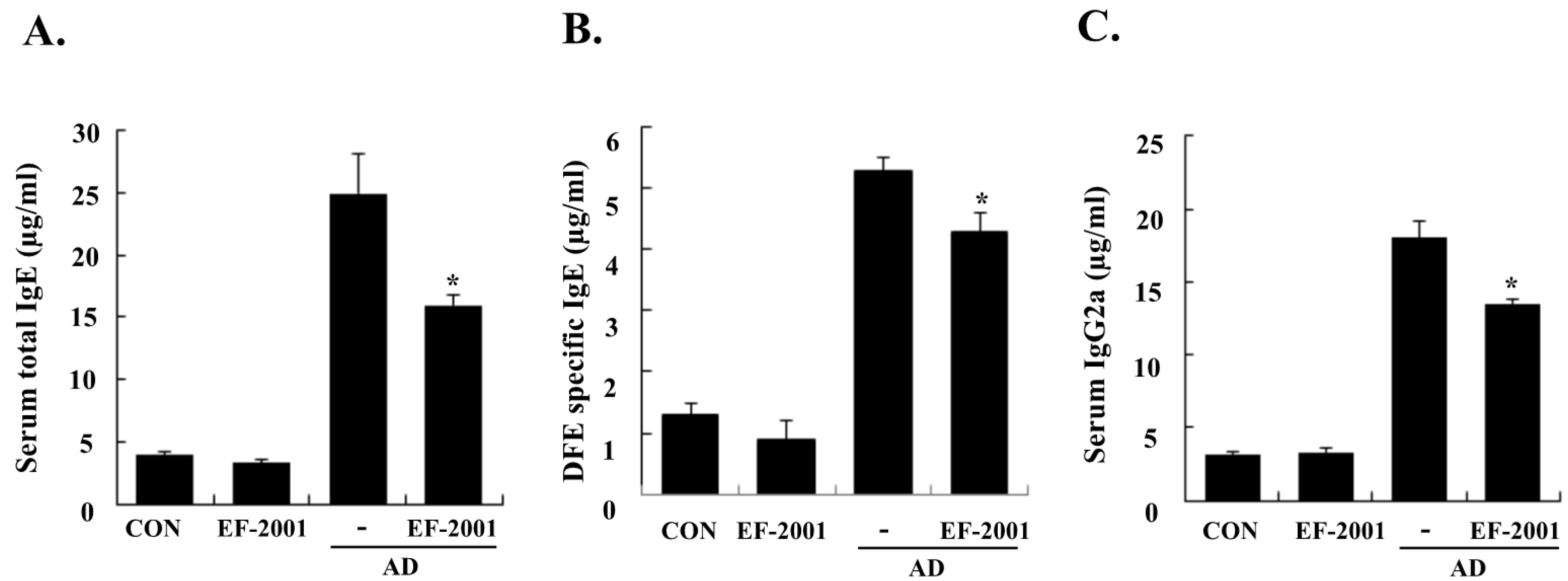
3.2. Effect of EF-2001 on Serum Ig Levels
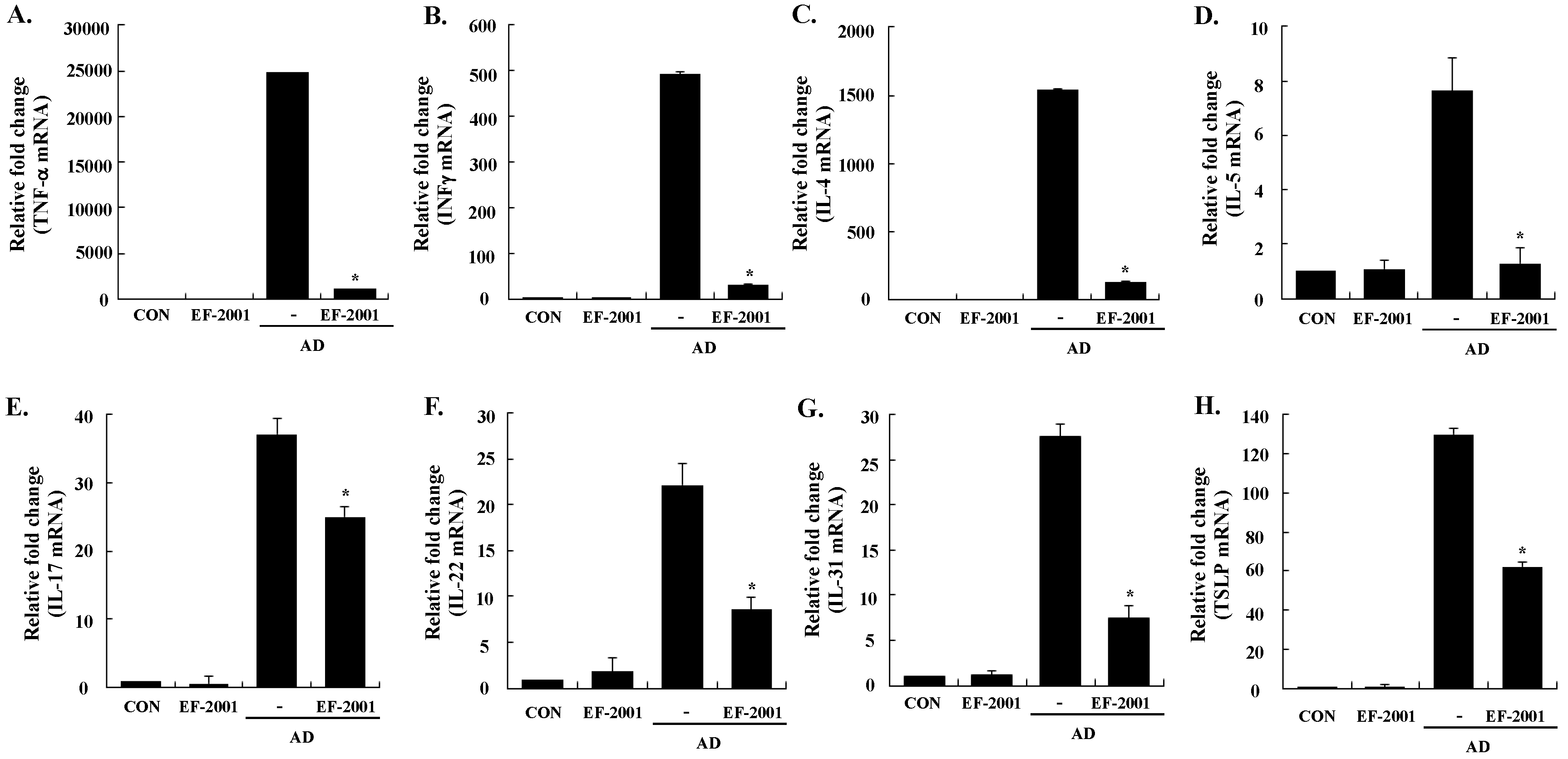
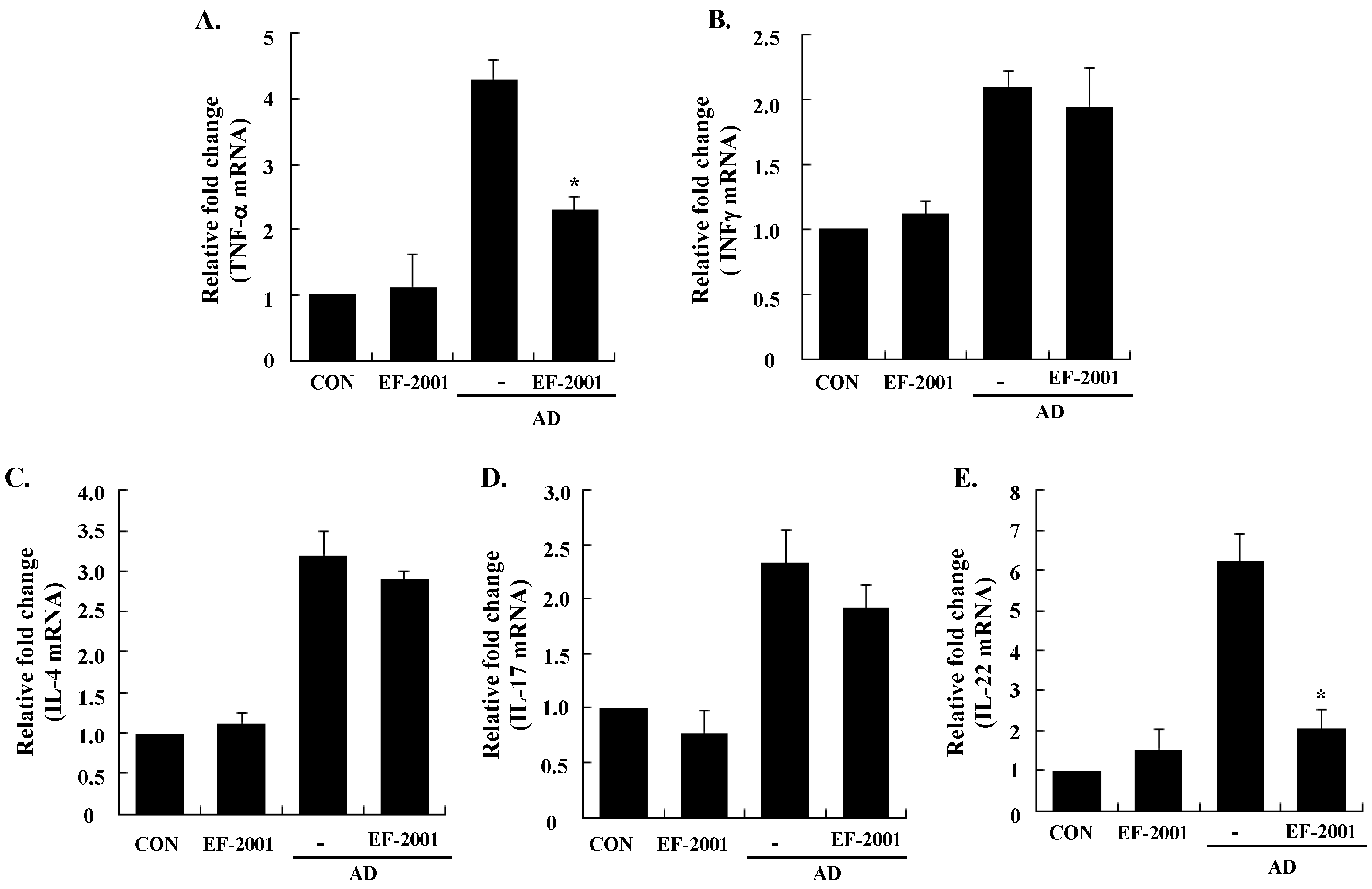
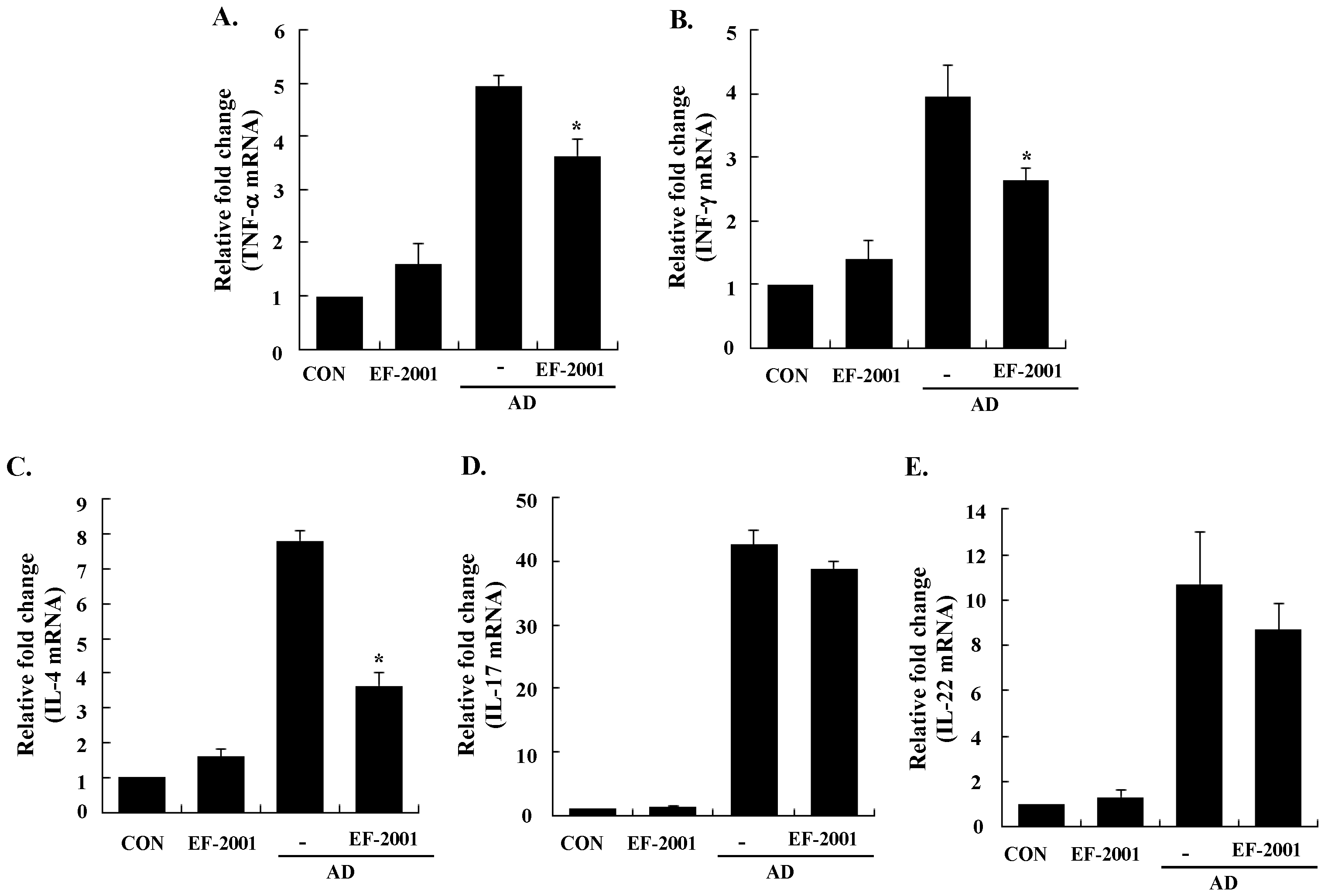
3.3. Effect of EF-2001 on the Expression of Various Pathogenic Cytokines
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, N.Y.; Ji, G.E. Effects of probiotics on the prevention of atopic dermatitis. Korean J. Pediatr. 2012, 55, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Roessler, A.; Friedrich, U.; Vogelsang, H.; Bauer, A.; Kaatz, M.; Hipler, U.C.; Schmidt, I.; Jahreis, G. The immune system in healthy adults and patients with atopic dermatitis seems to be affected differently by a probiotic intervention. Clin. Exp. Allergy 2001, 107, 129–134. [Google Scholar]
- Taverniti, V.; Guglielmetti, S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: Proposal of paraprobiotic concept). Genes. Nutr. 2011, 6, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, Y.; Guttman-Yassky, E. Immune pathways in atopic dermatitis, and definition of biomarkers through broad and targeted therapeutics. J. Clin. Med. 2015, 4, 858–873. [Google Scholar] [CrossRef] [PubMed]
- Montes-Torres, A.; Llamas-Velasco, M.; Pérez-Plaza, A.; Solano-López, G.; Sánchez-Pérez, J. Biological treatments in atopic dermatitis. J. Clin. Med. 2015, 4, 593–613. [Google Scholar] [CrossRef] [PubMed]
- Auriemma, M.; Vianale, G.; Amerio, P.; Reale, M. Cytokines and T cells in atopic dermatitis. Eur. Cytokine Netw. 2013, 24, 37–44. [Google Scholar] [PubMed]
- Baker, B.S. The role of microorganisms in atopic dermatitis. Clin. Exp. Immunol. 2006, 144, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Malajian, D.; Guttman-Yassky, E. New pathogenic and therapeutic paradigms in atopic dermatitis. Cytokine 2015, 73, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Fariñas, M.; Tintle, S.J.; Shemer, A.; Chiricozzi, A.; Nograles, K.; Cardinale, I.; Duan, S.; Bowcock, A.M.; Krueger, J.G.; Guttman-Yassky, E. Nonlesional atopic dermatitis skin is characterized by broad terminal differentiation defects and variable immune abnormalities. J. Allergy Clin. Immunol. 2011, 127, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Tintle, S.; Shemer, A.; Suárez-Fariñas, M.; Fujita, H.; Gilleaudeau, P.; Sullivan-Whalen, M.; Johnson-Huang, L.; Chiricozzi, A.; Cardinale, I.; Duan, S.; et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J. Allergy Clin. Immunol. 2011, 128, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Kanasugi, H.; Hasegawa, T.; Yamamoto, T.; Abe, S.; Yamaguchi, H. Optimal dose of Enterococcal preparation(FK-23) supplemented per orally for stimulation of leukocyte reconstitution in dogs treated with cyclophosphamide. J. Vet. Med. Sci. 1996, 56, 563–565. [Google Scholar] [CrossRef]
- Satonaka, K.; Ohashi, K.; Nohmi, T.; Yamamoto, T.; Abe, S.; Uchida, K.; Yamaguchi, H. Prophylactic effect on Enterococcus faecalis FK-23 preparation on experimental candidiasis in mice. Microbiol. Immunol. 1996, 40, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Mason, K.L.; Stepien, T.A.; Blum, J.E.; Holt, J.F.; Labbe, N.H.; Rush, J.S.; Raffa, K.F.; Handelsman, J. From commensal to pathogen: Translocation of Enterococcus faecalis from the midgut to hemocoel of Manduca sexta. mBio 2011, 2, e00065–11. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.; Phillips, C. The ecology, epidemiology and virulence of Enterococcus. Microbiology 2008, 155, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.-H.; Iwasa, M.; Iwasa, H.; Kobayashi, K.; Itokawa, Y.; Ishida, T. Radiation protection effect for EF2001 (Enterococcus faecalis 2001). Med. Biol. 2007, 151, 289–294. [Google Scholar]
- Yanagisawa, T.; Gu, Y.-H.; Tsuchihashi, E.; Umekawa, M.; Yamamoto, H.; Iwasa, T.; Suzuki, I. Analgesic and anti-neoplastic effects of the immunization-active fraction of Enterococcus faecalis 2001. J. Orient. Med. 2000, 5, 97–102. [Google Scholar]
- Choi, E.J.; Lee, S.; Kim, H.H.; Singh, T.S.; Choi, J.K.; Suh, W.M.; Lee, S.H.; Kim, S.H. Suppression of dust mite extract and 2,4-dinitrochlorobenzene-induced atopic dermatitis by the water extract of Lindera obtusiloba. J. Ethnopharmacol. 2011, 137, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Wang, Y.; Zhang, P.; Dong, Y.; Li, B. Subacute oral exposure to dibromoacetic acid induced immunotoxicity and apoptosis in the spleen and thymus of the mice. Toxicol. Sci. 2008, 105, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Metchnikoff, E. Essais optimists. The prolongation of life: Optimistic studies. In Classics in Longevity and Aging (Series); Mitchell, P.C., Metchnikoff, I.I., Eds.; Springer: Putnam, NY, USA, 1908. [Google Scholar]
- Besselink, M.G.; van Santvoort, H.C.; Buskens, E.; Boermeester, M.A.; van Goor, H.; Timmerman, H.M.; Nieuwenhuijs, V.B.; Bollen, T.L.; van Ramshorst, B.; Witteman, B.J.; et al. Probiotic prophylaxis in predicted severe acute pancreatitis: A randomised, double-blind, placebo-controlled trial. Lancet 2008, 371, 651–659. [Google Scholar] [CrossRef]
- Kochan, P.; Chmielarczyk, A.; Szymaniak, L.; Brykczynski, M.; Galant, K.; Zych, A.; Pakosz, K.; Giedrys-Kalemba, S.; Lenouvel, E.; Heczko, P.B. Lactobacillus rhamnosus administration causes sepsis in a cardiosurgical patient—Is the time right to revise probiotic safety guidelines? Clin. Microbiol. Infect. 2011, 17, 1589–1592. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, M.; Collins, M.D. Lactic acid bacteria and human clinical infection. J. Appl. Bacteriol. 1993, 75, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Ou, C.C.; Lin, S.L.; Tsai, J.J.; Lin, M.Y. Heat-killed lactic acid bacteria enhance immunomodulatory potential by skewing the immune response toward Th1 polarization. J. Food Sci. 2011, 76, M260–M267. [Google Scholar] [CrossRef] [PubMed]
- Murosaki, S.; Yamamoto, Y.; Ito, K.; Inokuchi, T.; Kusaka, H.; Ikeda, H.; Yoshikai, Y. Heat-killed Lactobacillus plantarum L-137 suppresses naturally fed antigen-specific IgE production by stimulation of IL-12 production in mice. J. Allergy Clin. Immunol. 1998, 102, 57–64. [Google Scholar] [CrossRef]
- Sawada, J.; Morita, H.; Tanaka, A.; Salminen, S.; He, F.; Matsuda, H. Ingestion of heat-treated Lactobacillus rhamnosus GG prevents development of atopic dermatitis in NC/NGA mice. Clin. Exp. Allergy 2007, 37, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Nakamura, F.; Kanzato, H.; Sawada, D.; Hirata, H.; Nishimura, A.; Kajimoto, O.; Fujiwara, S. Clinical effects of Lactobacillus acidophilus strain L-92 on perennial allergic rhinitis: A double-blind, placebo-controlled study. J. Dairy Sci. 2005, 88, 527–533. [Google Scholar] [CrossRef]
- O’Mahony, L.; Akdis, M.; Akdis, C.A. Regulation of the immune response and inflammation by histamine and histamine receptors. J. Allergy Clin. Immunol. 2011, 128, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Grendelmeier, P.; Simon, D.; Simon, H.U.; Akdis, C.A.; Wuthrich, B. Epidemiology, clinical features, and immunology of the “intrinsic” (non-IgE-mediated) type of atopic dermatitis (constitutional dermatitis). Allergy 2001, 56, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Bieber, T. Atopic dermatitis. Ann. Dermatol. 2010, 22, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Dokmeci, E.; Herrick, C.A. The immune system and atopic dermatitis. Semin. Cutan. Med. Surg. 2008, 27, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Homey, B.; Steinhoff, M.; Ruzicka, T.; Leung, D.Y. Cytokines and chemokines orchestrate atopic skin inflammation. J. Allergy Clin. Immunol. 2006, 118, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Nograles, K.E.; Zaba, L.C.; Shemer, A.; Fuentes-Duculan, J.; Cardinale, I.; Kikuchi, T.; Ramon, M.; Bergman, R.; Krueger, J.G.; Guttman-Yassky, E. IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J. Allergy Clin. Immunol. 2009, 123, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Albanesi, C.; Cavani, A.; Girolomoni, G. IL-17 is produced by nickel-specific T lymphocytes and regulates ICAM-1 expression and chemokine production in human keratinocytes: Synergistic or antagonist effects with IFN-gamma and TNF-α. J. Immunol. 1999, 162, 494–502. [Google Scholar] [PubMed]
- Guttman-Yassky, E.; Lowes, M.A.; Fuentes-Duculan, J.; Zaba, L.C.; Cardinale, I.; Nograles, K.E.; Khatcherian, A.; Novitskaya, I.; Carucci, J.A.; Bergman, R.; et al. Low expression of the IL-23/Th17 pathway in atopic dermatitis compared to psoriasis. J. Immunol. 2008, 181, 7420–7427. [Google Scholar] [CrossRef] [PubMed]
- Duhen, T.; Geiger, R.; Jarrossay, D.; Lanzavecchia, A.; Sallusto, F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat. Immunol. 2009, 10, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Eyerich, S.; Eyerich, K.; Pennino, D.; Carbone, T.; Nasorri, F.; Pallotta, S.; Cianfarani, F.; Odorisio, T.; Traidl-Hoffmann, C.; Behrendt, H.; et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J. Clin. Investig. 2009, 119, 3573–3585. [Google Scholar] [CrossRef] [PubMed]
- Fischer-Stabauer, M.; Boehner, A.; Eyerich, S.; Carbone, T.; Traidl-Hoffmann, C.; Schmidt-Weber, C.B.; Cavani, A.; Ring, J.; Hein, R.; Eyerich, K. Differential in situ expression of IL-17 in skin diseases. Eur. J. Dermatol. 2012, 22, 781–784. [Google Scholar] [PubMed]
- Nakae, S.; Komiyama, Y.; Nambu, A.; Sudo, K.; Iwase, M.; Homma, I.; Sekikawa, K.; Asano, M.; Iwakura, Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 2002, 17, 375–387. [Google Scholar] [CrossRef]
- Boniface, K.; Bernard, F.X.; Garcia, M.; Gurney, A.L.; Lecron, J.C.; Morel, F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J. Immunol. 2005, 174, 3695–3702. [Google Scholar] [CrossRef] [PubMed]
- Neis, M.M.; Peters, B.; Dreuw, A.; Wenzel, J.; Bieber, T.; Mauch, C.; Krieg, T.; Stanzel, S.; Heinrich, P.C.; Merk, H.F.; et al. Enhanced expression levels of IL-31 correlate with IL-4 and IL-13 in atopic and allergic contact dermatitis. J. Allergy Clin. Immunol. 2006, 118, 930–937. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Oyoshi, M.K.; Garibyan, L.; Kumar, L.; Ziegler, S.F.; Geha, R.S. TSLP acts on infiltrating effector T cells to drive allergic skin inflammation. Proc. Natl. Acad. Sci. USA 2008, 105, 11875–11880. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.H.; Li, H.Y.; Huang, C.H.; Lee, B.W.; Lee, Y.K.; Chua, K.Y. The effects of heat-killed wild-type Lactobacillus casei Shirota on allergic immune responses in an allergy mouse model. Int. Arch. Allergy Immunol. 2009, 148, 297–304. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, E.-J.; Iwasa, M.; Han, K.-I.; Kim, W.-J.; Tang, Y.; Hwang, Y.J.; Chae, J.R.; Han, W.C.; Shin, Y.-S.; Kim, E.-K. Heat-Killed Enterococcus faecalis EF-2001 Ameliorates Atopic Dermatitis in a Murine Model. Nutrients 2016, 8, 146. https://doi.org/10.3390/nu8030146
Choi E-J, Iwasa M, Han K-I, Kim W-J, Tang Y, Hwang YJ, Chae JR, Han WC, Shin Y-S, Kim E-K. Heat-Killed Enterococcus faecalis EF-2001 Ameliorates Atopic Dermatitis in a Murine Model. Nutrients. 2016; 8(3):146. https://doi.org/10.3390/nu8030146
Chicago/Turabian StyleChoi, Eun-Ju, Masahiro Iwasa, Kwon-Il Han, Wan-Jae Kim, Yujiao Tang, Young Joung Hwang, Jeong Ryong Chae, Weon Cheol Han, Yu-Su Shin, and Eun-Kyung Kim. 2016. "Heat-Killed Enterococcus faecalis EF-2001 Ameliorates Atopic Dermatitis in a Murine Model" Nutrients 8, no. 3: 146. https://doi.org/10.3390/nu8030146
APA StyleChoi, E.-J., Iwasa, M., Han, K.-I., Kim, W.-J., Tang, Y., Hwang, Y. J., Chae, J. R., Han, W. C., Shin, Y.-S., & Kim, E.-K. (2016). Heat-Killed Enterococcus faecalis EF-2001 Ameliorates Atopic Dermatitis in a Murine Model. Nutrients, 8(3), 146. https://doi.org/10.3390/nu8030146




