Dietary Flavonols Intake and Risk of Esophageal and Gastric Cancer: A Meta-Analysis of Epidemiological Studies
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Statistical Analysis
3. Results
3.1. The Characteristics of the Included Studies

3.2. Flavonols Intake and the Risk of Esophageal and Gastric Cancer
| Author, Year | Region, Period and Design | Cases/Controls | Baseline Intake of Flavonols | Flavonols Included | Flavonols Intake (mg/Day) | RR or OR and 95% CI | Adjustments | ||
|---|---|---|---|---|---|---|---|---|---|
| Petrick, et al. 2015 [17] | USA 1993–1995, PBCC | 465/662 | Control 14.46 ± 9.41 EAC 14.70 ± 9.68 ESCC 15.74 ± 10.41 | Total flavonols | 0–8.31 8.32–12.16 12.17–17.81 ≥17.82 | EC 1.00 0.57 (0.41–0.79) 0.68 (0.50–0.93) 0.87 (0.65–1.17) | EAC 1.00 0.56 (0.37–0.85) 0.67 (0.45-1.00) 0.80 (0.54–1.18) | ESCC 1.00 0.59 (0.36-0.98) 0.70 (0.43-1.14) 0.97 (0.62-1.53) | Age, sex, race, geographic centre, cigarette smoking, and dietary energy intake. |
| Verneulen, et al. 2013 [18] | 10 European countries 1992–2010, Cohort | 341 (477312) | Men2 7.1 ± 16.6 Women 27.2 ± 17.6 | Total flavonols | Q1 Q2 Q3 Q4 | 1.00 0.63 (0.44–0.90) 0.66 (0.45–0.96) 0.90 (0.61–1.34) | Age, sex, energy intake, BMI, smoking intensity, educational level, physical activity, alcohol, red and processed meat, fiber, vitamin C and carotenoids. | ||
| Bobe, et al. 2009 [19] | USA 1986–1989, PBCC | 493/1235 | W-Con:19.5 W-EAC: 21.0 W-ESCC: 28.6 B-Con: 20.7 B-ESCC: 26.2 | Isorhamnetin Kaempferol Myricetin Quercetin | <6.89 6.89–11.0 11.1–15.9 >15.9 | 1.00 1.17 (0.78–1.74) 1.11 (0.74–1.68) 1.05 (0.67–1.65) | 1.00 1.19 (0.66–2.17) 1.26 (0.67–2.38) 0.98 (0.47–2.01) | 1.00 1.15 (0.67–1.94) 1.02 (0.59–1.74) 1.10 (0.62–1.97) | Smoking duration and intensity, geographical area, age, BMI, hot tea, hard liquor, beer, red and white wine, caloric intake, education and income. |
| Rossi, et al. 2007 [20] | Italy 1992–1997, HBCC | 304/743 | 22.3 | Quercetin Myricetin Kaempferol | ≤15.9 16.0–20.4 20.5–25.4 25.5–31.9 >31.9 | 1.00 1.02 (0.59–1.76) 0.76 (0.43–1.32) 0.55 (0.30–0.98) 0.68 (0.38–1.24) | Age, sex, study centre, education, alcohol consumption, tobacco smoking, BMI and energy intake. | ||
| Petrick, et al. 2015 [17] | USA 1993–1995, PBCC | 589/662 | Contro l14.46 ± 9.41 GCA 16.04 ± 10.63 GCGA 14.9 ± 11.36 | Total flavonols | 0–8.31 8.32–12.16 12.17–17.81 ≥17.82 | GC 1.00 1.19 (0.90–1.58) 0.97 (0.77–1.24) 1.16 (0.87–1.55) | GCA 1.00 1.24 (0.81–1.91) 1.01 (0.65–1.57) 1.42 (0.93–2.17) | NCGA 1.00 1.16 (0.80–1.69) 0.96 (0.65–1.14) 0.98 (0.67–1.46) | Age, sex, race, geographic centre, cigarette smoking, and dietary energy intake. |
| Ekstrom, et al. 2011 [21] | Sweden 1989–1995, PBCC | 505/1116 | NM | Quercetin | 0.16–3.88 3.89–6.02 6.03–8.17 8.18–11.9 ≥11.9 | 1.00 0.68 (0.50–0.93) 0.50 (0.36–0.70) 0.49 (0.35–0.68) 0.61 (0.44–0.84) | 1.00 0.39 (0.18–0.83) 0.44 (0.21–0.91) 0.45 (0.22–0.95) 0.76 (0.40–1.44) | 1.00 0.76 (0.54–1.06) 0.52 (0.36–0.74) 0.50 (0.34–0.72) 0.57 (0.40–0.83) | Age, gender, socioeconomic status, number of siblings, body mass index, smoking and energy and salt intake. |
| Woo, et al. 2014 [22] | Korea 2011–2014, HBCC | 334/334 | Control 22.8 ± 19.5 Case 23.3 ± 21.4 | Isorhamnetin Kaempferol Myricetin Quercetin | (Median) T1(10.9) T2(14.4) T3(30.8) | GC 1.00 0.86 (0.55–1.36) 0.69 (0.39–1.20) | Men 1.00 0.89 (0.49–1.61) 0.65 (0.32–1.35) | Women 1.00 0.85 (0.37–1.97) 1.22 (0.47–3.16) | Total energy intake, H. pylori, age, sex, education, smoking, alcohol, BMI, physical activity, pickled vegetable, red and processed meat, fruits and vegetable. |
| Zamora–Ros, et al. 2012 [23] | 10 European countries 1992–2010, Cohort | 683 (477312) | Men 26.5 ± 16.4 Women 26.7 ± 17.4 | Isorhamnetin Kaempferol Myricetin Quercetin | Q1 Q2 Q3 Q4 | GC 1.00 0.81 (0.65–1.00) 0.78 (0.61–1.00) 0.71 (0.52–0.97) | Men 1.00 0.89 (0.66–1.18) 0.81 (0.57–1.13) 0.93 (0.63–1.37) | Women 1.00 0.72 (0.52–0.99) 0.75 (0.51–1.09) 0.45 (0.27–0.75) | Age, educational level, smoking status, physical activity, BMI, alcohol and energy intake, and daily consumption of fruit, vegetables, and red and processed meat. |
| Rossi, et al. 2010 [24] | Italy 1997–2007, HBCC | 230/547 | 22.9 ± 19.1 | Total flavonols | ≤13.2 13.3–16.4 16.5–20.3 20.3–32.3 >32.3 | 1.00 0.80 (0.51–1.27) 0.38 (0.22–0.66) 0.62 (0.39–1.01) 0.62 (0.38–1.02) | Sex, age, education, year of interview, BMI, tobacco smoking, and total energy intake. | ||
| Lagiou, et al. 2004 [25] | Greece 1981–1984, HBCC | 110/100 | NM | NM | Tper 10 mg | 1.00 0.77 (0.42–1.40) | Age, sex, place of birth, BMI, height, years of education, smoking, total energy intake, alcohol, fruits and vegetable. | ||
| Knekt, et al. 2002 [26] | Finland 1967–1994, Cohort | 74(9865) | 24.2 | Isorhamnetin Kaempferol Myricetin Quercetin | Q1 Q2 Q3 Q4 | 1.00 0.82 (0.44–1.52) 0.93 (0.49–1.78) 0.87 (0.44–1.75) | Sex, age, geographic area, occupation, smoking, and BMI. | ||
| Hirvonen, et al. 2001 [27] | Finland 1985–1993, Cohort | 111 (27110) | NM | Quercetin Myricetin Kaempferol | (Median) Q1(4.2) Q2(6.7) Q3(9.6) Q4(16.3) | 1.00 0.87 (0.51–1.50) 0.92 (0.54–1.60) 1.20 (0.71–1.90) | Age, dietary supplements of α-tocopherol and β-carotene. | ||
| Garcia-Closas, et al. 1999 [28] | Spain 1987–1989, HBCC | 354/354 | 9.0 ± 7.3 | Quercetin Myricetin Kaempferol | Q1 Q2 Q3 Q4 | 1.00 0.82 (0.47–1.42) 0.89 (0.66–1.20) 0.73 (0.53–1.01) | Total energy intake, intake of nitrites, nitrosamines, vitamin C, total carotenoids and other specific flavonoids. | ||

| Study | EC | GC | ||||||
|---|---|---|---|---|---|---|---|---|
| na | RR (95% CI) | Heterogeneity | na | RR (95% CI) | Heterogeneity | |||
| Pb | I2 (%) | Pb | I2 (%) | |||||
| Design | ||||||||
| Cohort | 1 | 0.90 (0.61–1.34) | - | - | 3 | 0.83 (0.65–1.06) | 0.208 | 36.2 |
| Case-control | 3 | 0.88 (0.70–1.10) | 0.515 | 0.0 | 6 | 0.79 (0.67–0.92) | 0.059 | 53.0 |
| PBCC | 2 | 0.92 (0.72–1.18) | 0.493 | 0.0 | 2 | 0.84 (0.45–1.59) | 0.004 | 88.2 |
| HBCC | 1 | 0.68 (0.38–1.24) | - | - | 4 | 0.70 (0.56–0.88) | 0.942 | 0.0 |
| Cancer type | ||||||||
| EAC | 2 | 0.84 (0.59–1.18) | 0.630 | 0.0 | ||||
| ESCC | 3 | 0.91 (0.67–1.24) | 0.491 | 0.0 | ||||
| GCA | 2 | 1.17 (0.82–1.67) | 0.111 | 60.7 | ||||
| NCGA | 2 | 0.73 (0.56–0.96) | 0.047 | 74.7 | ||||
| Gender | ||||||||
| Men | 0 | - | - | - | 3 | 0.96 (0.72–1.27) | 0.379 | 0.0 |
| Women | 0 | - | - | - | 2 | 0.56 (0.36–0.88) | 0.071 | 69.4 |
| Smoking | ||||||||
| Smokers | 2 | 0.73 (0.60–0.90) | 0.847 | 0.0 | 2 | 0.85 (0.73–0.99) | 0.149 | 52.0 |
| Nonsmokers | 2 | 1.25 (0.98–1.58) | 0.843 | 0.0 | 1 | 0.94 (0.76–1.17) | - | - |
| Population | ||||||||
| American | 2 | 0.92 (0.72–1.18) | 0.493 | 0.0 | 1 | 1.16 (0.87–1.55) | - | - |
| European | 2 | 0.83 (0.60–1.15) | 0.439 | 0.0 | 7 | 0.73 (0.62–0.85) | 0.442 | 0.0 |
| Asian | 0 | - | - | - | 1 | 0.69 (0.39–1.20) | - | - |
| Publication time | ||||||||
| 2010–2015 | 2 | 0.88 (0.70–1.11) | 0.892 | 0.0 | 5 | 0.78 (0.67–0.92) | 0.028 | 63.1 |
| Before 2010 | 2 | 0.90 (0.63–1.28) | 0.252 | 23.8 | 4 | 0.84 (0.66–1.06) | 0.418 | 0.0 |
3.3. Sensitivity Analysis
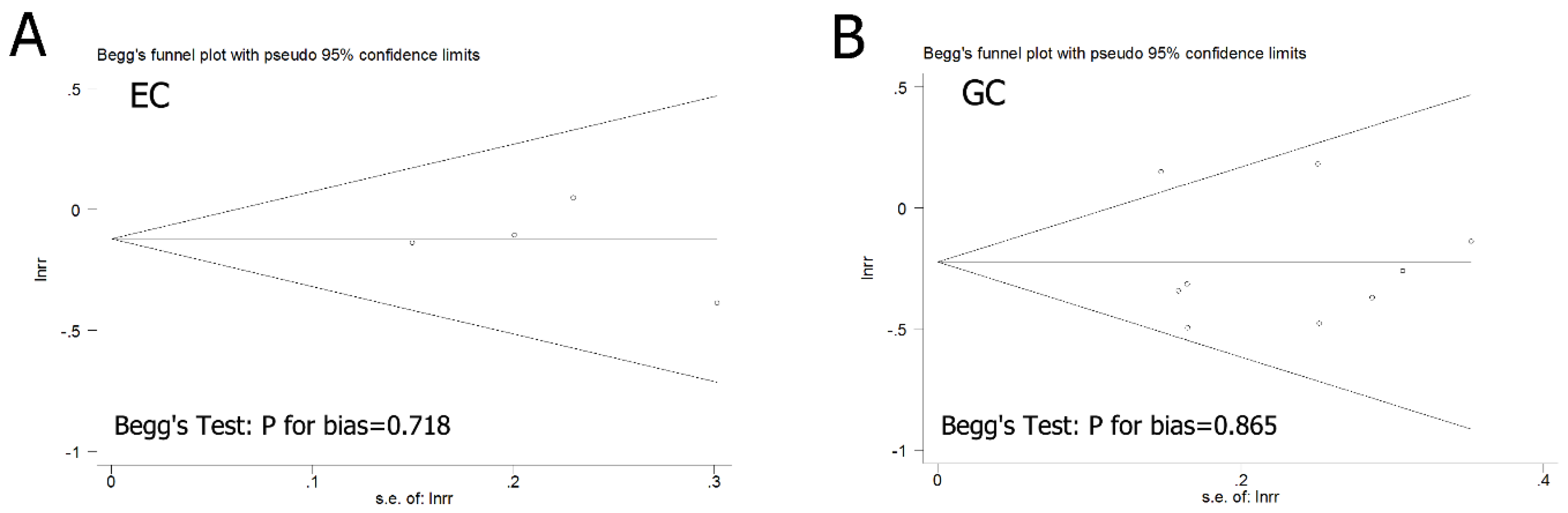
3.4. Publication Bias
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Dicker, D.; Pain, A.; Hamavid, H.; Moradi-Lakeh, M.; MacIntyre, M.F.; Allen, C.; Hansen, G.; Woodbrook, R.; et al. The Global Burden of Cancer 2013. JAMA Oncol. 2015, 1, 505–527. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Devesa, S.S.; Blot, W.J.; Fraumeni, J.F., Jr. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer 1998, 83, 2049–2053. [Google Scholar] [CrossRef]
- Simard, E.P.; Ward, E.M.; Siegel, R.; Jemal, A. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J. Clin. 2012, 62, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Wijnhoven, B.P.; Siersema, P.D.; Hop, W.C.; van Dekken, H.; Tilanus, H.W. Adenocarcinomas of the distal oesophagus and gastric cardia are one clinical entity. Rotterdam Oesophageal Tumour Study Group. Br. J. Surg. 1999, 86, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Abnet, C.C.; Corley, D.A.; Freedman, N.D.; Kamangar, F. Diet and upper gastrointestinal malignancies. Gastroenterology 2015, 148, 1234–1243.e4. [Google Scholar] [CrossRef] [PubMed]
- Somi, M.H.; Mousavi, S.M.; Naghashi, S.; Faramarzi, E.; Jafarabadi, M.A.; Ghojazade, M.; Majidi, A.; Naseri Alavi, S.A. Is there any relationship between food habits in the last two decades and gastric cancer in North-Western Iran? Asian Pac. J. Cancer Prev. 2015, 16, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.H.; Li, Y.H.; Leung, K.; Huang, C.Y.; Wang, X.R. Salt processed food and gastric cancer in a Chinese population. Asian Pac. J. Cancer Prev. 2014, 15, 5293–5298. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.M.; Zhao, D.; Nie, Z.L.; Zhao, H.; Zhou, B.; Gao, W.; Wang, L.S.; Yang, Z.J. Flavonol intake and stroke risk: A meta-analysis of cohort studies. Nutrition 2014, 30, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Xie, Q.; Zhang, Q.Y.; Peng, X.L.; Zhu, J.D.; Mi, M.T. Flavonoids, flavonoid subclasses and breast cancer risk: A meta-analysis of epidemiologic studies. PLoS ONE 2013, 8, e54318. [Google Scholar]
- Miles, S.L.; McFarland, M.; Niles, R.M. Molecular and physiological actions of quercetin: need for clinical trials to assess its benefits in human disease. Nutr. Rev. 2014, 72, 720–734. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Song, B.A.; Zhao, H.J.; Qi, X.B.; Huang, Y.J.; Liu, X.H. Novel myricetin derivatives: Design, synthesis and anticancer activity. Eur. J. Med. Chem. 2015, 97, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Greenland, S. Quantitative methods in the review of epidemiologic literature. Epidemiol. Rev. 1987, 9, 1–30. [Google Scholar] [PubMed]
- Mantel, N.; Haenszel, W. Statistical aspects of the analysis of data from retrospective studies of disease. J. Natl. Cancer Inst. 1959, 22, 719–748. [Google Scholar] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Ioannidis, J.P.; Ntzani, E.E.; Trikalinos, T.A.; Contopoulos-Ioannidis, D.G. Replication validity of genetic association studies. Nat. Genet. 2001, 29, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Petrick, J.L.; Steck, S.E.; Bradshaw, P.T.; Trivers, K.F.; Abrahamson, P.E.; Engel, L.S.; He, K.; Chow, W.H.; Mayne, S.T.; Risch, H.A.; et al. Dietary intake of flavonoids and oesophageal and gastric cancer: incidence and survival in the United States of America (USA). Br. J. Cancer 2015, 112, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, E.; Zamora-Ros, R.; Duell, E.J.; Luján-Barroso, L.; Boeing, H.; Aleksandrova, K.; Bueno-de-Mesquita, H.B.; Scalbert, A.; Romieu, I.; Fedirko, V.; et al. Dietary flavonoid intake and esophageal cancer risk in the European prospective investigation into cancer and nutrition cohort. Am. J. Epidemiol. 2013, 178, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Bobe, G.; Peterson, J.J.; Gridley, G.; Hyer, M.; Dwyer, J.T.; Brown, L.M. Flavonoid consumption and esophageal cancer among black and white men in the United States. Int. J. Cancer 2009, 125, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Garavello, W.; Talamini, R.; La Vecchia, C.; Franceschi, S.; Lagiou, P.; Zambon, P.; Dal Maso, L.; Bosetti, C.; Negri, E. Flavonoids and risk of squamous cell esophageal cancer. Int. J. Cancer 2007, 120, 1560–1564. [Google Scholar] [CrossRef] [PubMed]
- Ekström, A.M.; Serafini, M.; Nyrén, O.; Wolk, A.; Bosetti, C.; Bellocco, R. Dietary quercetin intake and risk of gastric cancer: Results from a population-based study in Sweden. Ann. Oncol. 2011, 22, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.D.; Lee, J.; Choi, I.J.; Kim, C.G.; Lee, J.Y.; Kwon, O.; Kim, J. Dietary flavonoids and gastric cancer risk in a Korean population. Nutrients 2014, 6, 4961–4973. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Agudo, A.; Luján-Barroso, L.; Romieu, I.; Ferrari, P.; Knaze, V.; Bueno-de-Mesquita, H.B.; Leenders, M.; Travis, R.C.; Navarro, C.; et al. Dietary flavonoid and lignan intake and gastric adenocarcinoma risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Am. J. Clin. Nutr. 2012, 96, 1398–1408. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Rosato, V.; Bosetti, C.; Lagiou, P.; Parpinel, M.; Bertuccio, P.; Negri, E.; La Vecchia, C. Flavonoids, proanthocyanidins, and the risk of stomach cancer. Cancer Causes Control. 2010, 21, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Lagiou, P.; Samoli, E.; Lagiou, A.; Peterson, J.; Tzonou, A.; Dwyer, J.; Trichopoulos, D. Flavonoids, vitamin C and adenocarcinoma of the stomach. Cancer Causes Control. 2004, 15, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Knekt, P.; Kumpulainen, J.; Järvinen, R.; Rissanen, H.; Heliövaara, M.; Reunanen, A.; Hakulinen, T.; Aromaa, A. Flavonoid intake and risk of chronic diseases. Am. J. Clin. Nutr. 2002, 76, 560–568. [Google Scholar] [PubMed]
- Hirvonen, T.; Virtamo, J.; Korhonen, P.; Albanes, D.; Pietinen, P. Flavonol and flavone intake and the risk of cancer in male smokers (Finland). Cancer Causes Control. 2001, 12, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Closas, R.; Gonzalez, C.A.; Agudo, A.; Riboli, E. Intake of specific carotenoids and flavonoids and the risk of gastric cancer in Spain. Cancer Causes Control. 1999, 10, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Fedewa, S.A.; Sauer, A.G.; Siegel, R.L.; Jemal, A. Prevalence of major risk factors and use of screening tests for cancer in the United States. Cancer Epidemiol. Biomark. Prev. 2015, 24, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, E.; Sankari, L.S.; Malathi, L.; Krupaa, J.R. Naturally occurring products in cancer therapy. J. Pharm. Bioallied. Sci. 2015, 7, S181–S183. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.L.; Russo, M.; Spagnuolo, C. The pleiotropic flavonoid quercetin: from its metabolism to the inhibition of protein kinases in chronic lymphocytic leukemia. Food Funct. 2014, 5, 2393–2401. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, Y.M.; Zhang, P.Y. Protective effects of curcumin and quercetin during benzo(a)pyrene induced lung carcinogenesis in mice. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1736–1743. [Google Scholar] [PubMed]
- Sak, K. Dependence of DPPH radical scavenging activity of dietary flavonoid quercetin on reaction environment. Mini Rev. Med. Chem. 2014, 14, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, N.; Izumi, H.; Miyamoto, R.; Kondo, H.; Tawara, A.; Sasaguri, Y.; Kohno, K. Quercetin induces the expression of peroxiredoxins 3 and 5 via the Nrf2/NRF1 transcription pathway. Invest. Ophthalmol. Vis. Sci. 2011, 52, 1055–1563. [Google Scholar] [CrossRef] [PubMed]
- Men, K.; Duan, X.; Wei, X.W.; Gou, M.L.; Huang, M.J.; Chen, L.J.; Qian, Z.Y.; Wei, Y.Q. Nanoparticle-delivered quercetin for cancer therapy. Anticancer Agents Med. Chem. 2014, 14, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.L.; Li, C.J.; Huang, L.H.; Chen, C.Y.; Tsai, C.H.; Lin, C.N.; Hsu, H.Y. Quercetin 3-O-methyl ether protects FL83B cells from copper induced oxidative stress through the PI3K/Akt and MAPK/Erk pathway. Toxicol. Appl. Pharmacol. 2012, 264, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Ji, X.J.; Zhou, Y.X.; Yao, X.Q.; Liu, Y.Q.; Zhang, F.; Yin, X.X. Quercetin inhibits the mTORC1/p70S6K signaling-mediated renal tubular epithelial-mesenchymal transition and renal fibrosis in diabetic nephropathy. Pharmacol. Res. 2015, 99, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Touvier, M.; Druesne-Pecollo, N.; Kesse-Guyot, E.; Andreeva, V.A.; Fezeu, L.; Galan, P.; Hercberg, S.; Latino-Martel, P. Dual association between polyphenol intake and breast cancer risk according to alcohol consumption level: a prospective cohort study. Breast Cancer Res. Treat. 2013, 137, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Milenkovic, D.; Vanden Berghe, W.; Boby, C.; Leroux, C.; Declerck, K.; Szarc vel Szic, K.; Heyninck, K.; Laukens, K.; Bizet, M.; Defrance, M.; et al. Dietary flavanols modulate the transcription of genes associated with cardiovascular pathology without changes in their DNA methylation state. PLoS ONE 2014, 9, e95527. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wulan, H.; Song, Z.; Paik, P.A.; Tsao, M.L.; Goodman, G.M.; MacEachern, P.T.; Downey, R.S.; Jankowska, A.J.; Rabinowitz, Y.M.; et al. Regulatory B Cell Function Is Suppressed by Smoking and Obesity in H. pylori-Infected Subjects and Is Correlated with Elevated Risk of Gastric Cancer. PLoS ONE 2015, 10, e0134591. [Google Scholar] [CrossRef] [PubMed]
- De Stefani, E.; Ronco, A.; Mendilaharsu, M.; Deneo-Pellegrini, H. Diet and risk of cancer of the upper aerodigestive tract--II. Nutrients.Oral Oncol. 1999, 35, 22–26. [Google Scholar] [CrossRef]
- Woo, H.D.; Kim, J. Dietary flavonoid intake and risk of stomach and colorectal cancer. World J. Gastroenterol. 2013, 19, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Huang, S.; Su, Y. Dietary Flavonols Intake and Risk of Esophageal and Gastric Cancer: A Meta-Analysis of Epidemiological Studies. Nutrients 2016, 8, 91. https://doi.org/10.3390/nu8020091
Xie Y, Huang S, Su Y. Dietary Flavonols Intake and Risk of Esophageal and Gastric Cancer: A Meta-Analysis of Epidemiological Studies. Nutrients. 2016; 8(2):91. https://doi.org/10.3390/nu8020091
Chicago/Turabian StyleXie, Yan, Shifeng Huang, and Yuxi Su. 2016. "Dietary Flavonols Intake and Risk of Esophageal and Gastric Cancer: A Meta-Analysis of Epidemiological Studies" Nutrients 8, no. 2: 91. https://doi.org/10.3390/nu8020091
APA StyleXie, Y., Huang, S., & Su, Y. (2016). Dietary Flavonols Intake and Risk of Esophageal and Gastric Cancer: A Meta-Analysis of Epidemiological Studies. Nutrients, 8(2), 91. https://doi.org/10.3390/nu8020091





