The Fto Gene Regulates the Proliferation and Differentiation of Pre-Adipocytes in Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Transfection
- GCAUGUCAGACCUUCCUAATT
- UUAGGAAGGUCUGACAUGCTT.
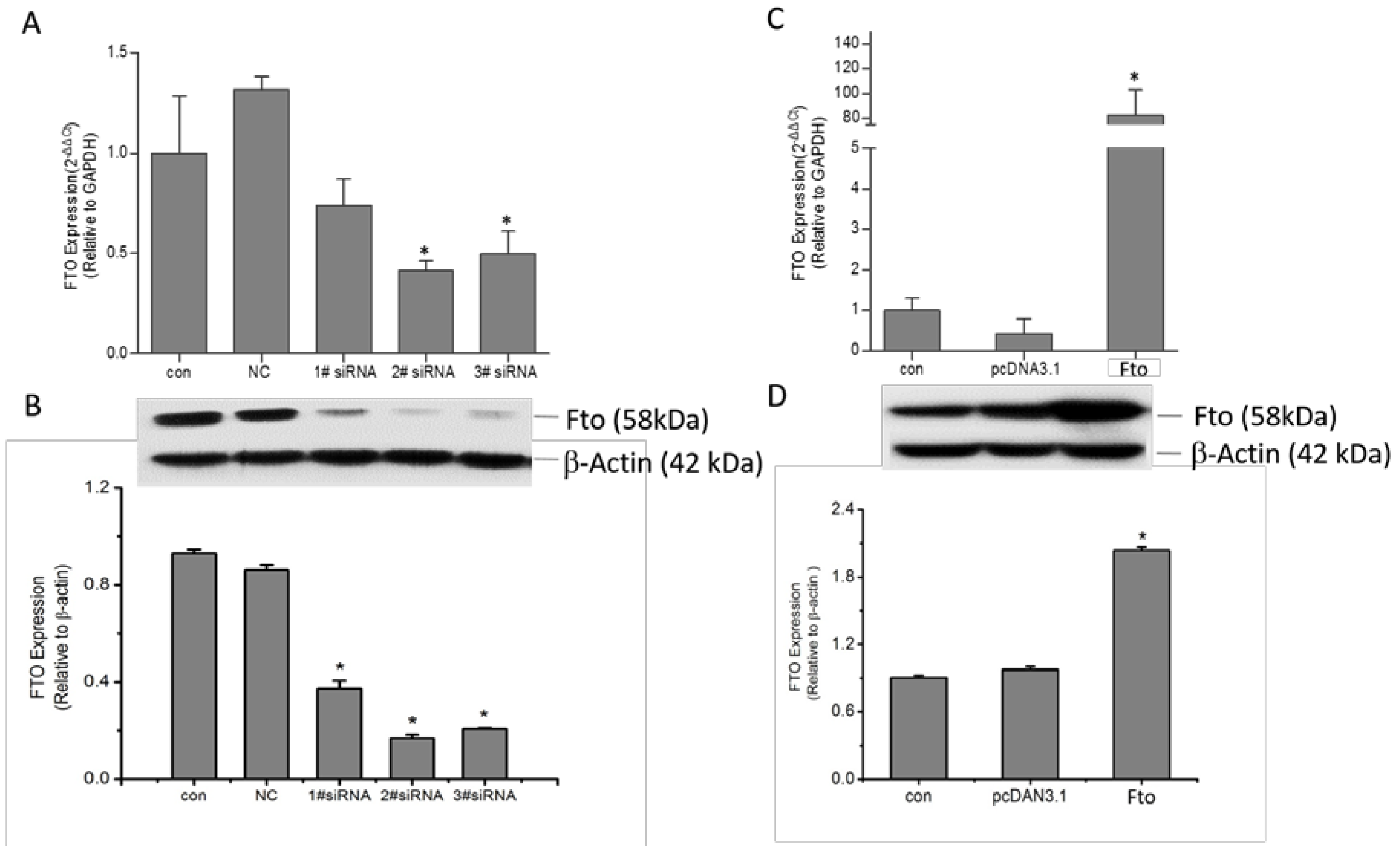
2.2. Reverse Transcription and Quantitative Real Time PCR
2.3. Western Blot Assay
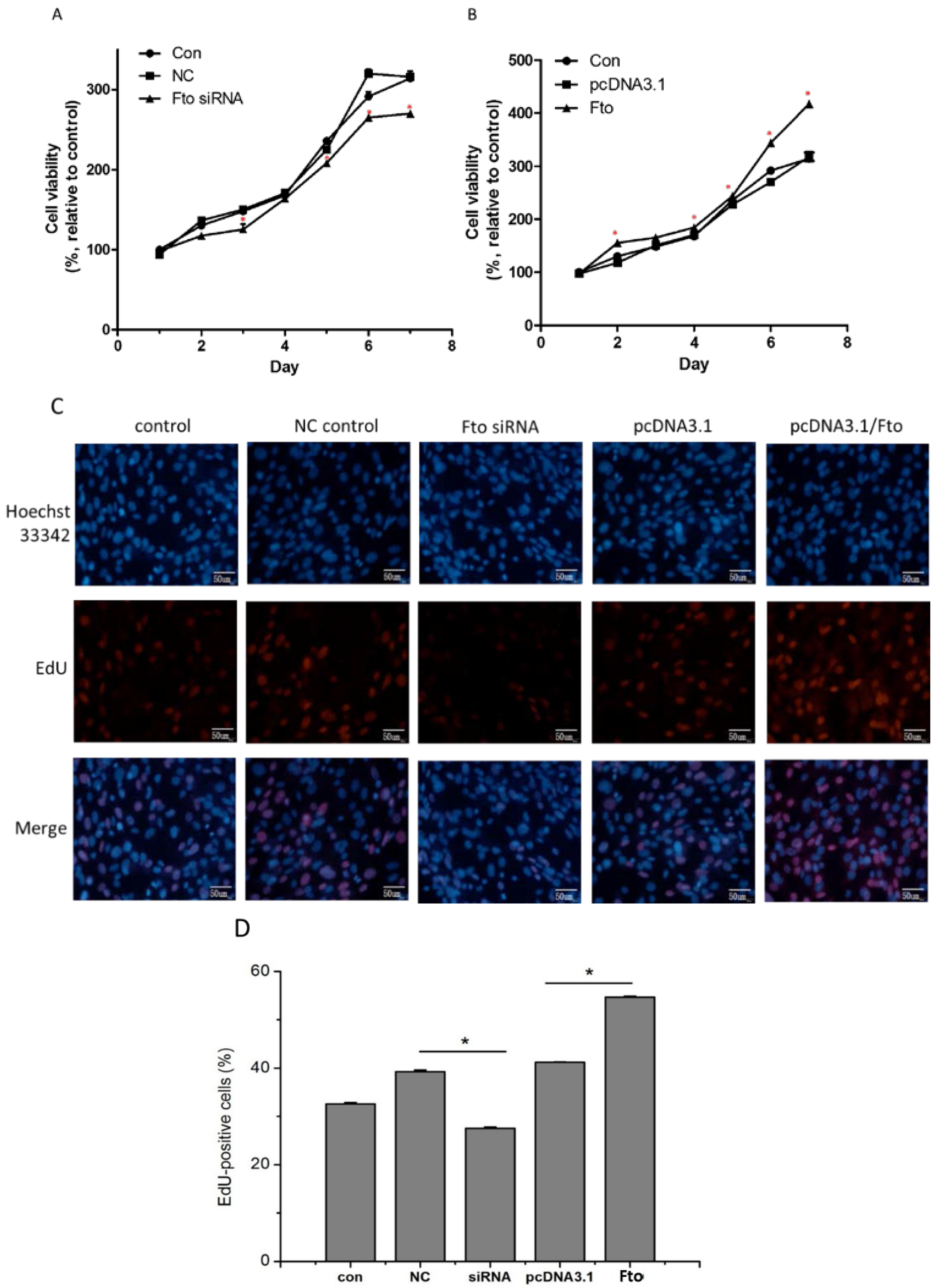
2.4. Cell Viability Assay
2.5. 5-Ethynyl-2’-deoxyuridine (EdU) Cell Proliferation Assay
2.6. Flow Cytometry Assay
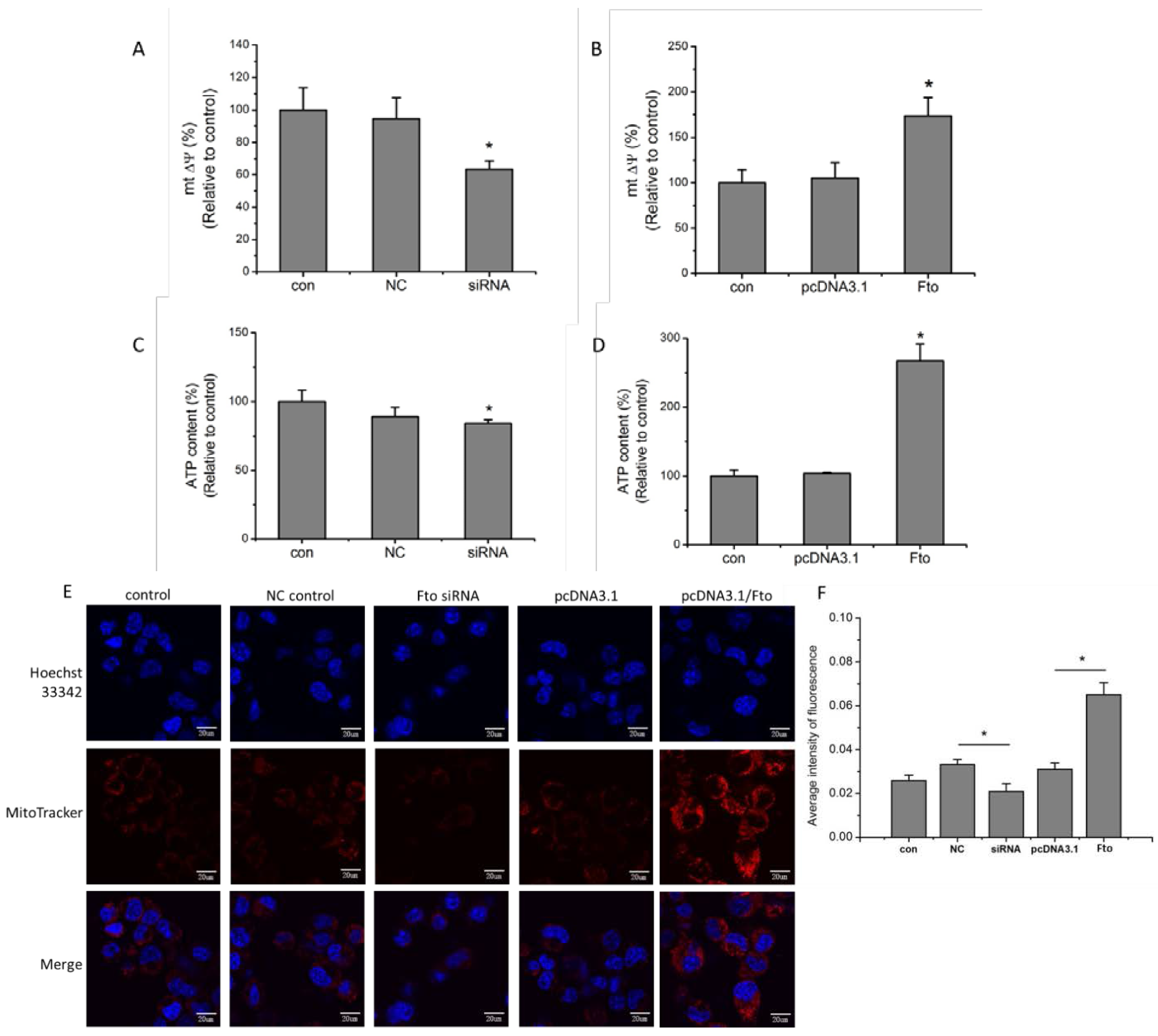
2.7. Live Cell Fluorescent Imaging of Mitochondria
2.8. ATP Assay/Determination of ATP Content of Cells
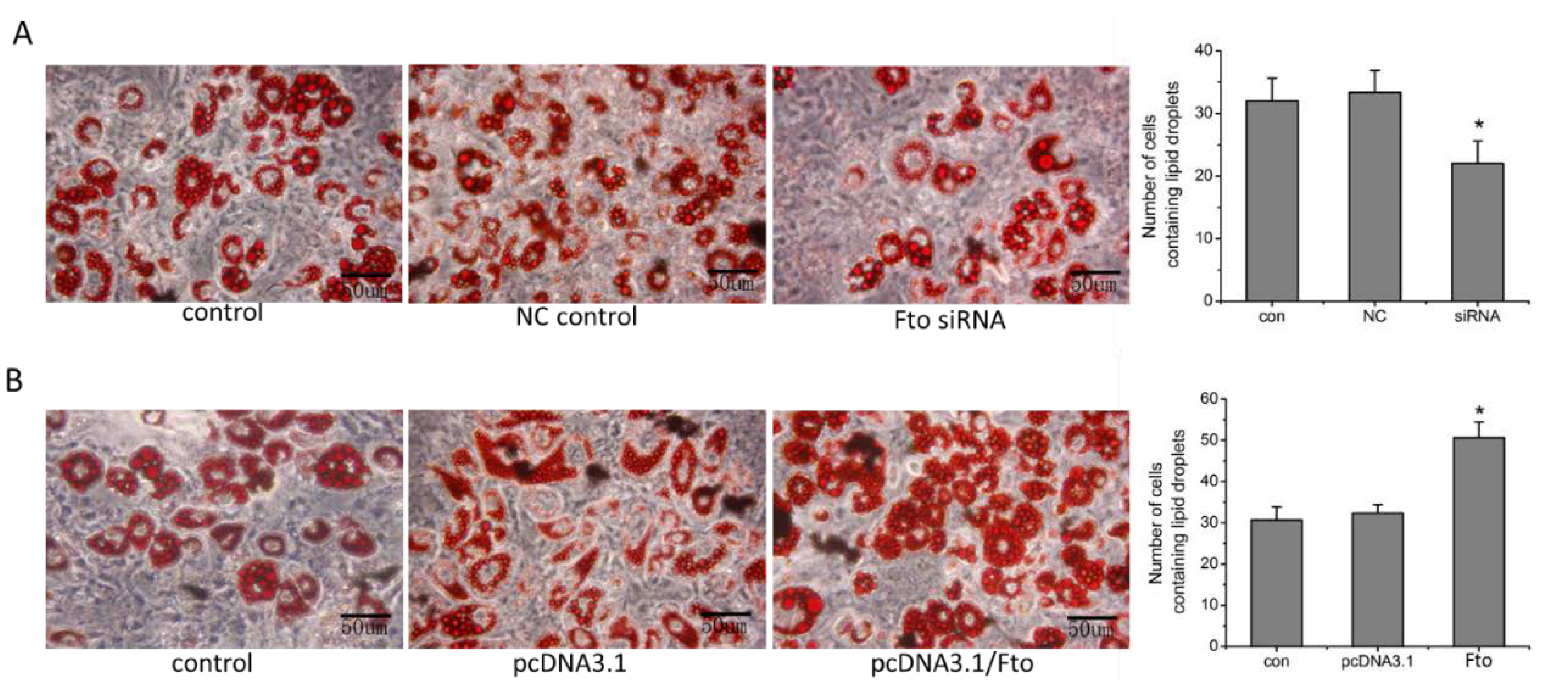
2.9. Cell Differentiation Assay and Oil Red O staining
2.10. Statistical Analysis
3. Results
3.1. Fto Gene Affects the Proliferation of 3T3-L1 Cells
3.2. Fto Gene Influences the Mitochondrial Membrane Potential and ATP Levels of Pre-adipocytes
3.3. Fto Gene Regulated the Differentiation of Pre-adipocyte
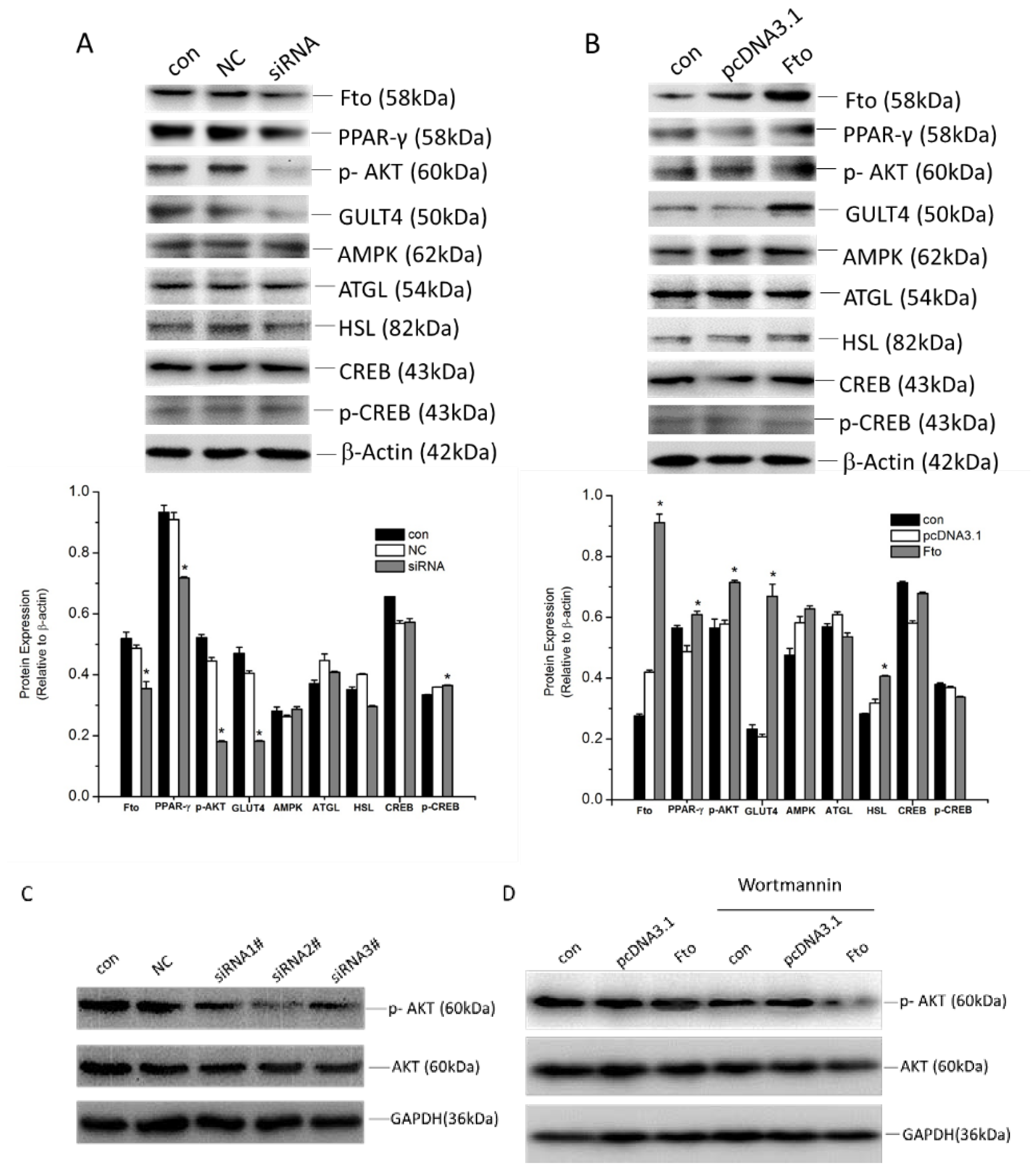
3.4. Fto Regulated the Differentiation and Proliferation of 3T3-L1 Cells via Multiple Mechanisms
4. Discussion
Acknowledgments
Author Contributions
Conflicts of interest
References
- Heindel, J.J.; Newbold, R.; Schug, T.T. Endocrine disruptors and obesity. Nat. Rev. Endocrinol. 2015, 11, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Abente, E.J.; Subramanian, M.; Ramachandran, V.; Najafi-Shoushtari, S.H. Micrornas in obesity-associated disorders. Arch. Biochem. Biophys. 2015, 589, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Hebert, J.R.; Allison, D.B.; Archer, E.; Lavie, C.J.; Blair, S.N. Scientific decision making, policy decisions, and the obesity pandemic. Mayo Clin. Proc. 2013, 88, 593–604. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global status report on noncommunicable diseases 2014. Available online: http://apps.who.int/iris/bitstream/10665/148114/1/9789241564854_eng.pdf?ua=1 (accessed on 15 February 2016).
- Butsch, W.S. Obesity medications: What does the future look like? Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 360–366. [Google Scholar] [CrossRef] [PubMed]
- McMorrow, A.M.; Connaughton, R.M.; Lithander, F.E.; Roche, H.M. Adipose tissue dysregulation and metabolic consequences in childhood and adolescent obesity: Potential impact of dietary fat quality. Proc. Nutr. Soc. 2015, 74, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Goossens, G.H.; Blaak, E.E. Adipose tissue dysfunction and impaired metabolic health in human obesity: A matter of oxygen? Front. Endocrinol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, J.M.; Stern, J.H.; Scherer, P.E. The cell biology of fat expansion. J. Cell Biol. 2015, 208, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Lee, P.; Chisholm, D.J.; James, D.E. Control of adipocyte differentiation in different fat depots; implications for pathophysiology or therapy. Front. Endocrinol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.T.; Wang, Z.W.; Higa, M.; Newgard, C.B.; Unger, R.H. Reversing adipocyte differentiation: Implications for treatment of obesity. Proc. Natl. Acad. Sci. USA 1999, 96, 2391–2395. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, X.; Shi, M.; Shi, H.; Yan, X.; Li, H.; Wang, S.; Wang, Y. LMO4 modulates proliferation and differentiation of 3T3-L1 preadipocytes. FEBS Lett. 2013, 587, 3032–3037. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, F.M. Adipocyte differentiation: From fibroblast to endocrine cell. Exp. Biol. Med. 2001, 226, 997–1002. [Google Scholar]
- Frayling, T.M.; Timpson, N.J.; Weedon, M.N.; Zeggini, E.; Freathy, R.M.; Lindgren, C.M.; Perry, J.R.; Elliott, K.S.; Lango, H.; Rayner, N.W.; et al. A common variant in the fto gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007, 316, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Dina, C.; Meyre, D.; Gallina, S.; Durand, E.; Korner, A.; Jacobson, P.; Carlsson, L.M.; Kiess, W.; Vatin, V.; Lecoeur, C.; et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat. Genet. 2007, 39, 724–726. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Koch, L.; Emmerling, C.; Vierkotten, J.; Peters, T.; Bruning, J.C.; Ruther, U. Inactivation of the Fto gene protects from obesity. Nature 2009, 458, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yang, Y.; Sun, B.F.; Zhao, Y.L.; Yang, Y.G. FTO and obesity: Mechanisms of association. Curr. Diabetes Rep. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Church, C.; Moir, L.; McMurray, F.; Girard, C.; Banks, G.T.; Teboul, L.; Wells, S.; Bruning, J.C.; Nolan, P.M.; Ashcroft, F.M.; et al. Overexpression of Fto leads to increased food intake and results in obesity. Nat. Genet. 2010, 42, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yang, Y.; Sun, B.F.; Shi, Y.; Yang, X.; Xiao, W.; Hao, Y.J.; Ping, X.L.; Chen, Y.S.; Wang, W.J.; et al. FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res. 2014, 24, 1403–1419. [Google Scholar] [CrossRef]
- Tews, D.; Fischer-Posovszky, P.; Fromme, T.; Klingenspor, M.; Fischer, J.; Ruther, U.; Marienfeld, R.; Barth, T.F.; Moller, P.; Debatin, K.M.; et al. FTO deficiency induces UCP-1 expression and mitochondrial uncoupling in adipocytes. Endocrinology 2013, 154, 3141–3151. [Google Scholar] [CrossRef] [PubMed]
- Merkestein, M.; Laber, S.; McMurray, F.; Andrew, D.; Sachse, G.; Sanderson, J.; Li, M.; Usher, S.; Sellayah, D.; Ashcroft, F.M.; et al. FTO influences adipogenesis by regulating mitotic clonal expansion. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Zhou, J.; Wang, L.; Qin, S.; Xu, X. Effects of FTO gene on the radiosensitivity of A172 cells and its mechanism. Chin. J. Radiol. Med. Prot. 2015, 35, 252–256. [Google Scholar]
- Jiao, Y.; Sun, K.K.; Zhao, L.; Xu, J.Y.; Wang, L.L.; Fan, S.J. Suppression of human lung cancer cell proliferation and metastasis in vitro by the transducer of ErbB-2.1 (TOB1). Acta Pharmacol. Sin. 2012, 33, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Parker, V.E.R.; Knox, R.G.; Zhang, Q.; Wakelam, M.J.O.; Semple, R.K. Phosphoinositide 3-kinase-related overgrowth: Cellular phenotype and future therapeutic options. Lancet 2015, 385. [Google Scholar] [CrossRef]
- Paxinou, E.; Weisse, M.; Chen, Q.; Souza, J.M.; Hertkorn, C.; Selak, M.; Daikhin, E.; Yudkoff, M.; Sowa, G.; Sessa, W.C.; et al. Dynamic regulation of metabolism and respiration by endogenously produced nitric oxide protects against oxidative stress. Proc. Natl. Acad. Sci. USA 2001, 98, 11575–11580. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, E.; Church, C.D.; Holtrup, B.; Colman, L.; Rodeheffer, M.S. Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nat. Cell Biol. 2015, 17, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Cristancho, A.G.; Lazar, M.A. Forming functional fat: A growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 2011, 12, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, Y.; Ma, J.; Guo, F.; Cao, Q.; Zhang, Y.; Zhou, B.; Chai, J.; Zhao, W.; Zhao, R. The demethylase activity of FTO (fat mass and obesity associated protein) is required for preadipocyte differentiation. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Ren, W.; Li, A.; Ding, Y.; Guo, W.; Su, D.; Hu, C.; Xu, K.; Chen, H.; Xu, X.; et al. Fat mass and obesity-associated gene enhances oxidative stress and lipogenesis in nonalcoholic fatty liver disease. Dig. Dis. Sci. 2013, 58, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, M.; Suh, J.M.; Hah, N.; Liddle, C.; Atkins, A.R.; Downes, M.; Evans, R.M. PPARγ signaling and metabolism: The good, the bad and the future. Nat. Med. 2013, 99, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Janani, C.; Ranjitha Kumari, B.D. PPAR gamma gene—A review. Diabetes Metab. Syndr. Clin. Res. Rev. 2015, 9, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Bravard, A.; Veilleux, A.; Disse, E.; Laville, M.; Vidal, H.; Tchernof, A.; Rieusset, J. The expression of FTO in human adipose tissue is influenced by fat depot, adiposity, and insulin sensitivity. Obesity 2013, 21, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Fischer-Posovszky, P.; Tews, D.; Horenburg, S.; Debatin, K.M.; Wabitsch, M. Differential function of Akt1 and Akt2 in human adipocytes. Mol. Cell Endocrinol. 2012, 358, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, K.N.; Li, R.; Shao, R.; Chen, C. Activation of PI3K/Akt/mTOR pathway and dual inhibitors of PI3K and mTOR in endometrial cancer. Curr. Med. Chem. 2014, 21, 3070–3080. [Google Scholar] [CrossRef] [PubMed]
- Mosca, E.; Barcella, M.; Alfieri, R.; Bevilacqua, A.; Canti, G.; Milanesi, L. Systems biology of the metabolic network regulated by the Akt pathway. Biotechnol. Adv. 2012, 30, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.H.; Sul, H.S. Insulin signaling in fatty acid and fat synthesis: A transcriptional perspective. Curr. Opin. Pharmacol. 2010, 10, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Chiang, S.H.; Saltiel, A.R. Insulin signaling and the regulation of glucose transport. Mol. Med. 2004, 10, 65–71. [Google Scholar] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, Y.; Zhang, J.; Lu, L.; Xu, J.; Qin, L. The Fto Gene Regulates the Proliferation and Differentiation of Pre-Adipocytes in Vitro. Nutrients 2016, 8, 102. https://doi.org/10.3390/nu8020102
Jiao Y, Zhang J, Lu L, Xu J, Qin L. The Fto Gene Regulates the Proliferation and Differentiation of Pre-Adipocytes in Vitro. Nutrients. 2016; 8(2):102. https://doi.org/10.3390/nu8020102
Chicago/Turabian StyleJiao, Yang, Jingying Zhang, Lunjie Lu, Jiaying Xu, and Liqiang Qin. 2016. "The Fto Gene Regulates the Proliferation and Differentiation of Pre-Adipocytes in Vitro" Nutrients 8, no. 2: 102. https://doi.org/10.3390/nu8020102
APA StyleJiao, Y., Zhang, J., Lu, L., Xu, J., & Qin, L. (2016). The Fto Gene Regulates the Proliferation and Differentiation of Pre-Adipocytes in Vitro. Nutrients, 8(2), 102. https://doi.org/10.3390/nu8020102



