Meta-Analysis of Milk Consumption and the Risk of Cognitive Disorders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Selection Criteria and Data Extraction
2.3. Quality Assessment
2.4. Statistical Analysis
3. Results
3.1. Study Identification and Selection
3.2. Study Characteristics
3.3. Quality Assessment
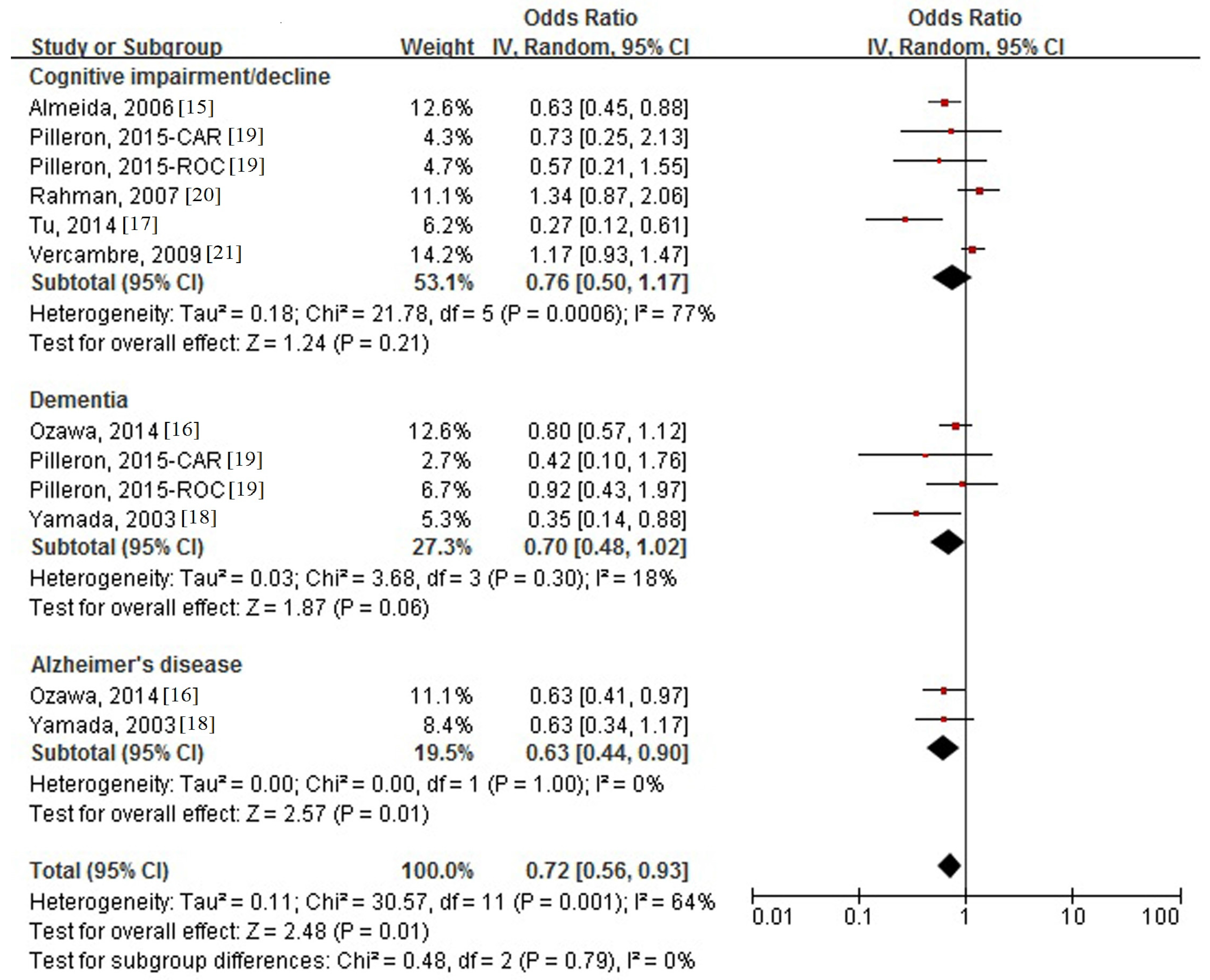
3.4. Association between Milk Intake and Cognitive Disorders
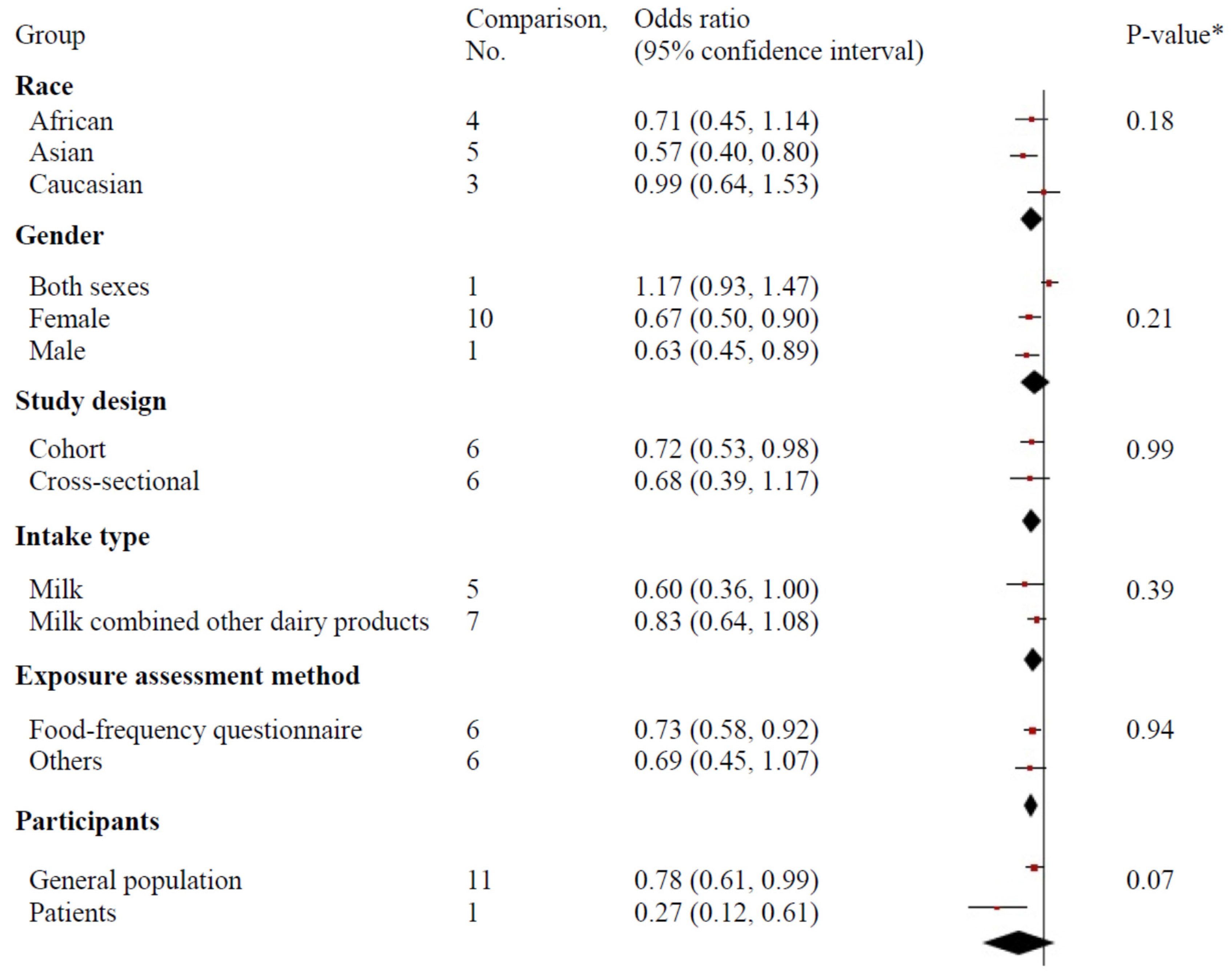
3.5. Subgroup Analysis and Meta-Regression
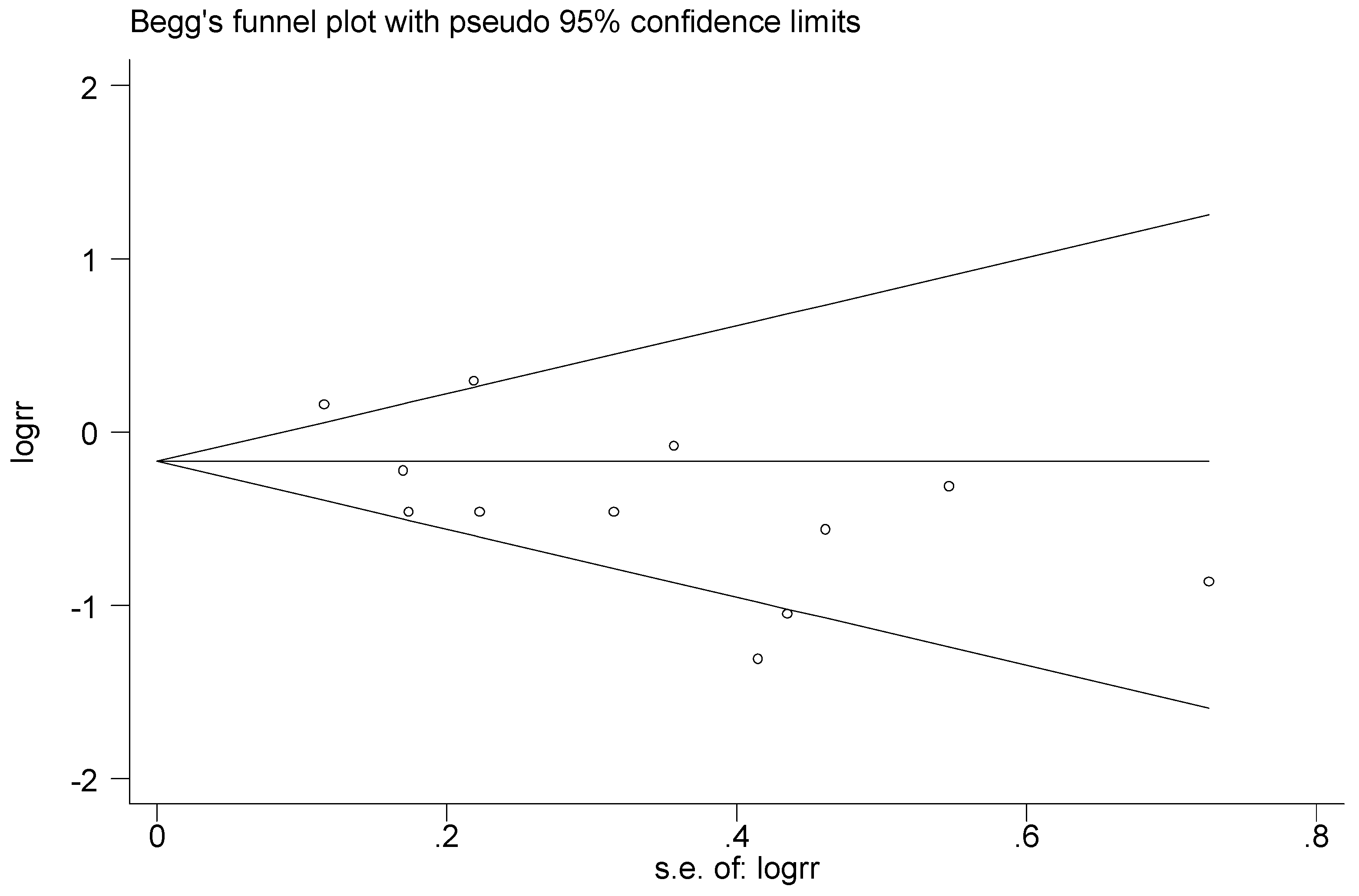
3.6. Publication Bias and Sensitivity Analysis
4. Discussion
5. Conclusions
Author Contributors
Conflict of interest
Appendix A
| Source: PubMed (Searched on: 30 October 2016) |
| #1 dairy [Title/Abstract] |
| #2 milk [Title/Abstract] |
| #3 yogurt [title/abstract] |
| #4 dementia [title/abstract] |
| #5 AD [title/abstract] |
| #6 Alzheimer* [title/abstract] |
| #7 aphronesia [title/abstract] |
| #8 cognitive* [title/abstract] |
| #9 “humans” [MeSH Terms] |
| #10 English [lang] |
| #11 #1 OR #2 OR #3 |
| #12 #4 OR #5 OR #6 OR #7 OR #8 |
| #13 #9 AND #10 AND #11 AND #12 |
| Source: Embase (Searched on: 30 October 2016) |
| #1 dairy:ti,ab |
| #2 milk:ti,ab |
| #3 yogurt:ti,ab |
| #4 dementia:ti,ab |
| #5 AD:ti,ab |
| #6 Alzheimer*:ti,ab |
| #7 aphronesia:ti,ab |
| #8 cognitive*:ti,ab |
| #9 “human“/de |
| #10 #1 OR #2 OR #3 |
| #11 #4 OR #5 OR #6 OR #7 OR #8 |
| #12 #9 AND #10 AND #11 |
| First Author, Published Year | Design Bias | Selection Bias | Information Bias | Confounding | Analysis Bias | Total |
|---|---|---|---|---|---|---|
| Almeida, 2006 [15] | 3 | 4 | 4 | 3 | 2 | 16 |
| Ozawa, 2014 [16] | 3 | 4 | 5 | 3 | 2 | 17 |
| Pilleron, 2015 [19] | 1 | 3 | 5 | 3 | 2 | 14 |
| Rahman, 2007 [20] | 1 | 2 | 4 | 3 | 2 | 12 |
| Tu, 2014 [17] | 1 | 3 | 4 | 3 | 2 | 13 |
| Vercambre, 2009 [21] | 3 | 4 | 4 | 3 | 2 | 16 |
| Yamada, 2003 [18] | 3 | 4 | 4 | 3 | 2 | 16 |
References
- Kinsella, K.; Velkoff, V.A. US Census Bureau, Series P95/01-1, an Aging World: 2001; Government Printing Office: Washington, DC, USA, 2001.
- Alzheimer’s Association. 2013 Alzheimer’s disease facts and figures. Alzheimers Dement. 2013, 9, 208–245. [Google Scholar]
- Zhang, Y.; Chen, J.; Qiu, J.; Li, Y.; Wang, J.; Jiao, J. Intakes of fish and polyunsaturated fatty acids and mild-to-severe cognitive impairment risks: A dose-response meta-analysis of 21 cohort studies. Am. J. Clin. Nutr. 2016, 103, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Sun, D.; He, Y. Coffee intake and the incident risk of cognitive disorders: A dose-response meta-analysis of nine prospective cohort studies. Clin. Nutr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.S.; Yu, J.T.; Tan, C.C.; Wang, H.F.; Meng, X.F.; Wang, C.; Jiang, T.; Zhu, X.C.; Tan, L. Efficacy and adverse effects of Ginkgo biloba for cognitive impairment and dementia: A systematic review and meta-analysis. J. Alzheimers Dis. 2015, 43, 589–603. [Google Scholar] [PubMed]
- Rita, C.B.; Apolinario, D.; da Silva Bandeira, V.; Busse, A.L.; Magaldi, R.M.; Jacob-Filho, W.; Cozzolino, S.M. Effects of Brazil nut consumption on selenium status and cognitive performance in older adults with mild cognitive impairment: A randomized controlled pilot trial. Eur. J. Nutr. 2016, 55, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, K.; Du, H.Z.; Yang, J.; Chen, G.C.; Zhang, Z.; Li, Z.N.; Qin, L.Q. Effects of milk proteins on blood pressure: A meta-analysis of randomized control trials. Hypertens. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Norat, T.; Romundstad, P.; Vatten, L.J. Dairy products and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis of cohort studies. Am. J. Clin. Nutr. 2013, 98, 1066–1083. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.D.; Bylsma, L.C.; Vargas, A.J.; Cohen, S.S.; Doucette, A.; Mohamed, M.; Irvin, S.R.; Miller, P.E.; Watson, H.; Fryzek, J.P. Dairy consumption and CVD: A systematic review and meta-analysis. Br. J Nutr. 2016, 115, 737–750. [Google Scholar] [CrossRef] [PubMed]
- De Goede, J.; Soedamah-Muthu, S.S.; Pan, A.; Gijsbers, L.; Geleijnse, J.M. Dairy consumption and risk of stroke: A systematic review and updated dose-response meta-analysis of prospective cohort studies. J. Am. Heart Assoc. 2016, 5, e002787. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Crippa, A.; Orsini, N.; Wolk, A.; Michaelsson, K. Milk consumption and mortality from all causes, cardiovascular disease, and cancer: A systematic review and meta-analysis. Nutrients 2015, 7, 7749–7763. [Google Scholar] [CrossRef] [PubMed]
- Huth, P.J.; Park, K.M. Influence of dairy product and milk fat consumption on cardiovascular disease risk: A review of the evidence. Adv. Nutr. 2012, 3, 266–285. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Huang, J.; Wang, Y.; Zhang, D.; Qum, Y. Dairy foods and risk of stroke: A meta-analysis of prospective cohort studies. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Virtamo, J.; Wolk, A. Dairy consumption and risk of stroke in Swedish women and men. Stroke 2012, 43, 1775–1780. [Google Scholar] [CrossRef] [PubMed]
- Almeida, O.P.; Norman, P.; Hankey, G.; Jamrozik, K.; Flicker, L. Successful mental health aging: Results from a longitudinal study of older Australian men. Am. J. Geriatr. Psychiatry 2006, 14, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Ohara, T.; Ninomiya, T.; Hata, J.; Yoshida, D.; Mukai, N.; Nagata, M.; Uchida, K.; Shirota, T.; Kitazono, T.; et al. Milk and dairy consumption and risk of dementia in an elderly Japanese population: The Hisayama Study. J. Am. Geriatr. Soc. 2014, 62, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Tu, Q.; Ding, B.; Yang, X.; Bai, S.; Tu, J.; Liu, X.; Wang, R.; Tao, J.; Jin, H.; Wang, Y.; et al. The current situation on vascular cognitive impairment after ischemic stroke in Changsha. Arch. Gerontol. Geriatr. 2014, 58, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Kasagi, F.; Sasaki, H.; Masunari, N.; Mimori, Y.; Suzuki, G. Association between dementia and midlife risk factors: The radiation effects research foundation adult health study. J. Am. Geriatr. Soc. 2003, 51, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Pilleron, S.; Desport, J.C.; Jesus, P.; Mbelesso, P.; Ndamba-Bandzouzi, B.; Dartigues, J.F.; Clement, J.P.; Preux, P.M.; Guerchet, M. Diet, alcohol consumption and cognitive disorders in Central Africa: A study from the EPIDEMCA program. J. Nutr. Health Aging 2015, 19, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Sawyer, B.P.; Allman, R.M.; Zamrini, E. Dietary factors and cognitive impairment in community-dwelling elderly. J. Nutr. Health Aging 2007, 11, 49–54. [Google Scholar] [PubMed]
- Vercambre, M.N.; Boutron-Ruault, M.C.; Ritchie, K.; Clavel-Chapelon, F.; Berr, C. Long-term association of food and nutrient intakes with cognitive and functional decline: A 13-year follow-up study of elderly French women. Br. J. Nutr. 2009, 102, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 (Updated March 2011). Available online: http://handbook.cochrane.org/ (accessed on 27 October 2016).
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Shamliyan, T.A.; Kane, R.L.; Ansari, M.T.; Raman, G.; Berkman, N.D.; Grant, M.; Janes, G.; Maglione, M.; Moher, D.; Nasser, M.; et al. Development qualitycriteria to evaluate nontherapeutic studies of incidence, prevalence, or risk factors of chronic diseases: Pilot study of new checklists. J. Clin. Epidemiol. 2011, 64, 637–657. [Google Scholar] [CrossRef] [PubMed]
- National Heart Lung and Blood Institute (2014). Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. Available online: http://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/cohort (accessed on 30 October 2016).
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Biasin meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. FAOSTAT Data. Available online: http://faostat3.fao.org/home/index.html#DOWNLOAD (accessed on 18 July 2013).
- Jakobsen, M.U.; O’Reilly, E.J.; Heitmann, B.L.; Pereira, M.A.; Balter, K.; Fraser, G.E.; Goldbourt, U.; Hallmans, G.; Knekt, P.; Liu, S.; et al. Author information Major types of dietary fat and risk of coronary heart disease: A pooled analysis of 11 cohort studies. Am. J. Clin. Nutr. 2009, 89, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Rideout, T.C.; Marinangeli, C.P.; Martin, H.; Browne, R.W.; Rempel, C.B. Consumption of low-fat dairy foods for 6 months improves insulin resistance without adversely affecting lipids or bodyweight in healthy adults: A randomized free-living cross-over study. Nutr. J. 2013, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Beunza, J.J.; Delgado-Rodriguez, M.; Martinez, J.A.; Martinez-Gonzalez, M.A. Low-fat dairy consumption and reduced risk of hypertension: The Seguimiento Universidad de Navarra (SUN) cohort. Am. J. Clin. Nutr. 2005, 82, 972–979. [Google Scholar] [PubMed]
- Zemel, M.B. Role of calcium and dairy products in energy partitioning and weight management. Am. J. Clin. Nutr. 2004, 79, 907S–912S. [Google Scholar] [PubMed]
- Von Hurst, P.R.; Stonehouse, W.; Coad, J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient—A randomised, placebo controlled trial. Br. J. Nutr. 2010, 103, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Bar Yamin, H.; Barnea, M.; Genzer, Y.; Chapnik, N.; Froy, O. Long-term commercial cow’s milk consumption and its effects on metabolic parameters associated with obesity in young mice. Mol. Nutr. Food Res. 2014, 58, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Jeon, B.S.; Park, J.W.; Kim, B.K.; Kim, H.K.; Jung, T.S.; Hahm, J.R.; Kim, D.R.; Cho, Y.S.; Cha, J.Y. Fermented mushroom milk-supplemented dietary fibre prevents the onset of obesity and hypertriglyceridaemia in Otsuka Long-Evans Tokushima fatty rats. Diabetes Obes. Metab. 2005, 7, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Manson, J.E.; Buring, J.E.; Lee, I.M.; Sesso, H.D. Dietary intake of dairy products, calcium, and vitamin D and the risk of hypertension in middle-aged and older women. Hypertension 2008, 51, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A clinical trial of the effects of dietary patterns on blood pressure. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Zemel, M.B. The role of dairy foods in weight management. J. Am. Coll. Nutr. 2005, 24, 537S–546S. [Google Scholar] [CrossRef] [PubMed]
- Crichton, G.E.; Bryan, J.; Murphy, K.J.; Buckley, J. Review of dairy consumption and cognitive performance in adults: Findings and methodological issues. Dement. Geriatr. Cogn. Disord. 2010, 30, 352–361. [Google Scholar] [CrossRef] [PubMed]

| First Author, Year | Study Design | Country | Follow-Up (Years) | Male (%) | Baseline Age (Years) | Participants, No. | Exposure | Outcome | Adjustment * | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Method of Assessment | Category | Type | Method of Assessment | Case, No. | ||||||||
| Almeida, 2006 [15] | Cohort | Australia | 4.8 | 100.0 | 65− | 601 | Self-report questionnaire | Rare, regularly | Cognitive impairment | MMSE < 24 | 144 | 1–4 |
| Ozawa, 2014 [16] | Cohort | Japan | 17 | 42.2 | 60− | 1081 | 70-item semi-quantitative FFQ | Women: <45, 45–96, 97–197, ≥198 g/day; Man: <20, 20–75, 76–173, ≥174 g/day | Dementia, AD | DSM-III-R, NINCDS-ADRDA | 303, 166 1 | 1, 2, 4–16 |
| Pilleron, 2015 [19] CAR; ROC | Cross-sectional | Africa | - | 40.3; 41.4 | 65− | 841; 931 | 8-item FFQ | <1 serving/day, ≥1 serving/day | Dementia, Cognitive impairment | DSM-IV, Petersen criteria | 72, 62; 63, 56 2 | 1, 2, 4, 17, 18 |
| Rahman, 2007 [20] | Cross-sectional | USA | - | 32.7 | 65− | 1056 | Self-report questionnaire | <1 time/week, ≥1 time/week | Cognitive impairment | Mental status questionnaire <9 | 175 | 1, 2, 4, 12–15 |
| Tu, 2014 [17] | Cross-sectional | China | - | 58.6 | 40− | 689 | Self-report questionnaire | Low, high intake | Cognitive impairment | MMSE < 28 and MoCA-CS < 27 | 221 | 1, 13, 16, 19–28 |
| Vercambre, 2009 [21] | Cohort | France | 13 | 0 | 63− | 4809 | Self-administrated questionnaire | Tertiles | Cognitive decline | DECO < 33 | 598 | 1, 2, 4, 6, 7, 9–11, 29-35 |
| Yamada, 2003 [18] | Cohort | Japan | 25 | 26.8 | 30− | 1774 | Self-administrated questionnaire | <4 times/week, daily | Dementia, AD | DSM-IV, NINCDS-ADRDA | 114, 51 3 | 1, 2, 7, 9, 36, 37 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Sun, D. Meta-Analysis of Milk Consumption and the Risk of Cognitive Disorders. Nutrients 2016, 8, 824. https://doi.org/10.3390/nu8120824
Wu L, Sun D. Meta-Analysis of Milk Consumption and the Risk of Cognitive Disorders. Nutrients. 2016; 8(12):824. https://doi.org/10.3390/nu8120824
Chicago/Turabian StyleWu, Lei, and Dali Sun. 2016. "Meta-Analysis of Milk Consumption and the Risk of Cognitive Disorders" Nutrients 8, no. 12: 824. https://doi.org/10.3390/nu8120824
APA StyleWu, L., & Sun, D. (2016). Meta-Analysis of Milk Consumption and the Risk of Cognitive Disorders. Nutrients, 8(12), 824. https://doi.org/10.3390/nu8120824




