Patient Survival and Costs on Moderately Restricted Low-Protein Diets in Advanced CKD: Equivalent Survival at Lower Costs?
Abstract
:1. Introduction
2. Methods
2.1. Setting of Study
2.2. Diet and Control Protocols
2.3. Standardized Mortality Rates
2.4. Cost Analysis
2.5. Statistical Analysis
2.6. Ethical Issues
3. Results
3.1. Baseline Data
3.2. Survival Analysis
3.3. Cost Analysis
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Addis, T. Glomerular Nephritis: Diagnosis and Treatment; Macmillan: London, UK, 1949. [Google Scholar]
- Giovannetti, S.; Maggiore, Q. A low-nitrogen diet with proteins of high biological value for severe chronic uraemia. Lancet 1964, 1, 1000–1003. [Google Scholar] [CrossRef]
- Ko, G.J.; Obi, Y.; Tortorici, A.R.; Kalantar-Zadeh, K. Dietary protein intake and chronic kidney disease. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Mitch, W.E.; Remuzzi, G. Diets for patients with chronic kidney disease, still worth prescribing. J. Am. Soc. Nephrol. 2004, 15, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Mitch, W.E.; Remuzzi, G. Diets for patients with chronic kidney disease, should we reconsider? BMC Nephrol. 2016, 17, 80. [Google Scholar] [CrossRef] [PubMed]
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.; Yang, C.W. Chronic kidney disease: Global dimension and perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef]
- Vanholder, R.; Lameire, N.; Annemans, L.; van Biesen, W. Cost of renal replacement: How to help as many as possible while keeping expenses reasonable? Nephrol. Dial. Transplant. 2016, 31, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Nesrallah, G.E.; Mustafa, R.A.; Clark, W.F.; Bass, A.; Barnieh, L.; Hemmelgarn, B.R.; Klarenbach, S.; Quinn, R.R.; Hiremath, S.; Ravani, P.; et al. Canadian Society of Nephrology. Canadian Society of Nephrology 2014 clinical practice guidelines for timing the initiation of chronic dialysis. CMAJ 2014, 186, 112–117. [Google Scholar] [PubMed]
- Kalantar-Zadeh, K.; Moore, L.W.; Tortorici, A.R.; Chou, J.A.; St-Jules, D.E.; Aoun, A.; Rojas-Bautista, V.; Tschida, A.K.; Rhee, C.M.; Shah, A.A.; et al. North American experience with Low protein diet for Non-dialysis-dependent chronic kidney disease. BMC Nephrol. 2016, 17, 90. [Google Scholar] [CrossRef] [PubMed]
- Eyre, S.; Faxén-Irving, G.; Attman, P.O.; Evans, M.; Windahl, K.; Wegener, S.; Andersén, C.; Nykvist-Raanaes, K.; Einemo, S.; Carrero, J. A practical approach to low protein diets in Sweden—45 years of clinical use. BMC Nephrol. 2016, 17, 89. [Google Scholar] [CrossRef] [PubMed]
- Cuppari, L.; Nerbass, F.B.; Avesani, C.M.; Kamimura, M.A. A practical approach to dietary interventions for nondialysis-dependent CKD patients: The experience of a reference nephrology center in Brazil. BMC Nephrol. 2016, 17, 85. [Google Scholar] [CrossRef] [PubMed]
- Ameh, O.I.; Cilliers, L.; Okpechi, I.G. A practical approach to the nutritional management of chronic kidney disease patients in Cape Town, South Africa. BMC Nephrol. 2016, 17, 68. [Google Scholar] [CrossRef] [PubMed]
- Fouque, D.; Laville, M. Low protein diets for chronic kidney disease in non diabetic adults. Cochrane Database Syst. 2009, 3. [Google Scholar] [CrossRef]
- Kovesdy, C.P.; Kalantar-Zadeh, K. Back to the future: Restricted protein intake for conservative management of CKD, triple goals of renoprotection, uremia mitigation, and nutritional health. Int. Urol. Nephrol. 2016, 48, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.W. Dietary protein restriction as a treatment for slowing chronic kidney disease progression: The case against. Nephrology 2006, 11, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Kopple, J.D.; Wang, X.; Beck, G.J.; Collins, A.J.; Kusek, J.W.; Greene, T.; Levey, A.S.; Sarnak, M.J. Effect of a very low-protein diet on outcomes: Long-term follow-up of the Modification of Diet in Renal Disease (MDRD) study. Am. J. Kidney Dis. 2009, 53, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Obi, Y.; Qader, H.; Kovesdy, C.P.; Kalantar-Zadeh, K. Latest consensus and update on protein-energy wasting in chronic kidney disease. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Ash, S.; Campbell, K.L.; Bogard, J.; Millichamp, A. Nutrition prescription to achieve positive outcomes in chronic Kidney disease: A systematic review. Nutrients 2014, 6, 416–451. [Google Scholar] [CrossRef] [PubMed]
- Brunori, G.; Viola, B.F.; Parrinello, G.; de Biase, V.; Como, G.; Franco, V.; Garibotto, G.; Zubani, R.; Cancarini, G.C. Efficacy and safety of a very-low-protein diet when postponing dialysis in the elderly: A prospective randomized multicenter controlled study. Am. J. Kidney Dis. 2007, 49, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Garneata, L.; Stancu, A.; Dragomir, D.; Stefan, G.; Mircescu, G. Ketoanalogue-supplemented vegetarian very low-protein diet and CKD progression. J. Am. Soc. Nephrol. 2016, 27, 2164–2176. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, G.B.; Ferraresi, M.; Deagostini, M.C.; Vigotti, F.N.; Consiglio, V.; Scognamiglio, S.; Moro, I.; Clari, R.; Fassio, F.; Biolcati, M.; et al. Vegetarian low-protein diets supplemented with keto analogues: A niche for the few or an option for many? Nephrol. Dial. Transplant. 2013, 28, 2295–2305. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, C.; Piccoli, G.B.; Calella, P.; Brunori, G.; Pasticci, F.; Egidi, M.F.; Capizzi, I.; Bellizzi, V.; Cupisti, A. “Dietaly”: Practical issues for the nutritional management of CKD patients in Italy. BMC Nephrol. 2016, 17, 102. [Google Scholar] [CrossRef] [PubMed]
- Iseki, K.; Yamagata, K. A practical approach of salt and protein restriction for CKD patients in Japan. BMC Nephrol. 2016, 17, 87. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.V. The changing role of dietary protein restriction in management of chronic Kidney disease (CKD). J. Assoc. Physician India 2015, 63, 34–40. [Google Scholar]
- Piccoli, G.B.; Deagostini, M.C.; Vigotti, F.N.; Ferraresi, M.; Moro, I.; Consiglio, V.; Scognamiglio, S.; Mongilardi, E.; Clari, R.; Aroasio, E.; et al. Which low-protein diet for which CKD patient? An observational, personalized approach. Nutrition 2014, 30, 992–999. [Google Scholar] [PubMed]
- D’Alessandro, C.; Rossi, A.; Innocenti, M.; Ricchiuti, G.; Bozzoli, L.; Sbragia, G.; Meola, M.; Cupisti, A. Dietary protein restriction for renal patients: Don’t forget protein-free foods. J. Ren. Nutr. 2013, 23, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, G.B.; Leone, F.; Attini, R.; Parisi, S.; Fassio, F.; Deagostini, M.C.; Ferraresi, M.; Clari, R.; Ghiotto, S.; Biolcati, M.; et al. Association of low-protein supplemented diets with fetal growth in pregnant women with CKD. Clin. J. Am. Soc. Nephrol. 2014, 9, 864–873. [Google Scholar] [CrossRef] [PubMed]
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am. J. Kidney Dis. 2002, 39, S1–S266. [Google Scholar]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Maroni, B.J.; Steinman, T.I.; Mitch, W.E. A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int. 1985, 27, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Cooper, B.A.; Branley, P.; Bulfone, L.; Pharm, B.; Collins, J.F.; Craig, J.C.; Fraenkel, M.B.; Harris, A.; Johnson, D.W.; Kesselhu, J.; et al. A randomized, controlled trial of early versus late initiation of dialysis. N. Engl. J. Med. 2010, 363, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Thorsteinsdottir, B.; Swetz, K.M.; Albright, R.C. The ethics of chronic dialysis for the older patient: Time to reevaluate the norms. Clin. J. Am. Soc. Nephrol. 2015, 10, 2094–2099. [Google Scholar] [CrossRef] [PubMed]
- United Stated Renal Data System. Available online: http://www.usrds.org (accessed on 15 August 2015).
- Nordio, M.; Limido, A.; Maggiore, U.; Nichelatti, M.; Postorino, M.; Quintaliani, G. Italian dialysis and transplantation registry. Survival in patients treated by long-term dialysis compared with the general population. Am. J. Kidney Dis. 2012, 59, 819–828. [Google Scholar] [PubMed]
- Société Francophone de Néphrologie Dialyse et Transplantation. Available online: http://www.soc-nephrologie.org (accessed on 15 August 2015).
- Vanholder, R.; Davenport, A.; Hannedouche, T.; Kooman, J.; Kribben, A.; Lameire, N.; Lonnemann, G.; Magner, P.; Mendelssohn, D.; Saggi, S.J.; et al. Reimbursement of dialysis: A comparison of seven countries. J. Am. Soc. Nephrol. 2012, 23, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, G.B.; Ventrella, F.; Capizzi, I.; Vigotti, F.N.; Mongilardi, E.; Grassi, G.; Loi, V.; Cabiddu, G.; Avagnina, P.; Versino, E. Low-protein diets in diabetic chronic Kidney disease (CKD) patients: Are they feasible and worth the effort? Nutrients 2016, 8, 649. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, G.B.; Vigotti, F.N.; Leone, F.; Capizzi, I.; Daidola, G.; Cabiddu, G.; Avagnina, P. Low-protein diets in CKD: How can we achieve them? A narrative, pragmatic review. Clin. Kidney J. 2015, 8, 61–70. [Google Scholar] [PubMed]
- Chauveau, P.; Couzi, L.; Vendrely, B.; de Précigout, V.; Combe, C.; Fouque, D.; Aparicio, M. Long-term outcome on renal replacement therapy in patients who previously received a keto acidsupplemented very-low-protein diet. Am. J. Clin. Nutr. 2009, 90, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Bellizzi, V.; Chiodini, P.; Cupisti, A.; Viola, B.F.; Pezzotta, M.; de Nicola, L.; Minutolo, R.; Barsotti, G.; Piccoli, G.B.; di Iorio, B. Very low-protein diet plus ketoacids in chronic kidney disease and risk of death during end-stage renal disease: A historical cohort controlled study. Nephrol. Dial. Transpl. 2015, 30, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Duenhas, M.; Gonçalves, E.; Dias, M.; Leme, G.; Laranja, S. Reduction of morbidity related to emergency access to dialysis with very low protein diet supplemented with ketoacids (VLPD+KA). Clin. Nephrol. 2013, 79, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Cianciaruso, B.; Capuano, A.; D’Amaro, E.; Ferrara, N.; Nastasi, A.; Conte, G.; Bellizzi, V.; Andreucci, V.E. Dietary compliance to a low protein and phosphate diet in patients with chronic renal failure. Kidney Int. Suppl. 1989, 27, S173–S176. [Google Scholar] [PubMed]
- Scalone, L.; Borghetti, F.; Brunori, G.; Viola, B.F.; Brancati, B.; Sottini, L.; Mantovani, L.G.; Cancarini, G. Cost-benefit analysis of supplemented very low-protein diet versus dialysis in elderly CKD5 patients. Nephrol. Dial. Transpl. 2010, 25, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Mennini, F.; Russo, S.; Marcellusi, A.; Quintaliani, G.; Fouque, D. Economic effects of treatment of chronic kidney disease with low-protein diet. J. Ren. Nutr. 2014, 24, 313–321. [Google Scholar] [CrossRef] [PubMed]
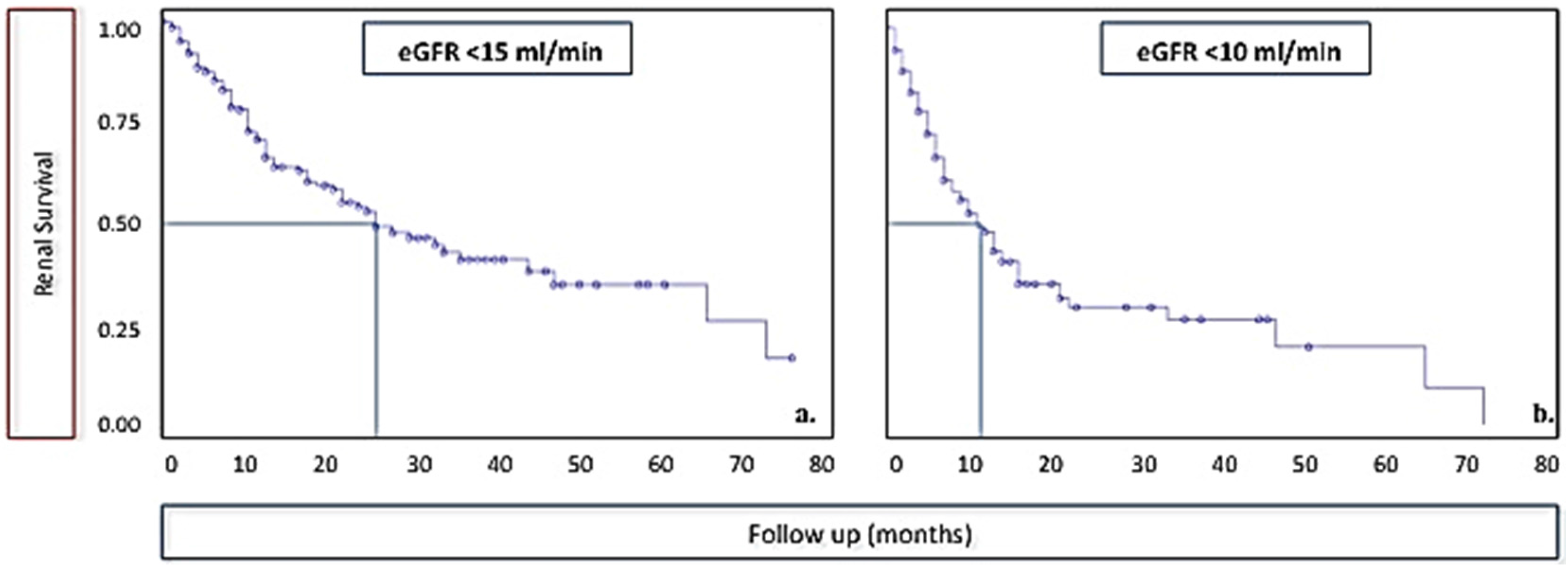
| All Cases | Pts Who Reached 10 mL of GFR (Data at Start of Diet) | |
|---|---|---|
| N | 449 | 148 |
| Years of observation | 847 | 205 * |
| Males (%) | 276 (61.5%) | 88 (59.5%) |
| Females (%) | 173 (38.5%) | 60 (40.5%) |
| Age: median (min–max) | 70 (19–97) | 68.5 (23–93) |
| Age over 65 (%) | 274 (61%) | 82 (55.4%) |
| Age over 80 (%) | 73 (16.3%) | 16 (10.8%) |
| Charlson: median (min–max) | 7 (2–13) | 6.5 (2–12) |
| Charlson ≥ 7 (%) | 246 (54.8%) | 74 (50%) |
| Charlson ≥ 10 (%) | 71 (15.8%) | 13 (8.8%) |
| Diabetes (%) | 149 (33.2%) | 51 (34.5%) |
| Cardiopathy (%) | 208 (46.3%) | 58 (39.2%) |
| Neoplasia (%) | 97 (21.6%) | 27 (18.2%) |
| sCreatinine (mg/dL) median (min–max) | 2.8 (0.6–16) | 4.0 (1.5–16) |
| e-GFR-EPI (mL/min) median (min–max) | 20 (3–127) | 13.8 (3.0–49.5) |
| Proteinuria (g/day) median (min–max) | 0.8 (0.1–11) | 1.5 (0.1–11) |
| Proteinuria ≥ 1 g/day (%) | 202 (45.4%) | 92 (63.4%) |
| Glomerulonephritis-systemic disease (%) | 95 (21.2%) | 38 (25.7%) |
| Nephroangiosclerosis and/or diabetes (%) | 265 (59.0%) | 80 (54.1%) |
| ADPKD (%) | 24 (5.3%) | 10 (6.8%) |
| Vegan Supplemented | With Protein-Free Food | Other | All Cases | |
|---|---|---|---|---|
| Follow-up/Follow-up one year after discontinuation (years) | 375.8 | 335.7 | 136.3 | 847.1 |
| 449.9 | 347.7 | 147.2 | 944.1 | |
| Observed deaths: on diet/one year after discontinuation | 35 | 59 | 6 | 100 |
| 42 | 60 | 7 | 109 | |
| Expected deaths (USRDS): on diet/one year after discontinuation | 82.15 | 105.51 | 38.31 | 226.38 |
| 94.52 | 109.87 | 40.49 | 247.30 | |
| RR (CI) (USRDS): on diet/one year after discontinuation | 0.43 (0.30–0.59) | 0.56 (0.43–0.72) | 0.16 (0.06–0.34) | 0.44 (0.36–0.54) |
| 0.44 (0.32–0.60) | 0.55 (0.42–0.70) | 0.17 (0.07–0.36) | 0.44 (0.36–0.53) | |
| Expected deaths (Italian Reg.): on diet/one year after discontinuation | 50.67 | 64.55 | 22.45 | 137.70 |
| 59.25 | 67.18 | 23.60 | 150.03 | |
| RR (CI) (Italian Reg.): on diet/one year after discontinuation | 0.69 (0.48–0.96) | 0.91 (0.70–1.18) | 0.27 (0.10–0.58) | 0.73 (0.59–0.88) |
| 0.71 (0.51–0.96) | 0.89 (0.68–1.15) | 0.30 (0.12–0.61) | 0.73 (0.60–0.88) | |
| Expected deaths (French Reg.): on diet/1 year after discontinuation | 48.51 | 71.70 | 25.54 | 143.69 |
| 57.19 | 74.47 | 26.96 | 156.55 | |
| RR (CI) (French Reg.): on diet/one year after discontinuation | 0.72 (0.50–1.00) | 0.82 (0.63–1.06) | 0.23 (0.09–0.51) | 0.70 (0.57–0.85) |
| 0.73 (0.53–0.99) | 0.81 (0.62–1.04) | 0.26 (0.10–0.53) | 0.70 (0.57–0.84) | |
| age < 65 years | ||||
| Follow-up on diet/one year after discontinuation | 189.75 | 52.42 | 46.17 | 287.6 |
| 232.75 | 54.42 | 50.17 | 336.7 | |
| Observed deaths: on diet/one year after discontinuation | 5/6 | 3/3 | 2/2 | 11/12 |
| Expected deaths (USRDS): on diet/one year after discontinuation | 25.78/28.96 | 9.35/9.71 | 6.68/7.35 | 41.72/48.43 |
| RR (CI) (USRDS): on diet/one year after discontinuation | 0.19 (0.06–0.45) | 0.32 (0.07–0.94) | 0.30 (0.04–1.08) | 0.26 (0.13–0.47) |
| 0.21 (0.08/0.45) | 0.31 (0.06–0.90) | 0.17 (0.03–0.98) | 0.25 (0.13–0.43) | |
| Expected deaths (Italian Reg.): on diet/one year after discontinuation | 13.94/16.91 | 4.44/4.62 | 3.11/3.41 | 21.48/24.94 |
| RR (CI) (Italian Registry): on diet/one year after discontinuation | 0.36(0.12–0.84) | 0.68 (0.14–1.97) | 0.64 (0.78–2.32) | 0.51 (0.26–0.92) |
| 0.35(0.13–0.77) | 0.65 (0.13/1.90) | 0.59 (0.71–2.12) | 0.48 (0.62–0.94) | |
| Expected deaths (French Reg.): on diet/one year after discontinuation | 12.18/14.81 | 4.82/5.01 | 3.20/3.54 | 20.16/23.32 |
| RR (CI) (French Reg.): on diet/one year after discontinuation | 0.41 (0.13–0.96) | 0.62 (0.13–1.82) | 0.63 (0.08–2.26) | 0.50 (0.24–0.91) |
| 0.41 (0.15–0.88) | 0.60 (0.12–1.75) | 0.57 (0.07–2.04) | 0.47 (0.24–0.84) | |
| age ≥ 65 years | ||||
| Follow-up on diet/one year after discontinuation | 186.0 | 283.2 | 90.2 | 559.6 |
| 216.7 | 293.3 | 95.0 | 604.9 | |
| Observed deaths: on diet/one year after discontinuation | 30/36 | 56/57 | 4/5 | 89/97 |
| Expected deaths (USRDS): on diet/one year after discontinuation | 56.37/65.56 | 96.58/100.16 | 31.63/33.14 | 184.65/198.86 |
| RR (CI) (USRDS): on diet/one year after discontinuation | 0.53 (0.36–0.76) | 0.58 (0.44–0.75) | 0.13 (0.03–032) | 0.48 (0.39–0.59) |
| 0.55 (0.39–0.76) | 0.57 (0.43–0.74) | 0.15 (0.05–0.35) | 0.49 (0.40–0.59) | |
| Expected deaths (Italian Reg.): on diet/1 year after discontinuation | 36.73/42.35 | 60.11/62.56 | 19.34/20.19 | 116.22/125.09 |
| RR (CI) (Italian Registry): on diet/one year after discontinuation | 0.82 (0.55–1.17) | 0.93 (0.70–1.21) | 0.21 (0.06–0.53) | 0.77 (0.25–0.84) |
| 0.85 (0.60–1.18) | 0.91 (0.69–1.18) | 0.25 (0.08–0.58) | 0.78 (0.63–0.95) | |
| Expected deaths (French Reg.): on diet/one year after discontinuation | 36.34/42.38 | 66.88/69.46 | 22.34/23.42 | 125.56/135.26 |
| RR (CI) (French Reg.): on diet/one year after discontinuation | 0.83 (0.56–1.18) | 0.84 (0.63–1.09) | 0.18 (0.05–0.46) | 0.72 (0.56–0.88) |
| 0.85 (0.56–1.18) | 0.82 (0.62–1.06) | 0.21 (0.07–0.50) | 0.72 (0.59–0.88) | |
| e-GFR < 15 (mL/min) | e-GFR < 10 (mL/min) | |
|---|---|---|
| Follow-up (years) | 384.83 | 204.75 |
| Observed deaths | 64 | 29 |
| Expected deaths (USRDS) | 104.43 | 50.00 |
| RR (CI) (USRDS) | 0.61 (0.47–0.78) | 0.58 (0.39–0.83) |
| Expected deaths (Italian Reg.) | 63.79 | 30.16 |
| RR (CI) (Italian Reg.) | 1.00 (0.77–1.28) | 0.96 (0.64–1.38) |
| Expected deaths (French Reg.) | 67.85 | 31.51 |
| RR (CI) (French Reg.) | 0.94 (0.73–1.21) | 0.92 (0.62–1.32) |
| <65 years | ||
| Follow-up (years) | 127.83 | 80.42 |
| Observed deaths | 6 | 4 |
| Expected deaths (USRDS) | 18.04 | 11.65 |
| RR (CI) (USRDS) | 0.33 (0.12–0.72) | 0.34 (0.09–0.88) |
| Expected deaths (Italian Reg.) | 9.33 | 6.05 |
| RR (CI) (Italian Reg.) | 0.64 (0.24–1.40) | 0.66 (0.18–1.69) |
| Expected deaths (French Reg.) | 8.62 | 5.63 |
| RR (CI) (French Reg.) | 0.70 (0.26–1.52) | 0.71 (0.62–1.32) |
| ≥65 years | ||
| Follow-up (years) | 262.00 | 124.33 |
| Observed deaths | 58 | 25 |
| Expected deaths (USRDS) | 86.39 | 38.35 |
| RR (CI) (USRDS) | 0.67 (0.51–0.87) | 0.65 (0.42–0.96) |
| Expected deaths (Italian Reg.) | 54.46 | 24.11 |
| RR (CI) (Italian Reg.) | 1.06 (0.81–1.38) | 1.04 (0.67–1.53) |
| Expected deaths (French Reg.) | 59.23 | 25.88 |
| RR (CI) (French Reg.) | 0.98 (0.74–1.27) | 0.97 (0.63–1.43) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccoli, G.B.; Nazha, M.; Capizzi, I.; Vigotti, F.N.; Mongilardi, E.; Bilocati, M.; Avagnina, P.; Versino, E. Patient Survival and Costs on Moderately Restricted Low-Protein Diets in Advanced CKD: Equivalent Survival at Lower Costs? Nutrients 2016, 8, 758. https://doi.org/10.3390/nu8120758
Piccoli GB, Nazha M, Capizzi I, Vigotti FN, Mongilardi E, Bilocati M, Avagnina P, Versino E. Patient Survival and Costs on Moderately Restricted Low-Protein Diets in Advanced CKD: Equivalent Survival at Lower Costs? Nutrients. 2016; 8(12):758. https://doi.org/10.3390/nu8120758
Chicago/Turabian StylePiccoli, Giorgina Barbara, Marta Nazha, Irene Capizzi, Federica Neve Vigotti, Elena Mongilardi, Marilisa Bilocati, Paolo Avagnina, and Elisabetta Versino. 2016. "Patient Survival and Costs on Moderately Restricted Low-Protein Diets in Advanced CKD: Equivalent Survival at Lower Costs?" Nutrients 8, no. 12: 758. https://doi.org/10.3390/nu8120758
APA StylePiccoli, G. B., Nazha, M., Capizzi, I., Vigotti, F. N., Mongilardi, E., Bilocati, M., Avagnina, P., & Versino, E. (2016). Patient Survival and Costs on Moderately Restricted Low-Protein Diets in Advanced CKD: Equivalent Survival at Lower Costs? Nutrients, 8(12), 758. https://doi.org/10.3390/nu8120758







