Positive Regulation of Decidualization by l-Type Amino Acid Transporter 1 (lat1) in Pregnant Mice
Abstract
:1. Introduction
2. Experimental Section
2.1. Animals and Treatments
2.2. Primary Culture of Uterine Endometrium ESC and Induction of Decidualization In Vitro Extraction and Induction of Decidualization
2.2.1. Addition of Inhibitor
2.2.2. Transfection
2.3. Immunocytochemistry
2.4. Western Blot Analysis
2.5. Semiquantitative RT-PCR
2.6. Histochemistry
2.7. Statistical Analysis
3. Results
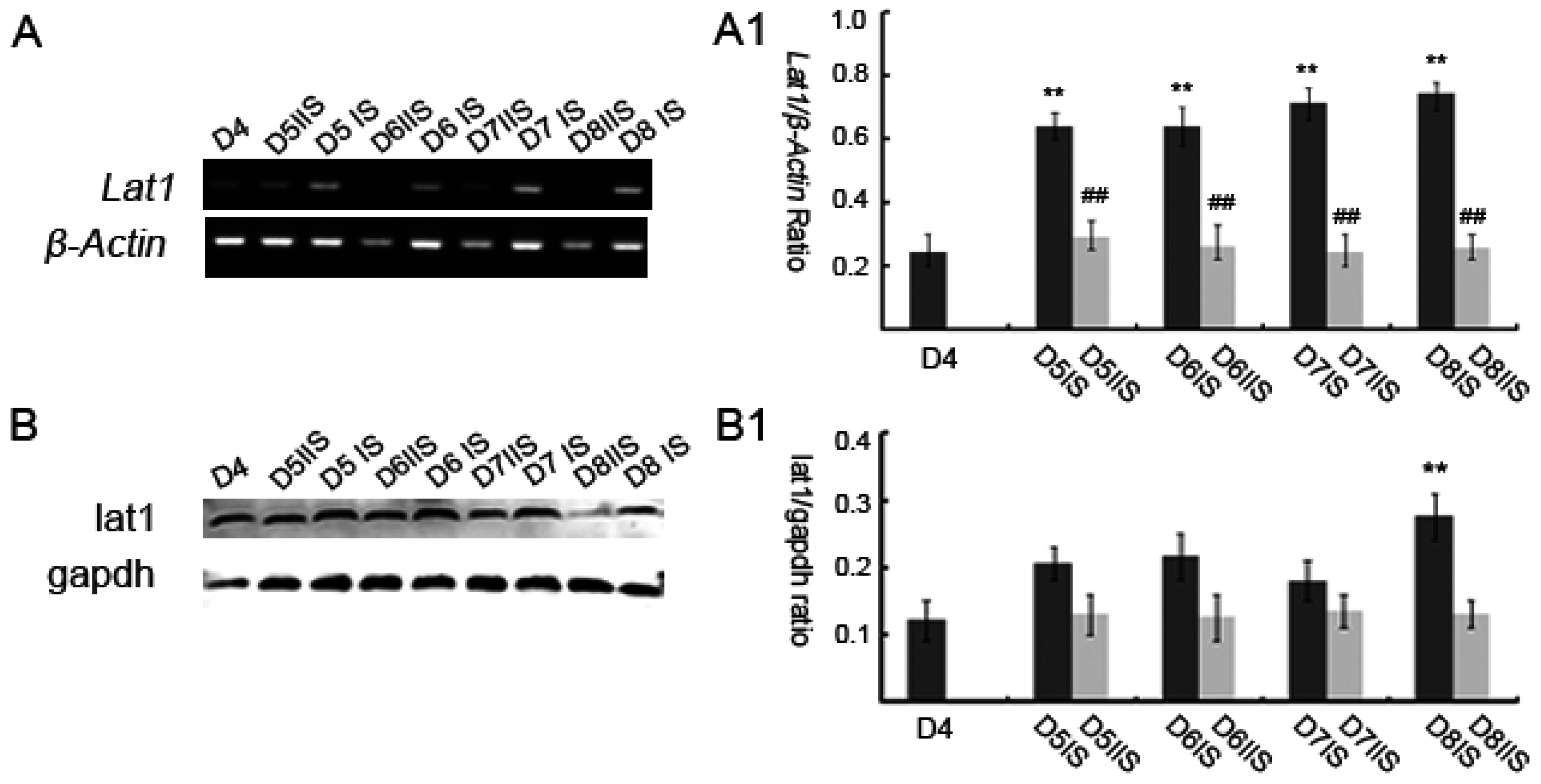
3.1. Expression of Lat1 in Mouse Uteri during Implantation
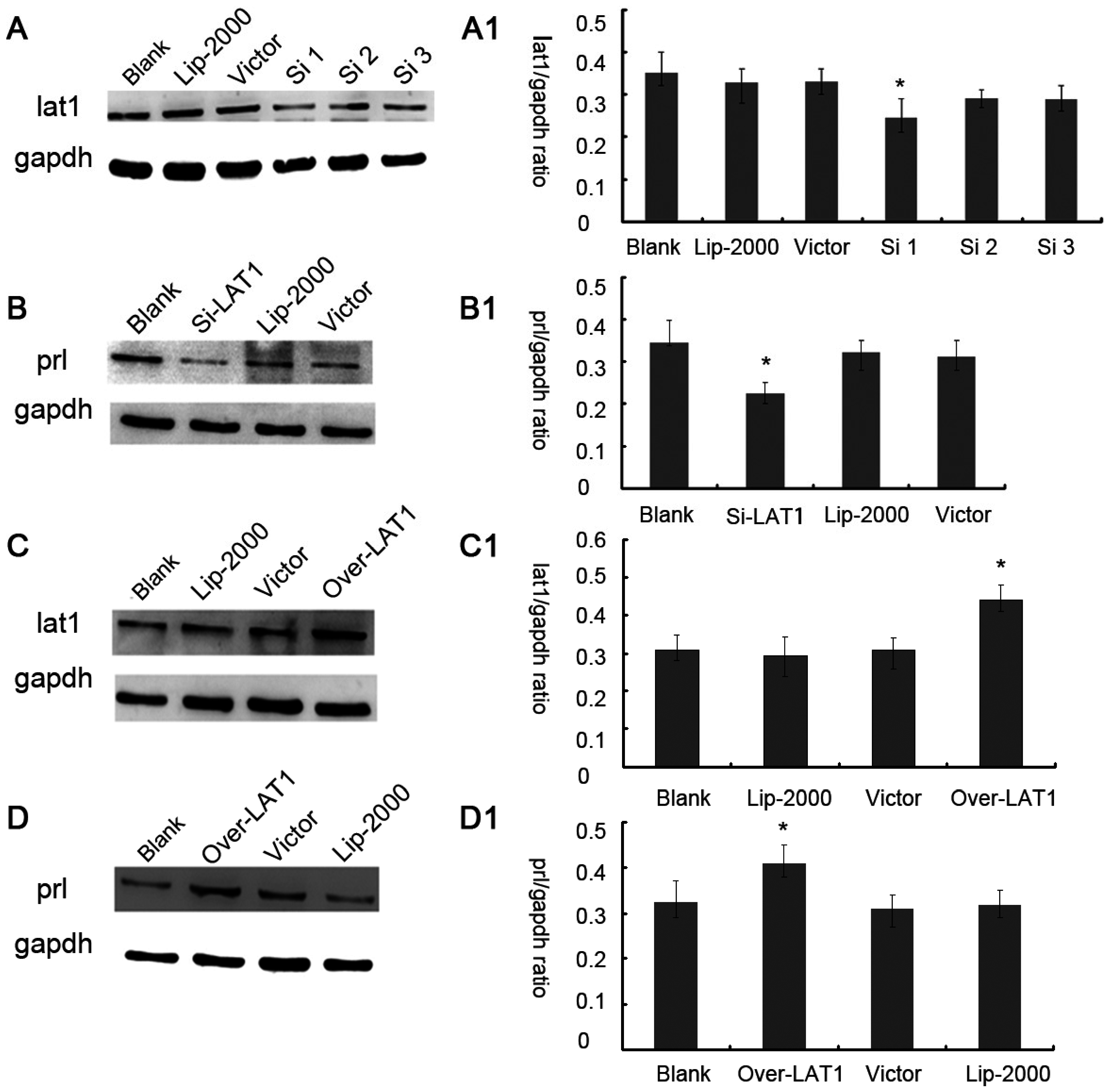
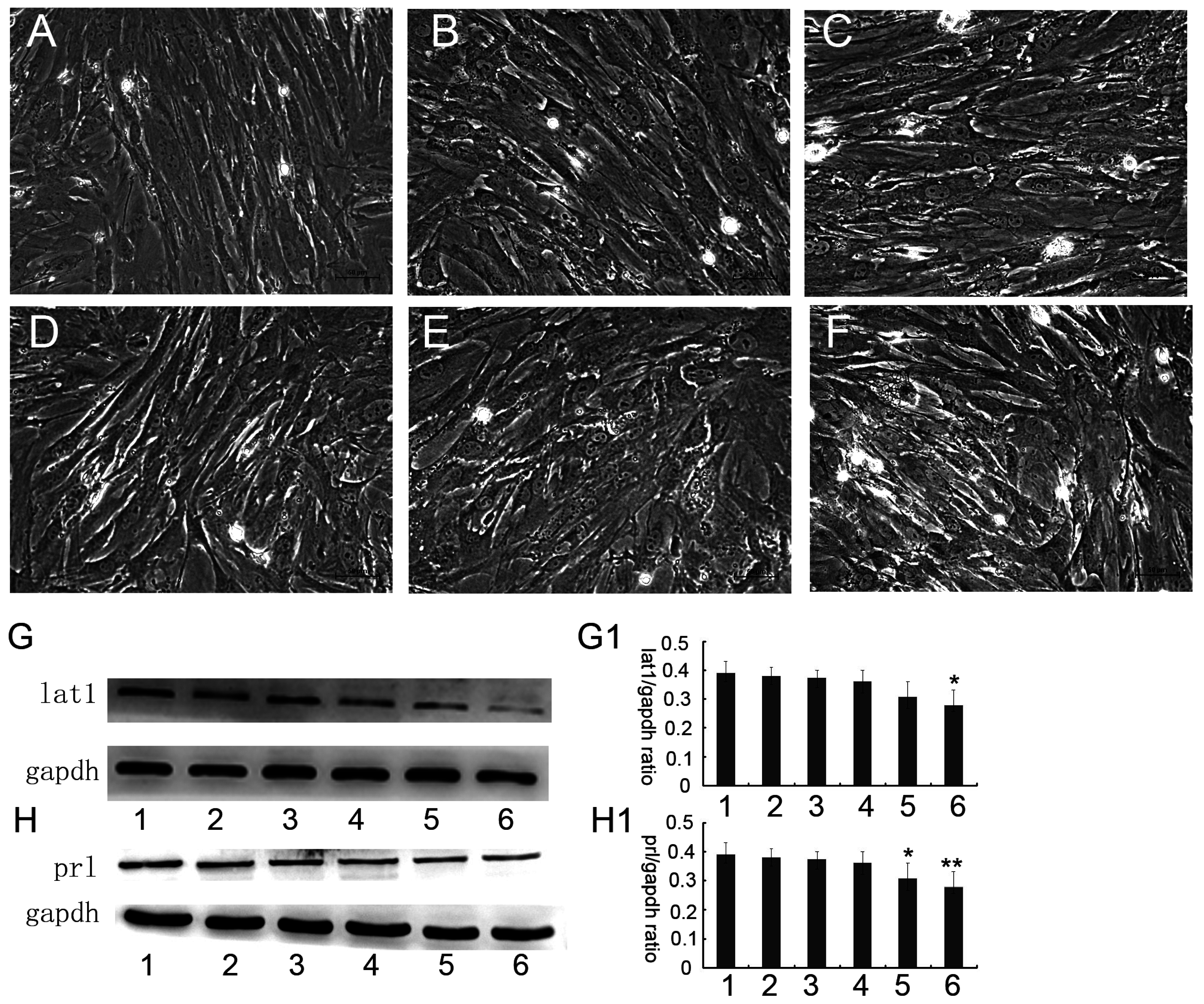
3.2. Up or Down Regulation Effect of lat1 on prl Expression in the Decidualization of ESCs
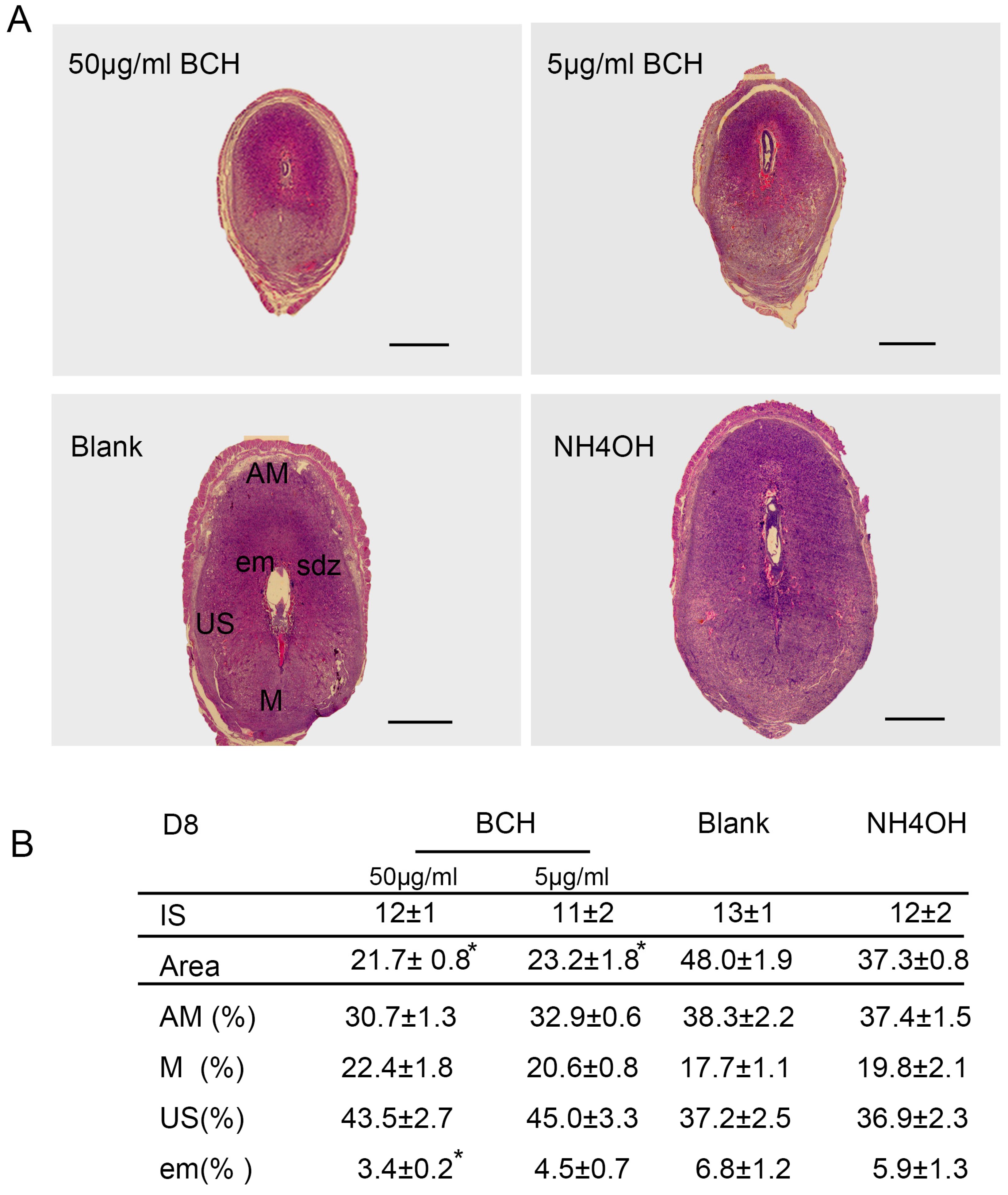
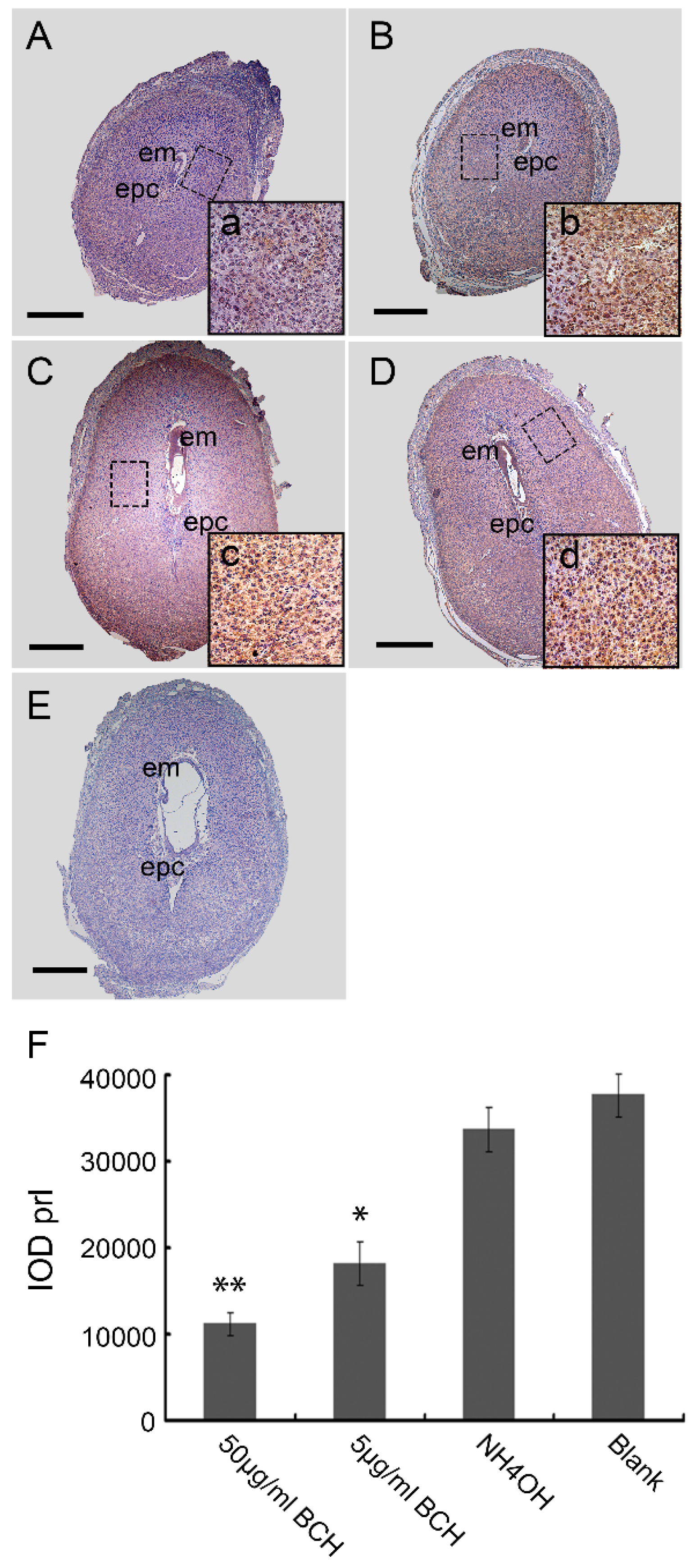
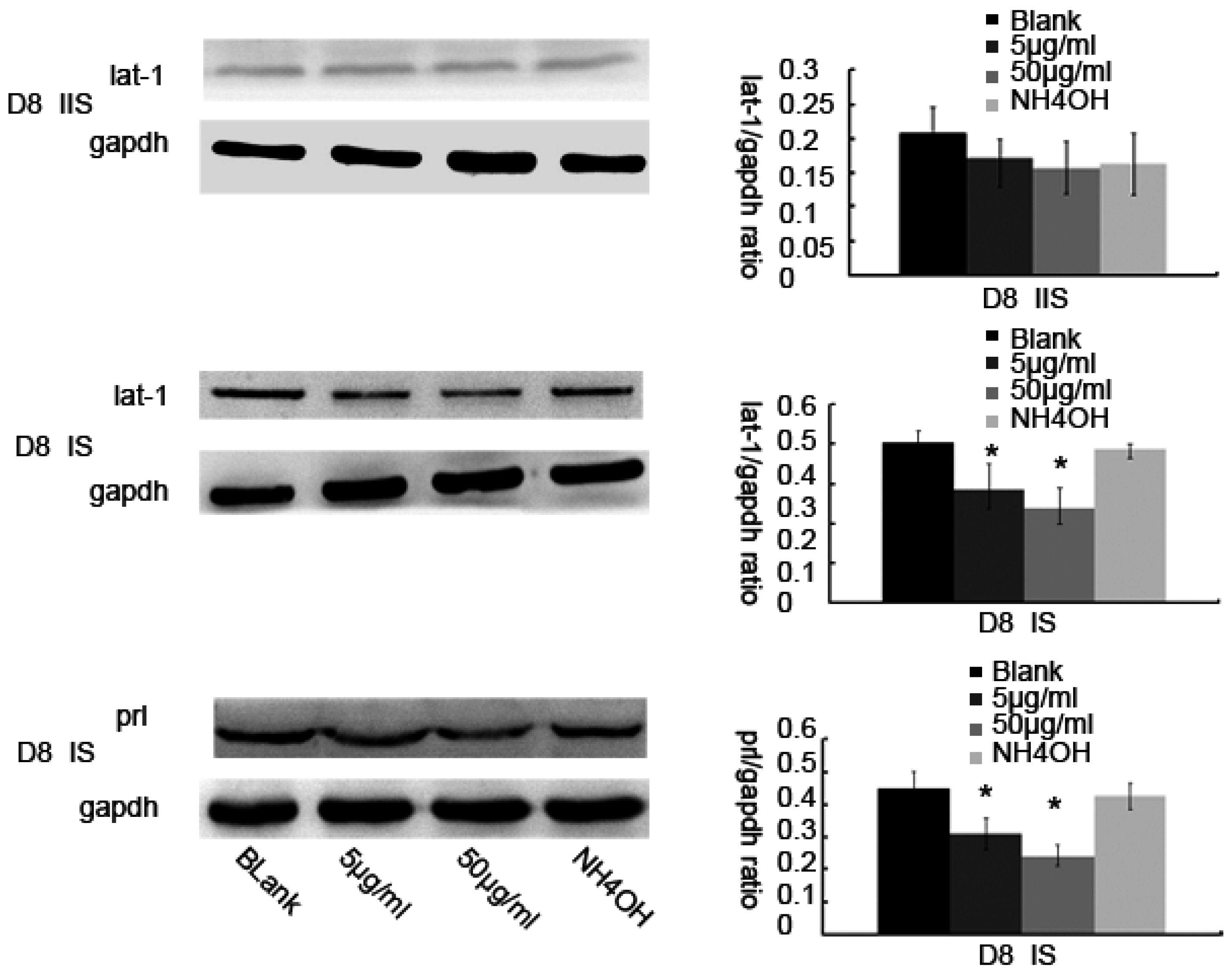
3.3. Inhibitor of lat1 Transportation Activity Restrained Decidualization In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zeng, X.; Mao, X.; Huang, Z.; Wang, F.; Wu, G.; Qiao, S. Arginine enhances embryo implantation in rats through PI3K/PKB/mTOR/NO signaling pathway during early pregnancy. Reproduction 2013, 145, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chrostowski, M.K.; McGonnigal, B.G.; Stabila, J.P.; Padbury, J.F. Role of the l-amino acid transporter-1 (lat-1) in mouse trophoblast cell invasion. Placenta 2010, 31, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Colonna, R.; Mangia, F. Mechanisms of amino acid uptake in cumulus-enclosed mouse oocytes. Biol. Reprod. 1983, 28, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Colonna, R.; Cecconi, S.; Buccione, R.; Mangia, F. Amino acid transport in growing mouse oocytes. Cell Biol. Int. Rep. 1983, 7, 1007–1015. [Google Scholar] [CrossRef]
- Colonna, R.; Cecconi, S.; Buccione, R.; Mangia, F. Stage-dependent modifications of amino acid uptake by antral and metaphase II mouse oocytes. Cell Biol. Int. Rep. 1984, 8, 3–9. [Google Scholar] [CrossRef]
- Prasad, P.D.; Wang, H.; Huang, W.; Kekuda, R.; Rajan, D.P.; Leibach, F.H.; Ganapathy, V. Human LAT1, a Subunit of System L Amino Acid Transporter: Molecular Cloning and Transport Function. Biol. Chem. Biophys. Res. Commun. 1999, 255, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Gwatkin, R.B. Amino Acid Requirements for Attachment and Outgrowth of the Mouse Blastocyst in vitro. J. Cell. Physiol. 1966, 68, 335–344. [Google Scholar] [CrossRef]
- Dey, S.K. Heparin-binding EGF-like growth factor gene is induced in the mouse uterine temporally by the blastocyst solely at the site of its apposition: A possible ligand for interaction with blastocyst EGF-receptor in implantation. Development 1994, 120, 1071–1083. [Google Scholar]
- Dey, S.K.; Lim, H.; Das, S.K.; Reese, J.; Paria, B.C.; Daikoku, T.; Wang, H. Molecular cues to implantation. Endocr. Rev. 2004, 25, 341–373. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Liu, Z.; Tan, D.; Zhang, Q.; Peng, H.; Wang, Y.; Tan, Y. Gamma-Amino Butyric Acid and the A-Type Receptor Suppress Decidualization of Mouse Uterine Stromal Cells by Down-Regulating Cyclin D3. Mol. Reprod. Dev. 2013, 80, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dey, S.K. Roadmap to embryo implantation: Clues from mouse models. Nat. Rev. Genet. 2006, 7, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K. Regional development of uterine decidualization: Molecular signaling by Hoxa-10. Mol. Reprod. Dev. 2010, 77, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Tan, D.; Luo, W.; Lu, J.; Tan, Y. Expression of CD82 in Human Trophoblast and Its Role in Trophoblast Invasion. PLoS ONE 2012, 7, e38487. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Tan, Y.; Tan, D.-M.; Lu, J.J.; Liang, H.; Luo, W.-P. Effects of LAT1 on Mouse Placenta Establishment in Early Phase. Acta Lab. Anim. Sci. Sin. 2015, 3, 256–260. [Google Scholar]
- Gao, F.; Bian, F.; Ma, X.; Kalinichenko, V.V.; Das, S.K. Control of regional decidualization in implantation: Role of FoxM1 downstream of Hoxa10 and cyclin D3. Sci. Rep. 2014, 5, 13863. [Google Scholar] [CrossRef] [PubMed]
- Mastroberardino, L.; Spindler, B.; Pfeiffer, R.; Skelly, P.J.; Loffing, J.; Shoemaker, C.B.; Verrey, F. Amino-acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature 1998, 395, 288–291. [Google Scholar] [PubMed]
- Kanai, Y.; Segawa, H.; Miyamoto, K.; Uchino, H.; Takeda, E.; Endou, H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98). J. Biol. Chem. 1998, 273, 23629–23632. [Google Scholar] [CrossRef] [PubMed]
- Kaira, K.; Oriuchi, N.; Imai, H.; Shimizu, K.; Yanagitani, N.; Sunaga, N.; Hisada, T.; Ishizuka, T.; Kanai, Y.; Nakajima, T.; et al. Prognostic significance of l-type amino acid transporter 1 (LAT1) and 4F2 heavy chain (CD98) expression in stage I pulmonary adenocarcinoma. Lung Cancer 2009, 66, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Raoof, D.A.; Thomas, D.G.; Greenson, J.K.; Giordano, T.J.; Robinson, G.S.; Bourner, M.J.; Bauer, C.T.; Orringer, M.B.; Beer, D.G. l-type amino acid transporter-1 overexpression and melphalan sensitivity in Barrett’s adenocarcinoma. Neoplasia 2004, 6, 74–84. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.; Su, L.; Li, P.; Liu, B.; Zhu, Z. Lat-1 functions as a promotor in gastric cancer associated with clinicopathologic features. Biomed. Pharmacother. 2013, 67, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Kaira, K.; Sunose, Y.; Ohshima, Y.; Ishioka, N.S.; Arakawa, K.; Ogawa, T.; Sunaga, N.; Shimizu, K.; Tominaga, H.; Oriuchi, N.; et al. Clinical significance of l-type amino acid transporter 1 expression as a prognostic marker and potential of new targeting therpy in biliary tract cancer. BMC Cancer 2012, 13, 5123–5128. [Google Scholar]
- Fukumoto, S.; Hanazono, K.; Komatsu, T.; Iwano, H.; Kadosawa, T.; Uchide, T. l-type amino acid transporter 1 (lat1) expression in canine mammary gland tumors. J. Vet. Med. Sci. 2013, 75, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Bailey, C.G.; Ng, C.; Tiffen, J.; Thoeng, A.; Minhas, V.; Lehman, M.L.; Hendy, S.C.; Buchanan, G.; Nelson, C.C.; et al. Androgen receptor and nutrient signaling pathways coordinate the demand for increased amino acid transport during prostate cancer progression. Cancer Res. 2011, 71, 7525–7536. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Li, M.; Cox, S.; Davis, M.K.; Tawfik, O.; Paria, B.C.; Das, S.K. HB-EGF directs stromal cell polyploidy and decidualization via cyclin D3 during implantation. Dev. Biol. 2004, 265, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Chrostowski, M.K.; McGonnigal, B.G.; Stabila, J.P.; Padbury, J.F. Lat-1 expression in pre- and post-implantation embryos and placenta. Placenta 2009, 30, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, S.; Hanazono, K.; Fu, D.R.; Endo, Y.; Kadosawa, T.; Iwano, H.; Uchide, T. A new treatment for human malignant melanoma targeting l-type amino acid transporter 1 (lat1): A pilot study in a canine model. Biochem. Biophys. Res. Commun. 2013, 439, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Nawashiro, H.; Otani, N.; Shinomiya, N.; Fukui, S.; Ooigawa, H.; Shima, K.; Matsuo, H.; Kanai, Y.; Endou, H. l-type amino acid transporter 1 as a potential molecular target in human astrocytic tumors. Int. J. Cancer 2006, 119, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Rosario, F.J.; Kanai, Y.; Powell, T.L.; Jansson, T. Mammalian target of rapamycinsignalling modulates amino acid uptake by regulating transporter cell surface abundance in primary human trophoblast cells. J. Physiol. 2013, 591, 609–625. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.A.S.; Russell, D.L.; Ochsner, S.; Hsieh, M.; Doyle, K.H.; Falender, A.E.; Lo, Y.K.; Sharma, S.C. Novel signalling pathways that control ovarian follicular development, ovulation and luteinization. Recent Prog. Horm. Res. 2002, 57, 195–220. [Google Scholar] [CrossRef] [PubMed]
- Roos, S.; Jansson, N.L.; Palmberg, I.; Saljo, K.; Powell, T.L.; Jansson, T. Mammalian target of rapamycin in the human placenta regulates leucine transport and is down regulated in restricted fetal growth. J. Physiol. 2007, 582, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Roos, S.; Kanai, Y.; Prasad, P.D.; Powell, T.L.; Jansson, T. Regulation of placental amino acid transporter activity by mammalian target of rapamycin. Am. J. Physiol. Cell Physiol. 2009, 296, C142–C150. [Google Scholar] [CrossRef] [PubMed]
- Jansson, T.; Scholtbach, B.; Pawell, T.L. Placental transport of leucine and lysine is reduced in intrauterine growth restriction. Pediatr. Res. 1998, 44, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Edinger, A.L.; Thompson, C.B. Akt maintains cell size and survival by increasing mTOR-dependent nutrient uptake. Mol. Biol. Cell. 2002, 13, 2276–2288. [Google Scholar] [CrossRef] [PubMed]
- Chand, A.L.; Legge, M. Amino acid transport system L activity in developing mouse ovarian follicles. Hum. Reprod. 2011, 26, 3102–3108. [Google Scholar] [CrossRef] [PubMed]
- Kimura, F.; Takakura, K.; Takebayashi, K.; Ishikawa, H.; Kasahara, K.; Goto, S.; Noda, Y. Messenger ribonucleic acid for the mouse decidual prolactin is present and induced during in vitro decidualization of endometrial ESC. Gynecol. Endocrinol. 2001, 15, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Shotwell, M.A.; Mattee, P.M.; Jayne, D.W.; Oxender, D.L. Regulation of amino acid transport system L in Chinese Hamster ovary cells. J. Biol. Chem. 1982, 257, 2974–2980. [Google Scholar] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Tan, D.; Ma, J.; Liang, H.; Zhang, Q.; Tan, Y.; Wang, J.; Luo, W. Positive Regulation of Decidualization by l-Type Amino Acid Transporter 1 (lat1) in Pregnant Mice. Nutrients 2016, 8, 704. https://doi.org/10.3390/nu8110704
Wang X, Tan D, Ma J, Liang H, Zhang Q, Tan Y, Wang J, Luo W. Positive Regulation of Decidualization by l-Type Amino Acid Transporter 1 (lat1) in Pregnant Mice. Nutrients. 2016; 8(11):704. https://doi.org/10.3390/nu8110704
Chicago/Turabian StyleWang, Xiaojie, Dongmei Tan, Jing Ma, Hao Liang, Qian Zhang, Yi Tan, Jiang Wang, and Wenping Luo. 2016. "Positive Regulation of Decidualization by l-Type Amino Acid Transporter 1 (lat1) in Pregnant Mice" Nutrients 8, no. 11: 704. https://doi.org/10.3390/nu8110704
APA StyleWang, X., Tan, D., Ma, J., Liang, H., Zhang, Q., Tan, Y., Wang, J., & Luo, W. (2016). Positive Regulation of Decidualization by l-Type Amino Acid Transporter 1 (lat1) in Pregnant Mice. Nutrients, 8(11), 704. https://doi.org/10.3390/nu8110704



