Chemopreventive Agents and Inhibitors of Cancer Hallmarks: May Citrus Offer New Perspectives?
Abstract
:1. Introduction
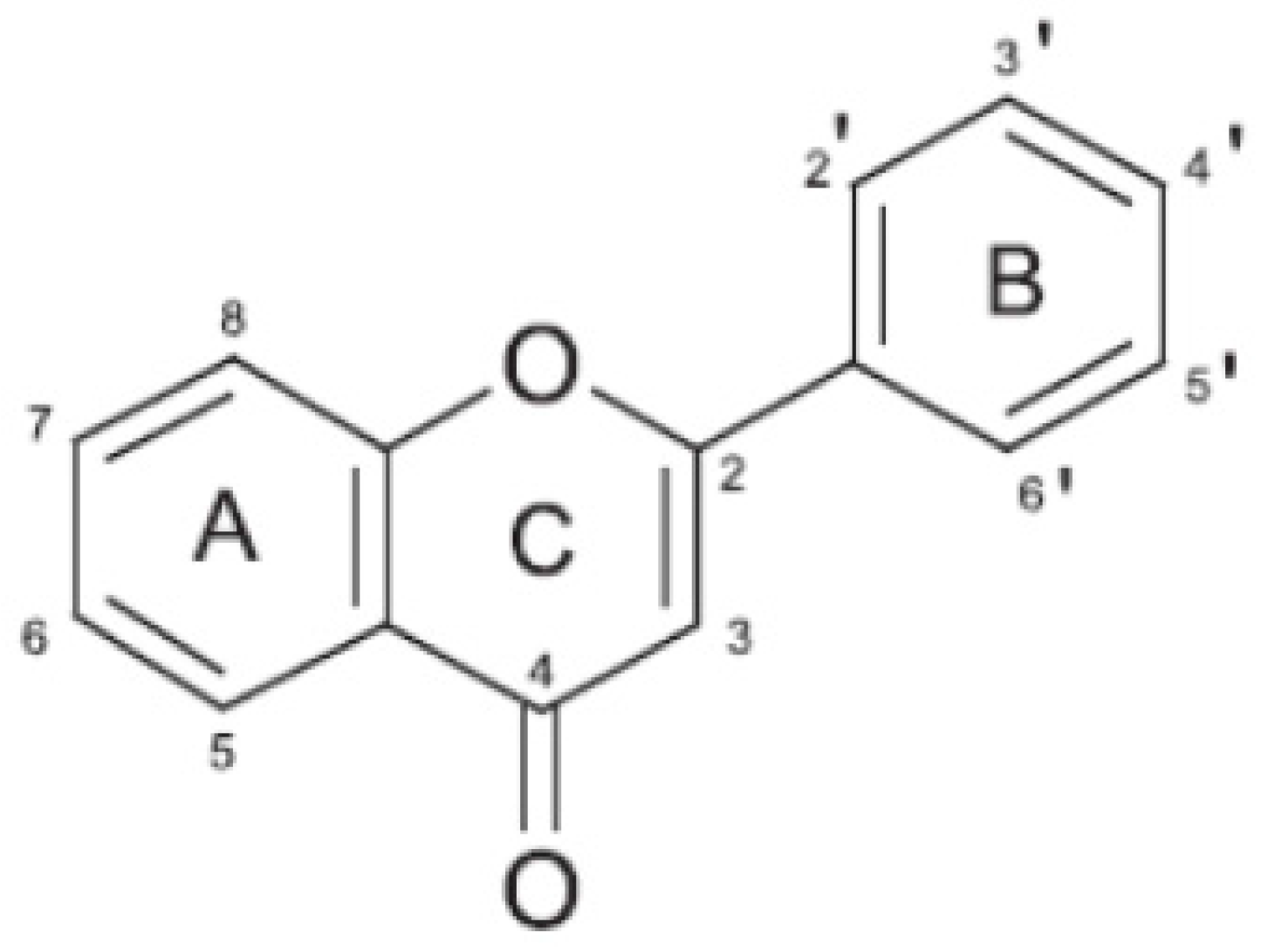
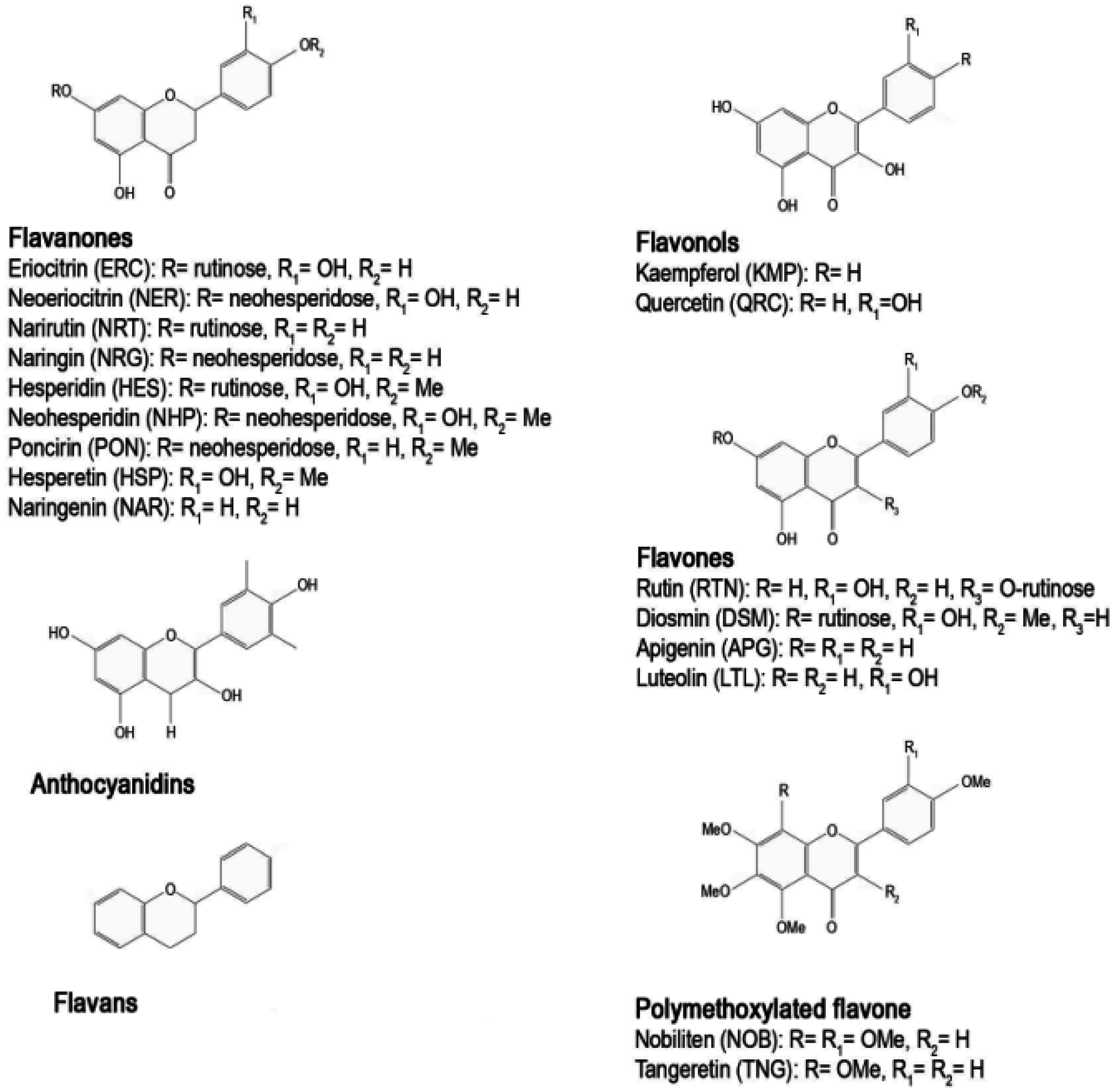
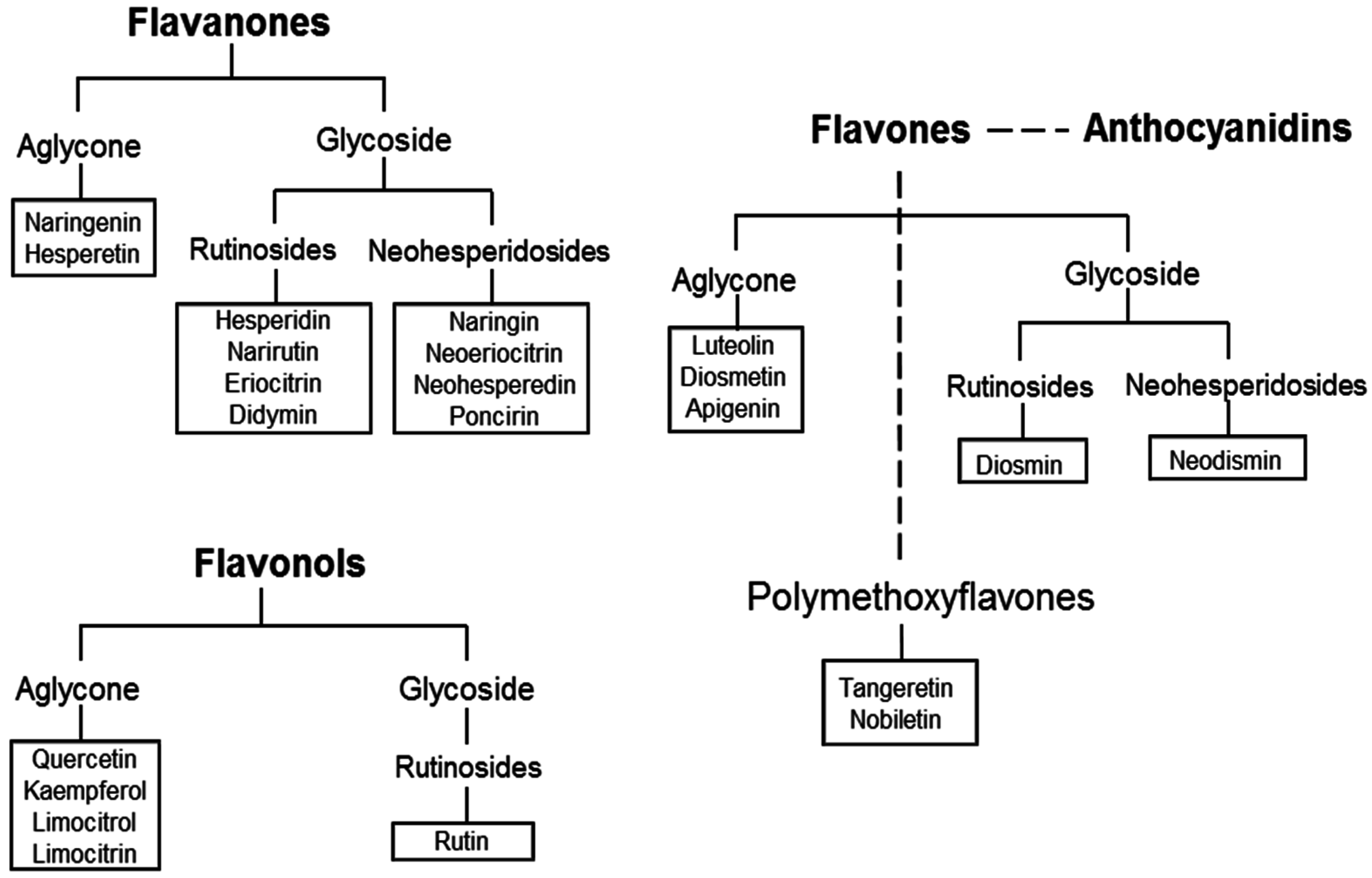
2. The Citrus Flavonoids
3. Preclinical Studies
3.1. Initiation Phase Inhibition by Citrus Flavonoids
3.2. Inhibition of Tumor Development
3.3. Inhibition of Tumor Progression: Focus on Angiogenesis and Metastatization
4. Anti-Cancer Properties of Citrus Juices and Extracts
5. Epidemiological Studies
6. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- International Agency for Research on Cancer (IARC). World Cancer Report 2014. Available online: http://publications.iarc.fr/Non-Series-Publications/World-Cancer-Reports/World-Cancer-Report-2014 (accessed on 5 August 2016).
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Gerber, M. The comprehensive approach to diet: A critical review. J. Nutr. 2001, 131, 3051S–3055S. [Google Scholar] [PubMed]
- Manson, M.M. Cancer prevention—The potential for diet to modulate molecular signalling. Trends Mol. Med. 2003, 9, 11–18. [Google Scholar] [CrossRef]
- Middleton, E.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Tomasetti, C.; Vogelstein, B. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science 2015, 347, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.R.M.R.; Kucuk, O.; Khuri, F.R.; Shin, D.M. Perspectives for cancer prevention with natural compounds. J. Clin. Oncol. 2009, 27, 2712–2725. [Google Scholar] [CrossRef] [PubMed]
- Gullett, N.P.; Ruhul Amin, A.R.; Bayraktar, S.; Pezzuto, J.M.; Shin, D.M.; Khuri, F.R.; Aggarwal, B.B.; Surh, Y.J.; Kucuk, O. Cancer prevention with natural compounds. Semin. Oncol. 2010, 37, 258–281. [Google Scholar] [CrossRef] [PubMed]
- Milner, J.A.; McDonald, S.S.; Anderson, D.E.; Greenwald, P. Molecular targets for nutrients involved with cancer prevention. Nutr. Cancer Int. J. 2001, 41, 1–16. [Google Scholar]
- Surh, Y.J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar]
- Micali, S.; Isgro, G.; Bianchi, G.; Miceli, N.; Calapai, G.; Navarra, M. Cranberry and recurrent cystitis: More than marketing? Crit. Rev. Food Sci. Nutr. 2014, 54, 1063–1075. [Google Scholar]
- Paterniti, I.; Cordaro, M.; Campolo, M.; Siracusa, R.; Cornelius, C.; Navarra, M.; Cuzzocrea, S.; Esposito, E. Neuroprotection by association of palmitoylethanolamide with luteolin in experimental alzheimer’s disease models: The control of neuroinflammation. CNS Neurol. Disord. Drug Targets 2014, 13, 1530–1541. [Google Scholar]
- Nogata, Y.; Sakamoto, K.; Shiratsuchi, H.; Ishii, T.; Yano, M.; Ohta, H. Flavonoid composition of fruit tissues of citrus species. Biosci. Biotechnol. Biochem. 2006, 70, 178–192. [Google Scholar]
- Benavente-Garcia, O.; Castillo, J. Update on uses and properties of citrus flavonoids: New findings in anticancer, cardiovascular, and anti-inflammatory activity. J. Agric. Food Chem. 2008, 56, 6185–6205. [Google Scholar]
- Pouget, C.; Lauthier, F.; Simon, A.; Fagnere, C.; Basly, J.P.; Delage, C.; Chulia, A.J. Flavonoids: Structural requirements for antiproliferative activity on breast cancer cells. Bioorg. Med. Chem. Lett. 2001, 11, 3095–3097. [Google Scholar]
- Yanez, J.; Vicente, V.; Alcaraz, M.; Castillo, J.; Benavente-Garcia, O.; Canteras, M.; Teruel, J.A.L. Cytotoxicity and antiproliferative activities of several phenolic compounds against three melanocytes cell lines: Relationship between structure and activity. Nutr. Cancer Int. J. 2004, 49, 191–199. [Google Scholar]
- Rodriguez, J.; Yanez, J.; Vicente, V.; Alcaraz, M.; Benavente-Garcia, O.; Castillo, J.; Lorente, J.; Lozano, J.A. Effects of several flavonoids on the growth of B16F10 and SK-MEL-1 melanoma cell lines: Relationship between structure and activity. Melanoma Res. 2002, 12, 99–107. [Google Scholar]
- Martinez, C.; Yanez, J.; Vicente, V.; Alcaraz, M.; Benavente-Garcia, O.; Castillo, J.; Lorente, J.; Lozano, J.A. Effects of several polyhydroxylated flavonoids on the growth of B16F10 melanoma and melan-a melanocyte cell lines: Influence of the sequential oxidation state of the flavonoid skeleton. Melanoma Res. 2003, 13, 3–9. [Google Scholar]
- Hursting, S.D.; Cantwell, M.M.; Sansbury, L.B.; Forman, M.R. Nutrition and cancer prevention: Targets, strategies, and the importance of early life interventions. In Proceedings of the 57th Nestlé Nutrition Workshop, Pediatric Program, Half Moon Bay, San Francisco, CA, USA, 24–28 May 2005; Lucas, A., Sampson, H.A., Eds.; Nestec Ltd.: Basel, Switzerland, 2006; pp. 153–202. [Google Scholar]
- Mandl, J.; Szarka, A.; Banhegyi, G. Vitamin C: Update on physiology and pharmacology. Br. J. Pharmacol. 2009, 157, 1097–1110. [Google Scholar]
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar]
- Manthey, J.A.; Grohmann, K.; Guthrie, N. Biological properties of citrus flavonoids pertaining to cancer and inflammation. Curr. Med. Chem. 2001, 8, 135–153. [Google Scholar]
- Nyberg, F.; Hou, S.M.; Pershagen, G.; Lambert, B. Dietary fruit and vegetables protect against somatic mutation in vivo, but low or high intake of carotenoids does not. Carcinogenesis 2003, 24, 689–696. [Google Scholar]
- Calomme, M.; Pieters, L.; Vlietinck, A.; Vanden Berghe, D. Inhibition of bacterial mutagenesis by citrus flavonoids. Planta Med. 1996, 62, 222–226. [Google Scholar]
- Kootstra, A. Protection from UV-B-induced DNA damage by flavonoids. Plant Mol. Biol. 1994, 26, 771–774. [Google Scholar]
- Barcelos, G.R.; Angeli, J.P.; Serpeloni, J.M.; Grotto, D.; Rocha, B.A.; Bastos, J.K.; Knasmuller, S.; Junior, F.B. Quercetin protects human-derived liver cells against mercury-induced DNA-damage and alterations of the redox status. Mutat. Res. 2011, 726, 109–115. [Google Scholar]
- Gao, K.; Henning, S.M.; Niu, Y.T.; Youssefian, A.A.; Seeram, N.P.; Xu, A.L.; Heber, D. The citrus flavonoid naringenin stimulates DNA repair in prostate cancer cells. J. Nutr. Biochem. 2006, 17, 89–95. [Google Scholar]
- Arul, D.; Subramanian, P. Inhibitory effect of naringenin (Citrus flavonone) on N-nitrosodiethylamine induced hepatocarcinogenesis in rats. Biochem. Biophys. Res. Commun. 2013, 434, 203–209. [Google Scholar]
- Subramanian, P.; Arul, D. Attenuation of ndea-induced hepatocarcinogenesis by naringenin in rats. Cell Biochem. Funct. 2013, 31, 511–517. [Google Scholar]
- Alvarez-Gonzalez, I.; Madrigal-Bujaidar, E.; Dorado, V.; Espinosa-Aguirre, J.J. Inhibitory effect of naringin on the micronuclei induced by ifosfamide in mouse, and evaluation of its modulatory effect on the CYP3A subfamily. Mutat. Res. 2001, 480, 171–178. [Google Scholar]
- Carino-Cortes, R.; Alvarez-Gonzalez, I.; Martino-Roaro, L.; Madrigal-Bujaidar, E. Effect of naringin on the DNA damage induced by daunorubicin in mouse hepatocytes and cardiocytes. Biol. Pharm. Bull. 2010, 33, 697–701. [Google Scholar]
- Sequetto, P.L.; Oliveira, T.T.; Maldonado, I.R.; Augusto, L.E.; Mello, V.J.; Pizziolo, V.R.; Almeida, M.R.; Silva, M.E.; Novaes, R.D. Naringin accelerates the regression of pre-neoplastic lesions and the colorectal structural reorganization in a murine model of chemical carcinogenesis. Food Chem. Toxicol. 2014, 64, 200–209. [Google Scholar]
- Ahmadi, A.; Hosseinimehr, S.J.; Naghshvar, F.; Hajir, E.; Ghahremani, M. Chemoprotective effects of hesperidin against genotoxicity induced by cyclophosphamide in mice bone marrow cells. Arch. Pharm. Res. 2008, 31, 794–797. [Google Scholar]
- Choi, J.S.; Park, K.Y.; Moon, S.H.; Rhee, S.H.; Young, H.S. Antimutagenic effect of plant flavonoids in the salmonella assay system. Arch. Pharm. Res. 1994, 17, 71–75. [Google Scholar]
- Kao, Y.C.; Zhou, C.; Sherman, M.; Laughton, C.A.; Chen, S. Molecular basis of the inhibition of human aromatase (estrogen synthetase) by flavone and isoflavone phytoestrogens: A site-directed mutagenesis study. Environ. Health Perspect. 1998, 106, 85–92. [Google Scholar]
- Harris, R.M.; Wood, D.M.; Bottomley, L.; Blagg, S.; Owen, K.; Hughes, P.J.; Waring, R.H.; Kirk, C.J. Phytoestrogens are potent inhibitors of estrogen sulfation: Implications for breast cancer risk and treatment. J. Clin. Endocrinol. Metab. 2004, 89, 1779–1787. [Google Scholar]
- Bear, W.L.; Teel, R.W. Effects of Citrus flavonoids on the mutagenicity of heterocyclic amines and on cytochrome P450 1A2 activity. Anticancer Res. 2000, 20, 3609–3614. [Google Scholar]
- Van Dross, R.; Xue, Y.; Knudson, A.; Pelling, J.C. The chemopreventive bioflavonoid apigenin modulates signal transduction pathways in keratinocyte and colon carcinoma cell lines. J. Nutr. 2003, 133, 3800S–3804S. [Google Scholar]
- Khan, T.H.; Jahangir, T.; Prasad, L.; Sultana, S. Inhibitory effect of apigenin on benzo(a)pyrene-mediated genotoxicity in swiss albino mice. J. Pharm. Pharmacol. 2006, 58, 1655–1660. [Google Scholar]
- Myhrstad, M.C.; Carlsen, H.; Nordstrom, O.; Blomhoff, R.; Moskaug, J.O. Flavonoids increase the intracellular glutathione level by transactivation of the gamma-glutamylcysteine synthetase catalytical subunit promoter. Free Radic. Biol. Med. 2002, 32, 386–393. [Google Scholar]
- Wei, H.; Tye, L.; Bresnick, E.; Birt, D.F. Inhibitory effect of apigenin, a plant flavonoid, on epidermal ornithine decarboxylase and skin tumor promotion in mice. Cancer Res. 1990, 50, 499–502. [Google Scholar]
- Birt, D.F.; Mitchell, D.; Gold, B.; Pour, P.; Pinch, H.C. Inhibition of ultraviolet light induced skin carcinogenesis in SKH-1 mice by apigenin, a plant flavonoid. Anticancer Res. 1997, 17, 85–91. [Google Scholar]
- Leonardi, T.; Vanamala, J.; Taddeo, S.S.; Davidson, L.A.; Murphy, M.E.; Patil, B.S.; Wang, N.; Carroll, R.J.; Chapkin, R.S.; Lupton, J.R.; et al. Apigenin and naringenin suppress colon carcinogenesis through the aberrant crypt stage in azoxymethane-treated rats. Exp. Biol. Med. (Maywood) 2010, 235, 710–717. [Google Scholar]
- Nandakumar, N.; Balasubramanian, M.P. Hesperidin protects renal and hepatic tissues against free radical-mediated oxidative stress during DMBA-induced experimental breast cancer. J. Environ. Pathol. Toxicol. Oncol. 2011, 30, 283–300. [Google Scholar]
- Aranganathan, S.; Selvam, J.P.; Sangeetha, N.; Nalini, N. Modulatory efficacy of hesperetin (Citrus flavanone) on xenobiotic-metabolizing enzymes during 1,2-dimethylhydrazine-induced colon carcinogenesis. Chem. Biol. Interact. 2009, 180, 254–261. [Google Scholar]
- Lakshmi, A.; Subramanian, S. Chemotherapeutic effect of tangeretin, a polymethoxylated flavone studied in 7,12-dimethylbenz(a)anthracene induced mammary carcinoma in experimental rats. Biochimie 2014, 99, 96–109. [Google Scholar]
- Murakami, A.; Nakamura, Y.; Torikai, K.; Tanaka, T.; Koshiba, T.; Koshimizu, K.; Kuwahara, S.; Takahashi, Y.; Ogawa, K.; Yano, M.; et al. Inhibitory effect of Citrus nobiletin on phorbol ester-induced skin inflammation, oxidative stress, and tumor promotion in mice. Cancer Res. 2000, 60, 5059–5066. [Google Scholar]
- Kandaswami, C.; Perkins, E.; Soloniuk, D.S.; Drzewiecki, G.; Middleton, E., Jr. Antiproliferative effects of citrus flavonoids on a human squamous cell carcinoma in vitro. Cancer Lett. 1991, 56, 147–152. [Google Scholar]
- Sugiyama, S.; Umehara, K.; Kuroyanagi, M.; Ueno, A.; Taki, T. Studies on the differentiation inducers of myeloid leukemic cells from Citrus species. Chem. Pharm. Bull. (Tokyo) 1993, 41, 714–719. [Google Scholar]
- Hirano, T.; Abe, K.; Gotoh, M.; Oka, K. Citrus flavone tangeretin inhibits leukaemic HL-60 cell growth partially through induction of apoptosis with less cytotoxicity on normal lymphocytes. Br. J. Cancer 1995, 72, 1380–1388. [Google Scholar]
- Morley, K.L.; Ferguson, P.J.; Koropatnick, J. Tangeretin and nobiletin induce G1 cell cycle arrest but not apoptosis in human breast and colon cancer cells. Cancer Lett. 2007, 251, 168–178. [Google Scholar]
- Pan, M.H.; Chen, W.J.; Lin-Shiau, S.Y.; Ho, C.T.; Lin, J.K. Tangeretin induces cell-cycle G1 arrest through inhibiting cyclin-dependent kinases 2 and 4 activities as well as elevating cdk inhibitors p21 and p27 in human colorectal carcinoma cells. Carcinogenesis 2002, 23, 1677–1684. [Google Scholar]
- Yoshimizu, N.; Otani, Y.; Saikawa, Y.; Kubota, T.; Yoshida, M.; Furukawa, T.; Kumai, K.; Kameyama, K.; Fujii, M.; Yano, M.; et al. Anti-tumour effects of nobiletin, a Citrus flavonoid, on gastric cancer include: Antiproliferative effects, induction of apoptosis and cell cycle deregulation. Aliment. Pharmacol. Ther. 2004, 20 (Suppl. 1), 95–101. [Google Scholar]
- Fan, K.; Kurihara, N.; Abe, S.; Ho, C.T.; Ghai, G.; Yang, K. Chemopreventive effects of orange peel extract (OPE) I: Ope inhibits intestinal tumor growth in Apc(min/+) mice. J. Med. Food 2007, 10, 11–17. [Google Scholar]
- Abe, S.; Fan, K.; Ho, C.T.; Ghai, G.; Yang, K. Chemopreventive effects of orange peel extract (OPE) II: OPE inhibits atypical hyperplastic lesions in rodent mammary gland. J. Med. Food 2007, 10, 18–24. [Google Scholar]
- Van Slambrouck, S.; Parmar, V.S.; Sharma, S.K.; de Bondt, B.; Fore, F.; Coopman, P.; Vanhoecke, B.W.; Boterberg, T.; Depypere, H.T.; Leclercq, G.; et al. Tangeretin inhibits extracellular-signal-regulated kinase (ERK) phosphorylation. FEBS Lett. 2005, 579, 1665–1669. [Google Scholar]
- Akao, Y.; Itoh, T.; Ohguchi, K.; Iinuma, M.; Nozawa, Y. Interactive effects of polymethoxy flavones from Citrus on cell growth inhibition in human neuroblastoma SH-SY5Y cells. Bioorg. Med. Chem. 2008, 16, 2803–2810. [Google Scholar]
- Rooprai, H.K.; Kandanearatchi, A.; Maidment, S.L.; Christidou, M.; Trillo-Pazos, G.; Dexter, D.T.; Rucklidge, G.J.; Widmer, W.; Pilkington, G.J. Evaluation of the effects of swainsonine, captopril, tangeretin and nobiletin on the biological behaviour of brain tumour cells in vitro. Neuropathol. Appl. Neurobiol. 2001, 27, 29–39. [Google Scholar]
- Arafa el, S.A.; Zhu, Q.; Barakat, B.M.; Wani, G.; Zhao, Q.; El-Mahdy, M.A.; Wani, A.A. Tangeretin sensitizes cisplatin-resistant human ovarian cancer cells through downregulation of phosphoinositide 3-kinase/Akt signaling pathway. Cancer Res. 2009, 69, 8910–8917. [Google Scholar]
- Dong, Y.; Cao, A.L.; Shi, J.R.; Yin, P.H.; Wang, L.; Ji, G.; Xie, J.Q.; Wu, D.Z. Tangeretin, a citrus polymethoxyflavonoid, induces apoptosis of human gastric cancer AGS cells through extrinsic and intrinsic signaling pathways. Oncol. Rep. 2014, 31, 1788–1794. [Google Scholar]
- Tang, M.; Ogawa, K.; Asamoto, M.; Hokaiwado, N.; Seeni, A.; Suzuki, S.; Takahashi, S.; Tanaka, T.; Ichikawa, K.; Shirai, T. Protective effects of Citrus nobiletin and auraptene in transgenic rats developing adenocarcinoma of the prostate (TRAP) and human prostate carcinoma cells. Cancer Sci. 2007, 98, 471–477. [Google Scholar]
- Tang, M.X.; Ogawa, K.; Asamoto, M.; Chewonarin, T.; Suzuki, S.; Tanaka, T.; Shirai, T. Effects of nobiletin on PhIP-induced prostate and colon carcinogenesis in F344 rats. Nutr. Cancer 2011, 63, 227–233. [Google Scholar]
- Suzuki, R.; Kohno, H.; Murakami, A.; Koshimizu, K.; Ohigashi, H.; Yano, M.; Tokuda, H.; Nishino, H.; Tanaka, T. Citrus nobiletin inhibits azoxymethane-induced large bowel carcinogenesis in rats. Biofactors 2004, 22, 111–114. [Google Scholar]
- Miyamoto, S.; Yasui, Y.; Ohigashi, H.; Tanaka, T.; Murakami, A. Dietary flavonoids suppress azoxymethane-induced colonic preneoplastic lesions in male C57BL/KSJ-DB/DB mice. Chem. Biol. Interact. 2010, 183, 276–283. [Google Scholar]
- Miyamoto, S.; Yasui, Y.; Tanaka, T.; Ohigashi, H.; Murakami, A. Suppressive effects of nobiletin on hyperleptinemia and colitis-related colon carcinogenesis in male ICR mice. Carcinogenesis 2008, 29, 1057–1063. [Google Scholar]
- Luo, G.; Guan, X.; Zhou, L. Apoptotic effect of Citrus fruit extract nobiletin on lung cancer cell line a549 in vitro and in vivo. Cancer Biol. Ther. 2008, 7, 966–973. [Google Scholar]
- Ohnishi, H.; Asamoto, M.; Tujimura, K.; Hokaiwado, N.; Takahashi, S.; Ogawa, K.; Kuribayashi, M.; Ogiso, T.; Okuyama, H.; Shirai, T. Inhibition of cell proliferation by nobiletin, a dietary phytochemical, associated with apoptosis and characteristic gene expression, but lack of effect on early rat hepatocarcinogenesis in vivo. Cancer Sci. 2004, 95, 936–942. [Google Scholar]
- Aoki, K.; Yokosuka, A.; Mimaki, Y.; Fukunaga, K.; Yamakuni, T. Nobiletin induces inhibitions of RAS activity and mitogen-activated protein kinase kinase/extracellular signal-regulated kinase signaling to suppress cell proliferation in C6 rat glioma cells. Biol. Pharm. Bull. 2013, 36, 540–547. [Google Scholar]
- Lien, L.M.; Wang, M.J.; Chen, R.J.; Chiu, H.C.; Wu, J.L.; Shen, M.Y.; Chou, D.S.; Sheu, J.R.; Lin, K.H.; Lu, W.J. Nobiletin, a polymethoxylated flavone, inhibits glioma cell growth and migration via arresting cell cycle and suppressing MAPK and Akt pathways. Phytother. Res. 2016, 30, 214–221. [Google Scholar]
- Moon, J.Y.; Cho, M.; Ahn, K.S.; Cho, S.K. Nobiletin induces apoptosis and potentiates the effects of the anticancer drug 5-fluorouracil in p53-mutated SNU-16 human gastric cancer cells. Nutr. Cancer Int. J. 2013, 65, 286–295. [Google Scholar]
- Hsiao, P.C.; Lee, W.J.; Yang, S.F.; Tan, P.; Chen, H.Y.; Lee, L.M.; Chang, J.L.; Lai, G.M.; Chow, J.M.; Chien, M.H. Nobiletin suppresses the proliferation and induces apoptosis involving MAPKs and caspase-8/-9/-3 signals in human acute myeloid leukemia cells. Tumor Biol. 2014, 35, 11903–11911. [Google Scholar]
- Wu, X.; Song, M.Y.; Wang, M.Q.; Zheng, J.K.; Gao, Z.L.; Xu, F.; Zhang, G.D.; Xiao, H. Chemopreventive effects of nobiletin and its colonic metabolites on colon carcinogenesis. Mol. Nutr. Food Res. 2015, 59, 2383–2394. [Google Scholar]
- Choi, E.J.; Kim, G.H. Apigenin induces apoptosis through a mitochondria/caspase-pathway in human breast cancer MDA-MB-453 cells. J. Clin. Biochem. Nutr. 2009, 44, 260–265. [Google Scholar]
- Seo, H.S.; Choi, H.S.; Kim, S.R.; Choi, Y.K.; Woo, S.M.; Shin, I.; Woo, J.K.; Park, S.Y.; Shin, Y.C.; Ko, S.K. Apigenin induces apoptosis via extrinsic pathway, inducing p53 and inhibiting STAT3 and NFκB signaling in HER2-overexpressing breast cancer cells. Mol. Cell. Biochem. 2012, 366, 319–334. [Google Scholar]
- Way, T.D.; Kao, M.C.; Lin, J.K. Apigenin induces apoptosis through proteasomal degradation of HER2/NEU in HER2/NEU-overexpressing breast cancer cells via the phosphatidylinositol 3-kinase/Akt-dependent pathway. J. Biol. Chem. 2004, 279, 4479–4489. [Google Scholar]
- Agrawal, A.; Yang, J.; Murphy, R.F.; Agrawal, D.K. Regulation of the p14ARF-MDM2-p53 pathway: An overview in breast cancer. Exp. Mol. Pathol. 2006, 81, 115–122. [Google Scholar]
- Takagaki, N.; Sowa, Y.; Oki, T.; Nakanishi, R.; Yogosawa, S.; Sakai, T. Apigenin induces cell cycle arrest and p21/WAF1 expression in a p53-independent pathway. Int. J. Oncol. 2005, 26, 185–189. [Google Scholar]
- Lepley, D.M.; Pelling, J.C. Induction of p21/WAF1 and G1 cell-cycle arrest by the chemopreventive agent apigenin. Mol. Carcinog. 1997, 19, 74–82. [Google Scholar]
- Plaumann, B.; Fritsche, M.; Rimpler, H.; Brandner, G.; Hess, R.D. Flavonoids activate wild-type p53. Oncogene 1996, 13, 1605–1614. [Google Scholar]
- Yin, F.; Giuliano, A.E.; Law, R.E.; Van Herle, A.J. Apigenin inhibits growth and induces G2/M arrest by modulating cyclin-CDK regulators and ERK MAP Kinase activation in breast carcinoma cells. Anticancer Res. 2001, 21, 413–420. [Google Scholar]
- King, J.C.; Lu, Q.Y.; Li, G.; Moro, A.; Takahashi, H.; Chen, M.; Go, V.L.; Reber, H.A.; Eibl, G.; Hines, O.J. Evidence for activation of mutated p53 by apigenin in human pancreatic cancer. Biochim. Biophys. Acta 2012, 1823, 593–604. [Google Scholar]
- Ujiki, M.B.; Ding, X.Z.; Salabat, M.R.; Bentrem, D.J.; Golkar, L.; Milam, B.; Talamonti, M.S.; Bell, R.H., Jr.; Iwamura, T.; Adrian, T.E. Apigenin inhibits pancreatic cancer cell proliferation through G2/M cell cycle arrest. Mol. Cancer 2006, 5, 76. [Google Scholar] [CrossRef]
- Johnson, J.L.; de Mejia, E.G. Flavonoid apigenin modified gene expression associated with inflammation and cancer and induced apoptosis in human pancreatic cancer cells through inhibition of GSK-3 beta/NFκB signaling cascade. Mol. Nutr. Food Res. 2013, 57, 2112–2127. [Google Scholar]
- Gupta, S.; Afaq, F.; Mukhtar, H. Involvement of nuclear factor-kappa B, BAX and BCL-2 in induction of cell cycle arrest and apoptosis by apigenin in human prostate carcinoma cells. Oncogene 2002, 21, 3727–3738. [Google Scholar]
- Shukla, S.; Gupta, S. Molecular mechanisms for apigenin-induced cell-cycle arrest and apoptosis of hormone refractory human prostate carcinoma DU145 cells. Mol. Carcinog. 2004, 39, 114–126. [Google Scholar]
- Wang, W.; Heideman, L.; Chung, C.S.; Pelling, J.C.; Koehler, K.J.; Birt, D.F. Cell-cycle arrest at G2/M and growth inhibition by apigenin in human colon carcinoma cell lines. Mol. Carcinog. 2000, 28, 102–110. [Google Scholar]
- Zhong, Y.; Krisanapun, C.; Lee, S.H.; Nualsanit, T.; Sams, C.; Peungvicha, P.; Baek, S.J. Molecular targets of apigenin in colorectal cancer cells: Involvement of p21, NAG-1 and p53. Eur. J. Cancer 2010, 46, 3365–3374. [Google Scholar]
- Shukla, S.; Gupta, S. Molecular targets for apigenin-induced cell cycle arrest and apoptosis in prostate cancer cell xenograft. Mol. Cancer Ther. 2006, 5, 843–852. [Google Scholar]
- Das, A.; Banik, N.L.; Ray, S.K. Mechanism of apoptosis with the involvement of calpain and caspase cascades in human malignant neuroblastoma SH-SY5Y cells exposed to flavonoids. Int. J. Cancer 2006, 119, 2575–2585. [Google Scholar]
- Torkin, R.; Lavoie, J.F.; Kaplan, D.R.; Yeger, H. Induction of caspase-dependent, p53-mediated apoptosis by apigenin in human neuroblastoma. Mol. Cancer Ther. 2005, 4, 1–11. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar]
- Kuntz, S.; Wenzel, U.; Daniel, H. Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer cell lines. Eur. J. Nutr. 1999, 38, 133–142. [Google Scholar]
- Dung, T.D.; Day, C.H.; Binh, T.V.; Lin, C.H.; Hsu, H.H.; Su, C.C.; Lin, Y.M.; Tsai, F.J.; Kuo, W.W.; Chen, L.M.; et al. PP2A mediates diosmin p53 activation to block HA22T cell proliferation and tumor growth in xenografted nude mice through PI3K-Akt-MDM2 signaling suppression. Food Chem. Toxicol. 2012, 50, 1802–1810. [Google Scholar]
- Lewinska, A.; Siwak, J.; Rzeszutek, I.; Wnuk, M. Diosmin induces genotoxicity and apoptosis in DU145 prostate cancer cell line. Toxicol. Vitr. 2015, 29, 417–425. [Google Scholar]
- Tanaka, T.; Makita, H.; Kawabata, K.; Mori, H.; Kakumoto, M.; Satoh, K.; Hara, A.; Sumida, T.; Fukutani, K.; Tanaka, T.; et al. Modulation of N-methyl-N-amylnitrosamine-induced rat oesophageal tumourigenesis by dietary feeding of diosmin and hesperidin, both alone and in combination. Carcinogenesis 1997, 18, 761–769. [Google Scholar]
- Tanaka, T.; Makita, H.; Ohnishi, M.; Mori, H.; Satoh, K.; Hara, A.; Sumida, T.; Fukutani, K.; Tanaka, T.; Ogawa, H. Chemoprevention of 4-nitroquinoline 1-oxide-induced oral carcinogenesis in rats by flavonoids diosmin and hesperidin, each alone and in combination. Cancer Res. 1997, 57, 246–252. [Google Scholar]
- Yang, M.; Tanaka, T.; Hirose, Y.; Deguchi, T.; Mori, H.; Kawada, Y. Chemopreventive effects of diosmin and hesperidin on N-butyl-N-(4-hydroxybutyl)nitrosamine-induced urinary-bladder carcinogenesis in male ICR mice. Int. J. Cancer 1997, 73, 719–724. [Google Scholar]
- Tanaka, T.; Makita, H.; Kawabata, K.; Mori, H.; Kakumoto, M.; Satoh, K.; Hara, A.; Sumida, T.; Tanaka, T.; Ogawa, H. Chemoprevention of azoxymethane-induced rat colon carcinogenesis by the naturally occurring flavonoids, diosmin and hesperidin. Carcinogenesis 1997, 18, 957–965. [Google Scholar]
- Rubio, S.; Quintana, J.; Lopez, M.; Eiroa, J.L.; Triana, J.; Estevez, F. Phenylbenzopyrones structure-activity studies identify betuletol derivatives as potential antitumoral agents. Eur. J. Pharmacol. 2006, 548, 9–20. [Google Scholar]
- Duraj, J.; Zazrivcova, K.; Bodo, J.; Sulikova, M.; Sedlak, J. Flavonoid quercetin, but not apigenin or luteolin, induced apoptosis in human myeloid leukemia cells and their resistant variants. Neoplasma 2005, 52, 273–279. [Google Scholar]
- Piantelli, M.; Rinelli, A.; Macri, E.; Maggiano, N.; Larocca, L.M.; Scerrati, M.; Roselli, R.; Iacoangeli, M.; Scambia, G.; Capelli, A.; et al. Type II estrogen binding sites and antiproliferative activity of quercetin in human meningiomas. Cancer 1993, 71, 193–198. [Google Scholar]
- Kuo, S.M. Antiproliferative potency of structurally distinct dietary flavonoids on human colon cancer cells. Cancer Lett. 1996, 110, 41–48. [Google Scholar]
- Araujo, J.R.; Goncalves, P.; Martel, F. Chemopreventive effect of dietary polyphenols in colorectal cancer cell lines. Nutr. Res. 2011, 31, 77–87. [Google Scholar]
- Paliwal, S.; Sundaram, J.; Mitragotri, S. Induction of cancer-specific cytotoxicity towards human prostate and skin cells using quercetin and ultrasound. Br. J. Cancer 2005, 92, 499–502. [Google Scholar]
- Huang, H.C.; Lin, C.L.; Lin, J.K. 1,2,3,4,6-penta-O-galloyl-beta-d-glucose, quercetin, curcumin and lycopene induce cell-cycle arrest in MDA-MB-231 and BT474 cells through downregulation of SKP2 protein. J. Agric. Food Chem. 2011, 59, 6765–6775. [Google Scholar]
- Li, J.; Zhu, F.; Lubet, R.A.; De Luca, A.; Grubbs, C.; Ericson, M.E.; D’Alessio, A.; Normanno, N.; Dong, Z.; Bode, A.M. Quercetin-3-methyl ether inhibits lapatinib-sensitive and -resistant breast cancer cell growth by inducing G(2)/M arrest and apoptosis. Mol. Carcinog. 2011, 52, 134–143. [Google Scholar]
- Staedler, D.; Idrizi, E.; Kenzaoui, B.H.; Juillerat-Jeanneret, L. Drug combinations with quercetin: Doxorubicin plus quercetin in human breast cancer cells. Cancer Chemother. Pharmacol. 2011, 68, 1161–1172. [Google Scholar]
- Scambia, G.; Ranelletti, F.O.; Panici, P.B.; De Vincenzo, R.; Bonanno, G.; Ferrandina, G.; Piantelli, M.; Bussa, S.; Rumi, C.; Cianfriglia, M.; et al. Quercetin potentiates the effect of adriamycin in a multidrug-resistant MCF-7 human breast-cancer cell line: P-glycoprotein as a possible target. Cancer Chemother. Pharmacol. 1994, 34, 459–464. [Google Scholar]
- Critchfield, J.W.; Welsh, C.J.; Phang, J.M.; Yeh, G.C. Modulation of adriamycin accumulation and efflux by flavonoids in HCT-15 colon cells. Activation of p-glycoprotein as a putative mechanism. Biochem. Pharmacol. 1994, 48, 1437–1445. [Google Scholar]
- Kanno, S.; Tomizawa, A.; Hiura, T.; Osanai, Y.; Shouji, A.; Ujibe, M.; Ohtake, T.; Kimura, K.; Ishikawa, M. Inhibitory effects of naringenin on tumor growth in human cancer cell lines and sarcoma S-180-implanted mice. Biol. Pharm. Bull. 2005, 28, 527–530. [Google Scholar]
- Kanno, S.; Tomizawa, A.; Ohtake, T.; Koiwai, K.; Ujibe, M.; Ishikawa, M. Naringenin-induced apoptosis via activation of NF-κB and necrosis involving the loss of ATP in human promyeloleukemia HL-60 cells. Toxicol. Lett. 2006, 166, 131–139. [Google Scholar]
- Frydoonfar, H.R.; McGrath, D.R.; Spigelman, A.D. The variable effect on proliferation of a colon cancer cell line by the Citrus fruit flavonoid naringenin. Colorectal Dis. 2003, 5, 149–152. [Google Scholar]
- Harmon, A.W.; Patel, Y.M. Naringenin inhibits glucose uptake in MCF-7 breast cancer cells: A mechanism for impaired cellular proliferation. Breast Cancer Res. Treat. 2004, 85, 103–110. [Google Scholar]
- Park, J.H.; Jin, C.Y.; Lee, B.K.; Kim, G.Y.; Choi, Y.H.; Jeong, Y.K. Naringenin induces apoptosis through downregulation of AKT and caspase-3 activation in human leukemia THP-1 cells. Food Chem. Toxicol. 2008, 46, 3684–3690. [Google Scholar]
- Shi, D.; Xu, Y.; Du, X.; Chen, X.; Zhang, X.; Lou, J.; Li, M.; Zhuo, J. Co-treatment of THP-1 cells with naringenin and curcumin induces cell cycle arrest and apoptosis via numerous pathways. Mol. Med. Rep. 2015, 12, 8223–8228. [Google Scholar]
- Ahamad, M.S.; Siddiqui, S.; Jafri, A.; Ahmad, S.; Afzal, M.; Arshad, M. Induction of apoptosis and antiproliferative activity of naringenin in human epidermoid carcinoma cell through ROS generation and cell cycle arrest. PLoS ONE 2014, 9, e110003. [Google Scholar]
- Naoghare, P.K.; Ki, H.A.; Paek, S.M.; Tak, Y.K.; Suh, Y.G.; Kim, S.G.; Leeb, K.H.; Song, J.M. Simultaneous quantitative monitoring of drug-induced caspase cascade pathways in carcinoma cells. Integr. Biol. 2010, 2, 46–57. [Google Scholar]
- Jin, C.Y.; Park, C.; Hwang, H.J.; Kim, G.Y.; Choi, B.T.; Kim, W.J.; Choi, Y.H. Naringenin up-regulates the expression of death receptor 5 and enhances TRAIL-induced apoptosis in human lung cancer A549 cells. Mol. Nutr. Food Res. 2011, 55, 300–309. [Google Scholar]
- Zhang, S.Z.; Yang, X.N.; Morris, M.E. Flavonoids are inhibitors of breast cancer resistance protein (ABCG2)-mediated transport. Mol. Pharmacol. 2004, 65, 1208–1216. [Google Scholar]
- Zhang, S.Z.; Yang, X.N.; Coburn, R.A.; Morris, M.E. Structure activity relationships and quantitative structure activity relationships for the flavonoid-mediated inhibition of breast cancer resistance protein. Biochem. Pharmacol. 2005, 70, 627–639. [Google Scholar]
- Romiti, N.; Tramonti, G.; Donati, A.; Chieli, E. Effects of grapefruit juice on the multidrug transporter p-glycoprotein in the human proximal tubular cell line HK-2. Life Sci. 2004, 76, 293–302. [Google Scholar]
- Tsai, T.H.; Lee, C.H.; Yeh, P.H. Effect of p-glycoprotein modulators on the pharmacokinetics of camptothecin using microdialysis. Br. J. Pharmacol. 2001, 134, 1245–1252. [Google Scholar]
- De Castro, W.V.; Mertens-Talcott, S.; Derendorf, H.; Butterweck, V. Effect of grapefruit juice, naringin, naringenin, and bergamottin on the intestinal carrier-mediated transport of talinolol in rats. J. Agric. Food Chem. 2008, 56, 4840–4845. [Google Scholar]
- Zhang, F.Y.; Du, G.J.; Zhang, L.; Zhang, C.L.; Lu, W.L.; Liang, W. Naringenin enhances the anti-tumor effect of doxorubicin through selectively inhibiting the activity of multidrug resistance-associated proteins but not p-glycoprotein. Pharm. Res. 2009, 26, 914–925. [Google Scholar]
- Park, H.S.; Oh, J.H.; Lee, J.H.; Lee, Y.J. Minor effects of the citrus flavonoids naringin, naringenin and quercetin, on the pharmacokinetics of doxorubicin in rats. Pharmazie 2011, 66, 424–429. [Google Scholar]
- Ekambaram, G.; Rajendran, P.; Magesh, V.; Sakthisekaran, D. Naringenin reduces tumor size and weight lost in N-methyl-N’-nitro-N-nitrosoguanidine-induced gastric carcinogenesis in rats. Nutr. Res. 2008, 28, 106–112. [Google Scholar]
- Ganapathy, E.; Peramaiyan, R.; Rajasekaran, D.; Venkataraman, M.; Dhanapal, S. Modulatory effect of naringenin on N-methyl-N′-nitro-N-nitrosoguanidine-and saturated sodium chloride-induced gastric carcinogenesis in male wistar rats. Clin. Exp. Pharmacol. Physiol. 2008, 35, 1190–1196. [Google Scholar]
- Sabarinathan, D.; Mahalakshmi, P.; Vanisree, A.J. Naringenin promote apoptosis in cerebrally implanted C6 glioma cells. Mol. Cell. Biochem. 2010, 345, 215–222. [Google Scholar]
- Miller, E.G.; Peacock, J.J.; Bourland, T.C.; Taylor, S.E.; Wright, J.A.; Patil, B.S.; Miller, E.G. Inhibition of oral carcinogenesis by Citrus flavonoids. Nutr. Cancer Int. J. 2008, 60, 69–74. [Google Scholar]
- Ramesh, E.; Alshatwi, A.A. Naringin induces death receptor and mitochondria-mediated apoptosis in human cervical cancer (SiHa) cells. Food Chem. Toxicol. 2013, 51, 97–105. [Google Scholar]
- Zeng, L.; Zhen, Y.; Chen, Y.; Zou, L.; Zhang, Y.; Hu, F.; Feng, J.; Shen, J.; Wei, B. Naringin inhibits growth and induces apoptosis by a mechanism dependent on reduced activation of NFκB/COX2caspase-1 pathway in HeLa cervical cancer cells. Int. J. Oncol. 2014, 45, 1929–1936. [Google Scholar]
- Yoshinaga, A.; Kajiya, N.; Oishi, K.; Kamada, Y.; Ikeda, A.; Chigwechokha, P.K.; Kibe, T.; Kishida, M.; Kishida, S.; Komatsu, M.; et al. Neu3 inhibitory effect of naringin suppresses cancer cell growth by attenuation of EGFR signaling through GM3 ganglioside accumulation. Eur. J. Pharmacol. 2016, 782, 21–29. [Google Scholar]
- Li, H.; Yang, B.; Huang, J.; Xiang, T.; Yin, X.; Wan, J.; Luo, F.; Zhang, L.; Li, H.; Ren, G. Naringin inhibits growth potential of human triple-negative breast cancer cells by targeting beta-catenin signaling pathway. Toxicol. Lett. 2013, 220, 219–228. [Google Scholar]
- Kim, D.I.; Lee, S.J.; Lee, S.B.; Park, K.; Kim, W.J.; Moon, S.K. Requirement for RAS/RAF/ERK pathway in naringin-induced G(1)-cell-cycle arrest via p21WAF1 expression. Carcinogenesis 2008, 29, 1701–1709. [Google Scholar]
- Xie, C.M.; Chan, W.Y.; Yu, S.; Zhao, J.; Cheng, C.H. Bufalin induces autophagy-mediated cell death in human colon cancer cells through reactive oxygen species generation and JNK activation. Free Radic. Biol. Med. 2011, 51, 1365–1375. [Google Scholar]
- Chen, Y.J.; Chi, C.W.; Su, W.C.; Huang, H.L. Lapatinib induces autophagic cell death and inhibits growth of human hepatocellular carcinoma. Oncotarget 2014, 5, 4845–4854. [Google Scholar]
- Raha, S.; Yumnam, S.; Hong, G.E.; Lee, H.J.; Saralamma, V.V.; Park, H.S.; Heo, J.D.; Lee, S.J.; Kim, E.H.; Kim, J.A.; et al. Naringin induces autophagy-mediated growth inhibition by downregulating the PI3K/AKT/MTOR cascade via activation of MAPK pathways in AGS cancer cells. Int. J. Oncol. 2015, 47, 1061–1069. [Google Scholar]
- Li, J.; Dong, Y.; Hao, G.; Wang, B.; Wang, J.; Liang, Y.; Liu, Y.; Zhen, E.; Feng, D.; Liang, G. Naringin suppresses the development of glioblastoma by inhibiting FAK activity. J. Drug Target. 2016. [Google Scholar] [CrossRef]
- Vanamala, J.; Leonardi, T.; Patil, B.S.; Taddeo, S.S.; Murphy, M.E.; Pike, L.M.; Chapkin, R.S.; Lupton, J.R.; Turner, N.D. Suppression of colon carcinogenesis by bioactive compounds in grapefruit. Carcinogenesis 2006, 27, 1257–1265. [Google Scholar]
- Camargo, C.A.; Gomes-Marcondes, M.C.C.; Wutzki, N.C.; Aoyama, H. Naringin inhibits tumor growth and reduces interleukin-6 and tumor necrosis factor alpha levels in rats with Walker 256 carcinosarcoma. Anticancer Res. 2012, 32, 129–133. [Google Scholar]
- Zhang, Y.S.; Li, Y.; Wang, Y.; Sun, S.Y.; Jiang, T.; Li, C.; Cui, S.X.; Qu, X.J. Naringin, a natural dietary compound, prevents intestinal tumorigenesis in Apc(min/+) mouse model. J. Cancer Res. Clin. Oncol. 2016, 142, 913–925. [Google Scholar]
- Garg, A.; Garg, S.; Zaneveld, L.J.; Singla, A.K. Chemistry and pharmacology of the Citrus bioflavonoid hesperidin. Phytother. Res. 2001, 15, 655–669. [Google Scholar]
- Chen, Y.C.; Shen, S.C.; Lin, H.Y. Rutinoside at C7 attenuates the apoptosis-inducing activity of flavonoids. Biochem. Pharmacol. 2003, 66, 1139–1150. [Google Scholar]
- Choi, E.J. Hesperetin induced G1-phase cell cycle arrest in human breast cancer MCF-7 cells: Involvement of CDK4 and p21. Nutr. Cancer Int. J. 2007, 59, 115–119. [Google Scholar]
- Sivagami, G.; Vinothkumar, R.; Preethy, C.P.; Riyasdeen, A.; Akbarsha, M.A.; Menon, V.P.; Nalini, N. Role of hesperetin (a natural flavonoid) and its analogue on apoptosis in HT-29 human colon adenocarcinoma cell line—A comparative study. Food Chem. Toxicol. 2012, 50, 660–671. [Google Scholar]
- Zarebczan, B.; Pinchot, S.N.; Kunnimalaiyaan, M.; Chen, H. Hesperetin, a potential therapy for carcinoid cancer. Am. J. Surg. 2011, 201, 329–333. [Google Scholar]
- Alshatwi, A.A.; Ramesh, E.; Periasamy, V.S.; Subash-Babu, P. The apoptotic effect of hesperetin on human cervical cancer cells is mediated through cell cycle arrest, death receptor, and mitochondrial pathways. Fundam. Clin. Pharmacol. 2013, 27, 581–592. [Google Scholar]
- Zhang, J.; Song, J.; Wu, D.; Wang, J.; Dong, W. Hesperetin induces the apoptosis of hepatocellular carcinoma cells via mitochondrial pathway mediated by the increased intracellular reactive oxygen species, ATP and calcium. Med. Oncol. 2015, 32, 101. [Google Scholar] [CrossRef]
- Aranganathan, S.; Nalini, N. Antiproliferative efficacy of hesperetin (Citrus flavanoid) in 1,2-dimethylhydrazine-induced colon cancer. Phytother. Res. 2013, 27, 999–1005. [Google Scholar]
- Nalini, N.; Aranganathan, S.; Kabalimurthy, J. Chemopreventive efficacy of hesperetin (Citrus flavonone) against 1,2-dimethylhydrazine-induced rat colon carcinogenesis. Toxicol. Mech. Methods 2012, 22, 397–408. [Google Scholar]
- Patil, J.R.; Murthy, K.N.C.; Jayaprakasha, G.K.; Chetti, M.B.; Patil, B.S. Bioactive compounds from mexican lime (Citrus aurantifolia) juice induce apoptosis in human pancreatic cells. J. Agric. Food Chem. 2009, 57, 10933–10942. [Google Scholar]
- Park, H.J.; Kim, M.J.; Ha, E.; Chung, J.H. Apoptotic effect of hesperidin through caspase3 activation in human colon cancer cells, SNU-C4. Phytomedicine 2008, 15, 147–151. [Google Scholar]
- Banjerdpongchai, R.; Wudtiwai, B.; Khaw-On, P.; Rachakhom, W.; Duangnil, N.; Kongtawelert, P. Hesperidin from Citrus seed induces human hepatocellular carcinoma HepG2 cell apoptosis via both mitochondrial and death receptor pathways. Tumour Biol. 2016, 37, 227–237. [Google Scholar]
- Yumnam, S.; Park, H.S.; Kim, M.K.; Nagappan, A.; Hong, G.E.; Lee, H.J.; Lee, W.S.; Kim, E.H.; Cho, J.H.; Shin, S.C.; et al. Hesperidin induces paraptosis like cell death in hepatoblastoma, hepg2 cells: Involvement of ERK1/2 MAPK. PLoS ONE 2014, 9, e101321. [Google Scholar]
- Nazari, M.; Ghorbani, A.; Hekmat-Doost, A.; Jeddi-Tehrani, M.; Zand, H. Inactivation of nuclear factor-κB by Citrus flavanone hesperidin contributes to apoptosis and chemo-sensitizing effect in ramos cells. Eur. J. Pharmacol. 2011, 650, 526–533. [Google Scholar]
- Ghorbani, A.; Nazari, M.; Jeddi-Tehrani, M.; Zand, H. The Citrus flavonoid hesperidin induces p53 and inhibits NF-kB activation in order to trigger apoptosis in NALM-6 cells: Involvement of ppar gamma-dependent mechanism. Eur. J. Nutr. 2012, 51, 39–46. [Google Scholar]
- Palit, S.; Kar, S.; Sharma, G.; Das, P.K. Hesperetin induces apoptosis in breast carcinoma by triggering accumulation of ROS and activation of ASK1/JNK pathway. J. Cell. Physiol. 2015, 230, 1729–1739. [Google Scholar]
- Natarajan, N.; Thamaraiselvan, R.; Lingaiah, H.; Srinivasan, P.; Periyasamy, B.M. Effect of flavonone hesperidin on the apoptosis of human mammary carcinoma cell line MCF-7. Biomed. Prev. Nutr. 2011, 1, 207–215. [Google Scholar]
- Wang, Y.X.; Yu, H.; Zhang, J.; Gao, J.; Ge, X.; Lou, G. Hesperidin inhibits HeLa cell proliferation through apoptosis mediated by endoplasmic reticulum stress pathways and cell cycle arrest. BMC Cancer 2015, 15. [Google Scholar] [CrossRef]
- El-Readi, M.Z.; Hamdan, D.; Farrag, N.; El-Shazly, A.; Wink, M. Inhibition of p-glycoprotein activity by limonin and other secondary metabolites from Citrus species in human colon and leukaemia cell lines. Eur. J. Pharmacol. 2010, 626, 139–145. [Google Scholar] [PubMed]
- Cooray, H.C.; Janvilisri, T.; van Veen, H.W.; Hladky, S.B.; Barrand, M.A. Interaction of the breast cancer resistance protein with plant polyphenols. Biochem. Biophys. Res. Commun. 2004, 317, 269–275. [Google Scholar]
- Lee, C.J.; Wilson, L.; Jordan, M.A.; Nguyen, V.; Tang, J.; Smiyun, G. Hesperidin suppressed proliferations of both human breast cancer and androgen-dependent prostate cancer cells. Phytother. Res. 2010, 24, S15–S19. [Google Scholar]
- Tanaka, T.; Makita, H.; Ohnishi, M.; Hirose, Y.; Wang, A.J.; Mori, H.; Satoh, K.; Hara, A.; Ogawa, H. Chemoprevention of 4-nitroquinoline 1-oxide-induced oral carcinogenesis by dietary curcumin and hesperidin—Comparison with the protective effect of beta-carotene. Cancer Res. 1994, 54, 4653–4659. [Google Scholar]
- Berkarda, B.; Koyuncu, H.; Soybir, G.; Baykut, F. Inhibitory effect of hesperidin on tumour initiation and promotion in mouse skin. Res. Exp. Med. 1998, 198, 93–99. [Google Scholar]
- Koyuncu, H.; Berkarda, B.; Baykut, F.; Soybir, G.; Alatli, C.; Gul, H.; Altun, M. Preventive effect of hesperidin against inflammation in CD-1 mouse skin caused by tumor promoter. Anticancer Res. 1999, 19, 3237–3241. [Google Scholar]
- Aranganathan, S.; Nalini, N. Efficacy of the potential chemopreventive agent, hesperetin (Citrus flavanone), on 1,2-dimethylhydrazine induced colon carcinogenesis. Food Chem. Toxicol. 2009, 47, 2594–2600. [Google Scholar]
- Choi, E.J.; Kim, G.H. Anti-/pro-apoptotic effects of hesperetin against 7,12-dimetylbenz(a)anthracene-induced alteration in animals. Oncol. Rep. 2011, 25, 545–550. [Google Scholar]
- Kamaraj, S.; Ramakrishnan, G.; Anandakumar, P.; Jagan, S.; Devaki, T. Antioxidant and anticancer efficacy of hesperidin in benzo(a)pyrene induced lung carcinogenesis in mice. Investig. New Drugs 2009, 27, 214–222. [Google Scholar]
- Ye, L.; Chan, F.L.; Chen, S.A.; Leung, L.K. The Citrus flavonone hesperetin inhibits growth of aromatase-expressing MCF-7 tumor in ovariectomized athymic mice. J. Nutr. Biochem. 2012, 23, 1230–1237. [Google Scholar]
- Hung, J.Y.; Hsu, Y.L.; Ko, Y.C.; Tsai, Y.M.; Yang, C.J.; Huang, M.S.; Kuo, P.L. Didymin, a dietary flavonoid glycoside from Citrus fruits, induces FAS-mediated apoptotic pathway in human non-small-cell lung cancer cells in vitro and in vivo. Lung Cancer 2010, 68, 366–374. [Google Scholar]
- Saralamma, V.V.G.; Nagappan, A.; Hong, G.E.; Lee, H.J.; Yumnam, S.; Raha, S.; Heo, J.D.; Lee, S.J.; Lee, W.S.; Kim, E.H.; et al. Poncirin induces apoptosis in AGS human gastric cancer cells through extrinsic apoptotic pathway by up-regulation of FAS ligand. Int. J. Mol. Sci. 2015, 16, 22676–22691. [Google Scholar]
- Wang, L.S.; Stoner, G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290. [Google Scholar]
- Schindler, R.; Mentlein, R. Flavonoids and vitamin E reduce the release of the angiogenic peptide vascular endothelial growth factor from human tumor cells. J. Nutr. 2006, 136, 1477–1482. [Google Scholar]
- Freitas, S.; Costa, S.; Azevedo, C.; Carvalho, G.; Freire, S.; Barbosa, P.; Velozo, E.; Schaer, R.; Tardy, M.; Meyer, R.; et al. Flavonoids inhibit angiogenic cytokine production by human glioma cells. Phytother. Res. 2011, 25, 916–921. [Google Scholar]
- Liu, L.Z.; Fang, J.; Zhou, Q.; Hu, X.W.; Shi, X.L.; Jiang, B.H. Apigenin inhibits expression of vascular endothelial growth factor and angiogenesis in human lung cancer cells: Implication of chemoprevention of lung cancer. Mol. Pharmacol. 2005, 68, 635–643. [Google Scholar]
- Fang, J.; Zhou, Q.; Liu, L.Z.; Xia, C.; Hu, X.W.; Shi, X.L.; Jiang, B.H. Apigenin inhibits tumor angiogenesis through decreasing HIF-1 alpha and VEGF expression. Carcinogenesis 2007, 28, 858–864. [Google Scholar]
- Kim, M.H. Flavonoids inhibit VEGF/BFGF-induced angiogenesis in vitro by inhibiting the matrix-degrading proteases. J. Cell. Biochem. 2003, 89, 529–538. [Google Scholar]
- Lamy, S.; Akla, N.; Ouanouki, A.; Lord-Dufour, S.; Beliveau, R. Diet-derived polyphenols inhibit angiogenesis by modulating the interleukin-6/Stat3 pathway. Exp. Cell Res. 2012, 318, 1586–1596. [Google Scholar]
- Lam, I.K.; Alex, D.; Wang, Y.H.; Liu, P.; Liu, A.L.; Du, G.H.; Lee, S.M. In vitro and in vivo structure and activity relationship analysis of polymethoxylated flavonoids: Identifying sinensetin as a novel antiangiogenesis agent. Mol. Nutr. Food Res. 2012, 56, 945–956. [Google Scholar]
- Kunimasa, K.; Ikekita, M.; Sato, M.; Ohta, T.; Yamori, Y.; Ikeda, M.; Kuranuki, S.; Oikawa, T. Nobiletin, a Citrus polymethoxyflavonoid, suppresses multiple angiogenesis-related endothelial cell functions and angiogenesis in vivo. Cancer Sci. 2010, 101, 2462–2469. [Google Scholar]
- Wang, Y.; Su, M.; Yin, J.; Zhang, H. Effect of nobiletin on K562 cells xenograft in nude mice. Zhongguo Zhong Yao Za Zhi 2009, 34, 1410–1414. [Google Scholar]
- Tan, W.F.; Lin, L.P.; Li, M.H.; Zhang, Y.X.; Tong, Y.G.; Xiao, D.; Ding, J. Quercetin, a dietary-derived flavonoid, possesses antiangiogenic potential. Eur. J. Pharmacol. 2003, 459, 255–262. [Google Scholar]
- Ahn, M.R.; Kunimasa, K.; Kumazawa, S.; Nakayama, T.; Kaji, K.; Uto, Y.; Hori, H.; Nagasawa, H.; Ohta, T. Correlation between antiangiogenic activity and antioxidant activity of various components from propolis. Mol. Nutr. Food Res. 2009, 53, 643–651. [Google Scholar]
- Weng, C.J.; Yen, G.C. Flavonoids, a ubiquitous dietary phenolic subclass, exert extensive in vitro anti-invasive and in vivo anti-metastatic activities. Cancer Metastas. Rev. 2012, 31, 323–351. [Google Scholar]
- Bracke, M.E.; Boterberg, T.; Depypere, H.T.; Stove, C.; Leclercq, G.; Mareel, M.M. The Citrus methoxyflavone tangeretin affects human cell-cell interactions. Flavonoids Cell Funct. 2002, 505, 135–139. [Google Scholar]
- Tan, T.W.; Chou, Y.E.; Yang, W.H.; Hsu, C.J.; Fong, Y.C.; Tang, C.H. Naringin suppress chondrosarcoma migration through inhibition vascular adhesion molecule-1 expression by modulating mir-126. Int. Immunopharmacol. 2014, 22, 107–114. [Google Scholar]
- Liao, A.C.H.; Kuo, C.C.; Huang, Y.C.; Yeh, C.W.; Hseu, Y.C.; Liu, J.Y.; Hsu, L.S. Naringenin inhibits migration of bladder cancer cells through downregulation of AKT and MMP-2. Mol. Med. Rep. 2014, 10, 1531–1536. [Google Scholar]
- Martinez Conesa, C.; Vicente Ortega, V.; Yanez Gascon, M.J.; Alcaraz Banos, M.; Canteras Jordana, M.; Benavente-Garcia, O.; Castillo, J. Treatment of metastatic melanoma B16F10 by the flavonoids tangeretin, rutin, and diosmin. J. Agric. Food Chem. 2005, 53, 6791–6797. [Google Scholar]
- Lentini, A.; Forni, C.; Provenzano, B.; Beninati, S. Enhancement of transglutaminase activity and polyamine depletion in B16-F10 melanoma cells by flavonoids naringenin and hesperitin correlate to reduction of the in vivo metastatic potential. Amino Acids 2007, 32, 95–100. [Google Scholar]
- Qin, L.; Jin, L.T.; Lu, L.L.; Lu, X.Y.; Zhang, C.L.; Zhang, F.Y.; Liang, W. Naringenin reduces lung metastasis in a breast cancer resection model. Protein Cell 2011, 2, 507–516. [Google Scholar]
- Miyata, Y.; Sato, T.; Imada, K.; Dobashi, A.; Yano, M.; Ito, A. A Citrus polymethoxyflavonoid, nobiletin, is a novel mek inhibitor that exhibits antitumor metastasis in human fibrosarcoma HT-1080 cells. Biochem. Biophys. Res. Commun. 2008, 366, 168–173. [Google Scholar]
- Sato, T.; Koike, L.; Miyata, Y.; Hirata, M.; Mimaki, Y.; Sashida, Y.; Yano, M.; Ito, A. Inhibition of activator protein-1 binding activity and phosphatidylinositol 3-kinase pathway by nobiletin, a polymethoxy flavonoid, results in augmentation of tissue inhibitor of metalloproteinases-1 production and suppression of production of matrix metalloproteinases-1 and-9 in human fibrosarcoma HT-1080 cells. Cancer Res. 2002, 62, 1025–1029. [Google Scholar]
- Kawabata, K.; Murakami, A.; Ohigashi, H. Nobiletin, a Citrus flavonoid, down-regulates matrix metalloproteinase-7 (matrilysin) expression in HT-29 human colorectal cancer cells. Biosci. Biotechnol. Biochem. 2005, 69, 307–314. [Google Scholar]
- Chien, S.Y.; Hsieh, M.J.; Chen, C.J.; Yang, S.F.; Chen, M.K. Nobiletin inhibits invasion and migration of human nasopharyngeal carcinoma cell lines by involving ERK1/2 and transcriptional inhibition of MMP-2. Expert Opin. Ther. Targets 2015, 19, 307–320. [Google Scholar]
- Baek, S.H.; Kim, S.M.; Nam, D.; Lee, J.H.; Ahn, K.S.; Choi, S.H.; Kim, S.H.; Shim, B.S.; Chang, I.M.; Ahn, K.S. Antimetastatic effect of nobiletin through the down-regulation of CXC chemokine receptor type 4 and matrix metallopeptidase-9. Pharm. Biol. 2012, 50, 1210–1218. [Google Scholar]
- Minagawa, A.; Otani, Y.; Kubota, T.; Wada, N.; Furukawa, T.; Kumai, K.; Kameyama, K.; Okada, Y.; Fujii, M.; Yano, M.; et al. The Citrus flavonoid, nobiletin, inhibits peritoneal dissemination of human gastric carcinoma in SCID mice. Jpn. J. Cancer Res. 2001, 92, 1322–1328. [Google Scholar]
- Lee, Y.C.; Cheng, T.H.; Lee, J.S.; Chen, J.H.; Liao, Y.C.; Fong, Y.; Wu, C.H.; Shih, Y.W. Nobiletin, a citrus flavonoid, suppresses invasion and migration involving FAK/PI3K/AKT and small GTPase signals in human gastric adenocarcinoma AGS cells. Mol. Cell. Biochem. 2011, 347, 103–115. [Google Scholar]
- Da, C.; Liu, Y.; Zhan, Y.; Liu, K.; Wang, R. Nobiletin inhibits epithelial-mesenchymal transition of human non-small cell lung cancer cells by antagonizing the TGF-BETA1/SMAD3 signaling pathway. Oncol. Rep. 2016, 35, 2767–2774. [Google Scholar]
- Lindenmeyer, F.; Li, H.; Menashi, S.; Soria, C.; Lu, H. Apigenin acts on the tumor cell invasion process and regulates protease production. Nutr. Cancer Int. J. 2001, 39, 139–147. [Google Scholar]
- Lee, W.J.; Chen, W.K.; Wang, C.J.; Lin, W.L.; Tseng, T.H. Apigenin inhibits hgf-promoted invasive growth and metastasis involving blocking PI3K/AKT pathway and beta 4 integrin function in MDA-MB-231 breast cancer cells. Toxicol. Appl. Pharmacol. 2008, 226, 178–191. [Google Scholar]
- Franzen, C.A.; Amargo, E.; Todorovic, V.; Desai, B.V.; Huda, S.; Mirzoeva, S.; Chiu, K.; Grzybowski, B.A.; Chew, T.L.; Green, K.J.; et al. The chemopreventive bioflavonoid apigenin inhibits prostate cancer cell motility through the focal adhesion kinase/Src signaling mechanism. Cancer Prev. Res. 2009, 2, 830–841. [Google Scholar]
- Hu, X.W.; Meng, D.; Fang, J. Apigenin inhibited migration and invasion of human ovarian cancer A2780 cells through focal adhesion kinase. Carcinogenesis 2008, 29, 2369–2376. [Google Scholar]
- Czyz, J.; Madeja, Z.; Irmer, U.; Korohoda, W.; Hulser, D.F. Flavonoid apigenin inhibits motility and invasiveness of carcinoma cells in vitro. Int. J. Cancer 2005, 114, 12–18. [Google Scholar]
- Tatsuta, M.; Iishi, H.; Baba, M.; Yano, H.; Murata, K.; Mukai, M.; Akedo, H. Suppression by apigenin of peritoneal metastasis of intestinal adenocarcinomas induced by azoxymethane in wistar rats. Clin. Exp. Metastas. 2001, 18, 657–662. [Google Scholar]
- Noh, H.J.; Sung, E.G.; Kim, J.Y.; Lee, T.J.; Song, I.H. Suppression of phorbol-12-myristate-13-acetate-induced tumor cell invasion by apigenin via the inhibition of p38 mitogen-activated protein kinase-dependent matrix metalloproteinase-9 expression. Oncol. Rep. 2010, 24, 277–283. [Google Scholar]
- Caltagirone, S.; Rossi, C.; Poggi, A.; Ranelletti, F.O.; Natali, P.G.; Brunetti, M.; Aiello, F.B.; Piantelli, M. Flavonoids apigenin and quercetin inhibit melanoma growth and metastatic potential. Int. J. Cancer 2000, 87, 595–600. [Google Scholar]
- Lin, C.W.; Hou, W.C.; Shen, S.C.; Juan, S.H.; Ko, C.H.; Wang, L.M.; Chen, Y.C. Quercetin inhibition of tumor invasion via suppressing PKC DELTA/ERK/AP-1-dependent matrix metalloproteinase-9 activation in breast carcinoma cells. Carcinogenesis 2008, 29, 1807–1815. [Google Scholar]
- Phromnoi, K.; Yodkeeree, S.; Anuchapreeda, S.; Limtrakul, P. Inhibition of MMP-3 activity and invasion of the MDA-MB-231 human invasive breast carcinoma cell line by bioflavonoids. Acta Pharmacol. Sin. 2009, 30, 1169–1176. [Google Scholar]
- Vijayababu, M.R.; Arunkumar, A.; Kanagaraj, P.; Venkataraman, P.; Krishnamoorthy, G.; Arunakaran, J. Quercetin downregulates matrix metalloproteinases 2 and 9 proteins expression in prostate cancer cells (PC-3). Mol. Cell. Biochem. 2006, 287, 109–116. [Google Scholar]
- Senthilkumar, K.; Arunkumar, R.; Elumalai, P.; Sharmila, G.; Gunadharini, D.N.; Banudevi, S.; Krishnamoorthy, G.; Benson, C.S.; Arunakaran, J. Quercetin inhibits invasion, migration and signalling molecules involved in cell survival and proliferation of prostate cancer cell line (PC-3). Cell Biochem. Funct. 2011, 29, 87–95. [Google Scholar]
- Chiu, W.T.; Shen, S.C.; Chow, J.M.; Lin, C.W.; Shia, L.T.; Chen, Y.C. Contribution of reactive oxygen species to migration/invasion of human glioblastoma cells U87 via ERK-dependent COX-2/PGE(2) activation. Neurobiol. Dis. 2010, 37, 118–129. [Google Scholar]
- Labbe, D.; Provencal, M.; Lamy, S.; Boivin, D.; Gingras, D.; Beliveau, R. The flavonols quercetin, kaempferol, and myricetin inhibit hepatocyte growth factor-induced medulloblastoma cell migration. J. Nutr. 2009, 139, 646–652. [Google Scholar]
- Lin, Y.S.; Tsai, P.H.; Kandaswami, C.C.; Cheng, C.H.; Ke, F.C.; Lee, P.P.; Hwang, J.J.; Lee, M.T. Effects of dietary flavonoids, luteolin, and quercetin on the reversal of epithelial-mesenchymal transition in A431 epidermal cancer cells. Cancer Sci. 2011, 102, 1829–1839. [Google Scholar]
- Zhang, W.; Zhang, F. Effects of quercetin on proliferation, apoptosis, adhesion and migration, and invasion of HeLa cells. Eur. J. Gynaecol. Oncol. 2009, 30, 60–64. [Google Scholar]
- Zhang, X.M.; Huang, S.P.; Xu, Q. Quercetin inhibits the invasion of murine melanoma B16-BL6 cells by decreasing pro-MMP-9 via the PKC pathway. Cancer Chemother. Pharmacol. 2004, 53, 82–88. [Google Scholar]
- Devipriya, S.; Ganapathy, V.; Shyamaladevi, C.S. Suppression of tumor growth and invasion in 9,10 dimethyl benz(a) anthracene induced mammary carcinoma by the plant bioflavonoid quercetin. Chem. Biol. Interact. 2006, 162, 106–113. [Google Scholar]
- Hsu, Y.L.; Hsieh, C.J.; Tsai, E.M.; Hung, J.Y.; Chang, W.A.; Hou, M.F.; Kuo, P.L. Didymin reverses phthalate ester-associated breast cancer aggravation in the breast cancer tumor microenvironment. Oncol. Lett. 2016, 11, 1035–1042. [Google Scholar]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004, 134, 3479S–3485S. [Google Scholar]
- So, F.V.; Guthrie, N.; Chambers, A.F.; Moussa, M.; Carroll, K.K. Inhibition of human breast cancer cell proliferation and delay of mammary tumorigenesis by flavonoids and citrus juices. Nutr. Cancer Int. J. 1996, 26, 167–181. [Google Scholar]
- Guthrie, N.; Carroll, K.K. Inhibition of mammary cancer by Citrus flavonoids. Flavonoids Cell Funct. 1998, 439, 227–236. [Google Scholar]
- Miyagi, Y.; Om, A.S.; Chee, K.M.; Bennink, M.R. Inhibition of azoxymethane-induced colon cancer by orange juice. Nutr. Cancer Int. J. 2000, 36, 224–229. [Google Scholar]
- Tanaka, T.; Kohno, H.; Murakami, M.; Shimada, R.; Kagami, S.; Sumida, T.; Azuma, Y.; Ogawa, H. Suppression of azoxymethane-induced colon carcinogenesis in male F344 rats by mandarin juices rich in beta-cryptoxanthin and hesperidin. Int. J. Cancer 2000, 88, 146–150. [Google Scholar]
- Kohno, H.; Taima, M.; Sumida, T.; Azuma, Y.; Ogawa, H.; Tanaka, T. Inhibitory effect of mandarin juice rich in beta-cryptoxanthin and hesperidin on 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced pulmonary tumorigenesis in mice. Cancer Lett. 2001, 174, 141–150. [Google Scholar]
- Tanaka, T.; Tanaka, T.; Tanaka, M.; Kuno, T. Cancer chemoprevention by Citrus pulp and juices containing high amounts of beta-cryptoxanthin and hesperidin. J. Biomed. Biotechnol. 2012, 2012, 516981. [Google Scholar]
- Kohno, H.; Maeda, M.; Honjo, S.; Murakami, M.; Shimada, R.; Masuda, S.; Sumida, T.; Azuma, Y.; Ogawa, H.; Tanaka, T. Prevention of colonic preneoplastic lesions by the β-cryptoxanthin and hesperidin rich powder prepared from Citrus unshiu marc. Juice in male f344 rats. J. Toxicol. Pathol. 1999, 12, 209–215. [Google Scholar]
- Celano, M.; Maggisano, V.; De Rose, R.F.; Bulotta, S.; Maiuolo, J.; Navarra, M.; Russo, D. Flavonoid fraction of Citrus reticulata juice reduces proliferation and migration of anaplastic thyroid carcinoma cells. Nutr. Cancer Int. J. 2015, 67, 1183–1190. [Google Scholar]
- Alshatwi, A.A.; Shafi, G.; Hasan, T.N.; Al-Hazzani, A.A.; Alsaif, M.A.; Alfawaz, M.A.; Lei, K.Y.; Munshi, A. Apoptosis-mediated inhibition of human breast cancer cell proliferation by lemon Citrus extract. Asian Pac. J. Cancer Prev. 2011, 12, 1555–1559. [Google Scholar]
- Kim, J.; Jayaprakasha, G.K.; Uckoo, R.M.; Patil, B.S. Evaluation of chemopreventive and cytotoxic effect of lemon seed extracts on human breast cancer (MCF-7) cells. Food Chem. Toxicol. 2012, 50, 423–430. [Google Scholar]
- Delle Monache, S.; Sanita, P.; Trapasso, E.; Ursino, M.R.; Dugo, P.; Russo, M.; Ferlazzo, N.; Calapai, G.; Angelucci, A.; Navarra, M. Mechanisms underlying the anti-tumoral effects of Citrus bergamia juice. PLoS ONE 2013, 8, e61484. [Google Scholar]
- Ferlazzo, N.; Cirmi, S.; Russo, M.; Trapasso, E.; Ursino, M.R.; Lombardo, G.E.; Gangemi, S.; Calapai, G.; Navarra, M. NF-κB mediates the antiproliferative and proapoptotic effects of bergamot juice in HepG2 cells. Life Sci. 2016, 146, 81–91. [Google Scholar]
- Navarra, M.; Ursino, M.R.; Ferlazzo, N.; Russo, M.; Schumacher, U.; Valentiner, U. Effect of Citrus bergamia juice on human neuroblastoma cells in vitro and in metastatic xenograft models. Fitoterapia 2014, 95, 83–92. [Google Scholar]
- Visalli, G.; Ferlazzo, N.; Cirmi, S.; Campiglia, P.; Gangemi, S.; Di Pietro, A.; Calapai, G.; Navarra, M. Bergamot juice extract inhibits proliferation by inducing apoptosis in human colon cancer cells. Anticancer Agents Med. Chem. 2014, 14, 1402–1413. [Google Scholar]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to virchow? Lancet 2001, 357, 539–545. [Google Scholar]
- Crawford, S. Anti-inflammatory/antioxidant use in long-term maintenance cancer therapy: A new therapeutic approach to disease progression and recurrence. Ther. Adv. Med. Oncol. 2014, 6, 52–68. [Google Scholar]
- Ferlazzo, N.; Visalli, G.; Smeriglio, A.; Cirmi, S.; Lombardo, G.E.; Campiglia, P.; di Pietro, A.; Navarra, M. Flavonoid fraction of orange and bergamot juices protect human lung epithelial cells from hydrogen peroxide-induced oxidative stress. Evid. Based Complement. Altern. Med. 2015, 2015, 957031. [Google Scholar]
- Ferlazzo, N.; Visalli, G.; Cirmi, S.; Lombardo, G.E.; Lagana, P.; di Pietro, A.; Navarra, M. Natural iron chelators: Protective role in A549 cells of flavonoids-rich extracts of Citrus juices in Fe3+-induced oxidative stress. Environ. Toxicol. Pharmacol. 2016, 43, 248–256. [Google Scholar]
- Risitano, R.; Currò, M.; Cirmi, S.; Ferlazzo, N.; Campiglia, P.; Caccamo, D.; Ientile, R.; Navarra, M. Flavonoid fraction of bergamot juice reduces LPS-induced inflammatory response through SIRT1-mediated NF-kB inhibition in THP-1 monocytes. PLoS ONE 2014, 9, e107431. [Google Scholar]
- Currò, M.; Risitano, R.; Ferlazzo, N.; Cirmi, S.; Gangemi, C.; Caccamo, D.; Ientile, R.; Navarra, M. Citrus bergamia juice extract attenuates beta-amyloid-induced pro-inflammatory activation of THP-1 cells through MAPK and AP-1 pathways. Sci. Rep. 2016, 6, 20809. [Google Scholar]
- Impellizzeri, D.; Bruschetta, G.; di Paola, R.; Ahmad, A.; Campolo, M.; Cuzzocrea, S.; Esposito, E.; Navarra, M. The anti-inflammatory and antioxidant effects of bergamot juice extract (BJe) in an experimental model of inflammatory bowel disease. Clin. Nutr. 2015, 34, 1146–1154. [Google Scholar]
- Impellizzeri, D.; Cordaro, M.; Campolo, M.; Gugliandolo, E.; Esposito, E.; Benedetto, F.; Cuzzocrea, S.; Navarra, M. Anti-inflammatory and antioxidant effects of flavonoid-rich fraction of bergamot juice (BJe) in a mouse model of intestinal ischemia/reperfusion injury. FASEB J. 2016, 30 (Suppl. 1), 720–725. [Google Scholar]
- Filocamo, A.; Bisignano, C.; Ferlazzo, N.; Cirmi, S.; Mandalari, G.; Navarra, M. In vitro effect of bergamot (Citrus bergamia) juice against cagA-positive and-negative clinical isolates of helicobacter pylori. BMC Complement. Altern. Med. 2015, 15. [Google Scholar] [CrossRef]
- Cirmi, S.; Bisignano, C.; Mandalari, G.; Navarra, M. Anti-infective potential of Citrus bergamia risso et poiteau (bergamot) derivatives: A systematic review. Phytother. Res. 2016. [Google Scholar] [CrossRef]
- Marino, A.; Paterniti, I.; Cordaro, M.; Morabito, R.; Campolo, M.; Navarra, M.; Esposito, E.; Cuzzocrea, S. Role of natural antioxidants and potential use of bergamot in treating rheumatoid arthritis. PharmaNutrition 2015, 3, 53–59. [Google Scholar]
- Mak, N.K.; WongLeung, Y.L.; Chan, S.C.; Wen, J.M.; Leung, K.N.; Fung, M.C. Isolation of anti-leukemia compounds from Citrus reticulata. Life Sci. 1996, 58, 1269–1276. [Google Scholar]
- Kim, M.J.; Park, H.J.; Hong, M.S.; Park, H.J.; Kim, M.S.; Leem, K.H.; Kim, J.B.; Kim, Y.J.; Kim, H.K. Citrus reticulata blanco induces apoptosis in human gastric cancer cells SNU-668. Nutr. Cancer 2005, 51, 78–82. [Google Scholar]
- Park, K.I.; Park, H.S.; Nagappan, A.; Hong, G.E.; Lee, D.H.; Kang, S.R.; Kim, J.A.; Zhang, J.; Kim, E.H.; Lee, W.S.; et al. Induction of the cell cycle arrest and apoptosis by flavonoids isolated from korean Citrus aurantium L. in non-small-cell lung cancer cells. Food Chem. 2012, 135, 2728–2735. [Google Scholar]
- Han, M.H.; Lee, W.S.; Lu, J.N.; Kim, G.; Jung, J.M.; Ryu, C.H.; Kim, G.Y.; Hwang, H.J.; Kwon, T.K.; Choi, Y.H. Citrus aurantium L. exhibits apoptotic effects on U937 human leukemia cells partly through inhibition of AKT. Int. J. Oncol. 2012, 40, 2090–2096. [Google Scholar]
- Adina, A.B.; Goenadi, F.A.; Handoko, F.F.; Nawangsari, D.A.; Hermawan, A.; Jenie, R.I.; Meiyanto, E. Combination of ethanolic extract of Citrus aurantifolia peels with doxorubicin modulate cell cycle and increase apoptosis induction on MCF-7 cells. Iran. J. Pharm. Res. 2014, 13, 919–926. [Google Scholar]
- Wang, B.; Lin, S.Y.; Shen, Y.Y.; Wu, L.Q.; Chen, Z.Z.; Li, J.; Chen, Z.; Qian, W.B.; Jiang, J.P. Pure total flavonoids from Citrus paradisi Macfadyen act synergistically with arsenic trioxide in inducing apoptosis of kasumi-1 leukemia cells in vitro. J. Zhejiang Univ. Sci. B 2015, 16, 580–585. [Google Scholar]
- Celia, C.; Trapasso, E.; Locatelli, M.; Navarra, M.; Ventura, C.A.; Wolfram, J.; Carafa, M.; Morittu, V.M.; Britti, D.; di Marzio, L.; et al. Anticancer activity of liposomal bergamot essential oil (BEO) on human neuroblastoma cells. Colloids Surf. B Biointerfaces 2013, 112, 548–553. [Google Scholar]
- Navarra, M.; Ferlazzo, N.; Cirmi, S.; Trapasso, E.; Bramanti, P.; Lombardo, G.E.; Minciullo, P.L.; Calapai, G.; Gangemi, S. Effects of bergamot essential oil and its extractive fractions on SH-SY5Y human neuroblastoma cell growth. J. Pharm. Pharmacol. 2015, 67, 1042–1053. [Google Scholar]
- Yuan, J.M.; Wang, X.L.; Xiang, Y.B.; Gao, Y.T.; Ross, R.K.; Yu, M.C. Preserved foods in relation to risk of nasopharyngeal carcinoma in Shanghai, China. Int. J. Cancer 2000, 85, 358–363. [Google Scholar]
- Bosetti, C.; la Vecchia, C.; Talamini, R.; Simonato, L.; Zambon, P.; Negri, E.; Trichopoulos, D.; Lagiou, P.; Bardini, R.; Franceschi, S. Food groups and risk of squamous cell esophageal cancer in northern Italy. Int. J. Cancer 2000, 87, 289–294. [Google Scholar]
- Steevens, J.; Schouten, L.J.; Goldbohm, R.A.; van den Brandt, P.A. Vegetables and fruits consumption and risk of esophageal and gastric cancer subtypes in the Netherlands cohort study. Int. J. Cancer 2011, 129, 2681–2693. [Google Scholar]
- Franceschi, S.; Favero, A.; Conti, E.; Talamini, R.; Volpe, R.; Negri, E.; Barzan, L.; la Vecchia, C. Food groups, oils and butter, and cancer of the oral cavity and pharynx. Br. J. Cancer 1999, 80, 614–620. [Google Scholar]
- Bosetti, C.; la Vecchia, C.; Talamini, R.; Negri, E.; Levi, F.; dal Maso, L.; Franceschi, S. Food groups and laryngeal cancer risk: A case-control study from Italy and Switzerland. Int. J. Cancer 2002, 100, 355–360. [Google Scholar]
- Maserejian, N.N.; Giovannucci, E.; Rosner, B.; Zavras, A.; Joshipura, K. Prospective study of fruits and vegetables and risk of oral premalignant lesions in men. Am. J. Epidemiol. 2006, 164, 556–566. [Google Scholar]
- Pavia, M.; Pileggi, C.; Nobile, C.G.A.; Angelillo, I.F. Association between fruit and vegetable consumption and oral cancer: A meta-analysis of observational studies. Am. J. Clin. Nutr. 2006, 83, 1126–1134. [Google Scholar]
- Pourfarzi, F.; Whelan, A.; Kaldor, J.; Malekzadeh, R. The role of diet and other environmental factors in the causation of gastric cancer in Iran-a population based study. Int. J. Cancer 2009, 125, 1953–1960. [Google Scholar]
- Foschi, R.; Pelucchi, C.; dal Maso, L.; Rossi, M.; Levi, F.; Talamini, R.; Bosetti, C.; Negri, E.; Serraino, D.; Giacosa, A.; et al. Citrus fruit and cancer risk in a network of case-control studies. Cancer Causes Control 2010, 21, 237–242. [Google Scholar]
- Gonzalez, C.A.; Lujan-Barroso, L.; Bueno-de-Mesquita, H.B.; Jenab, M.; Duell, E.J.; Agudo, A.; Tjonneland, A.; Boutron-Ruault, M.C.; Clavel-Chapelon, F.; Touillaud, M.; et al. Fruit and vegetable intake and the risk of gastric adenocarcinoma: A reanalysis of the european prospective investigation into cancer and nutrition (epic-eurgast) study after a longer follow-up. Int. J. Cancer 2012, 131, 2910–2919. [Google Scholar]
- Franceschi, S.; Favero, A.; la Vecchia, C.; Negri, E.; Conti, E.; Montella, M.; Giacosa, A.; Nanni, O.; Decarli, A. Food groups and risk of colorectal cancer in Italy. Int. J. Cancer 1997, 72, 56–61. [Google Scholar]
- Levi, F.; Pasche, C.; la Vecchia, C.; Lucchini, F.; Franceschi, S. Food groups and colorectal cancer risk. Br. J. Cancer 1999, 79, 1283–1287. [Google Scholar]
- Malin, A.S.; Qi, D.; Shu, X.O.; Gao, Y.T.; Friedmann, J.M.; Jin, F.; Zheng, W. Intake of fruits, vegetables and selected micronutrients in relation to the risk of breast cancer. Int. J. Cancer 2003, 105, 413–418. [Google Scholar]
- Ronco, A.L.; de Stefani, E.; Stoll, M. Hormonal and metabolic modulation through nutrition: Towards a primary prevention of breast cancer. Breast 2010, 19, 322–332. [Google Scholar]
- Jansen, R.J.; Robinson, D.P.; Stolzenberg-Solomon, R.Z.; Bamlet, W.R.; de Andrade, M.; Oberg, A.L.; Hammer, T.J.; Rabe, K.G.; Anderson, K.E.; Olson, J.E.; et al. Fruit and vegetable consumption is inversely associated with having pancreatic cancer. Cancer Causes Control 2011, 22, 1613–1625. [Google Scholar]
- Chan, J.M.; Wang, F.; Holly, E.A. Vegetable and fruit intake and pancreatic cancer in a population-based case-control study in the San Francisco bay area. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2093–2097. [Google Scholar]
- Jian, L.; Du, C.J.; Lee, A.H.; Binns, C.W. Do dietary lycopene and other carotenoids protect against prostate cancer? Int. J. Cancer 2005, 113, 1010–1014. [Google Scholar]
- Fortes, C.; Mastroeni, S.; Melchi, F.; Pilla, M.A.; Antonelli, G.; Camaioni, D.; Alotto, M.; Pasquini, P. A protective effect of the mediterranean diet for cutaneous melanoma. Int. J. Epidemiol. 2008, 37, 1018–1029. [Google Scholar]
- Li, W.Q.; Kuriyama, S.; Li, Q.; Nagai, M.; Hozawa, A.; Nishino, Y.; Tsuji, I. Citrus consumption and cancer incidence: The Ohsaki cohort study. Int. J. Cancer 2010, 127, 1913–1922. [Google Scholar]
- Dikshit, R.P.; Boffetta, P.; Bouchardy, C.; Merletti, F.; Crosignani, P.; Cuchi, T.; Ardanaz, E.; Brennan, P. Risk factors for the development of second primary tumors among men after laryngeal and hypopharyngeal carcinoma—A multicentric european study. Cancer 2005, 103, 2326–2333. [Google Scholar]
- Bae, J.M.; Lee, E.J.; Guyatt, G. Citrus fruit intake and stomach cancer risk: A quantitative systematic review. Gastric Cancer 2008, 11, 23–32. [Google Scholar]
- Bae, J.M.; Lee, E.J.; Guyatt, G. Citrus fruit intake and pancreatic cancer risk: A quantitative systematic review. Pancreas 2009, 38, 168–174. [Google Scholar]
- Song, J.K.; Bae, J.M. Citrus fruit intake and breast cancer risk: A quantitative systematic review. J. Breast Cancer 2013, 16, 72–76. [Google Scholar]
- Liang, S.; Lv, G.; Chen, W.; Jiang, J.; Wang, J. Citrus fruit intake and bladder cancer risk: A meta-analysis of observational studies. Int. J. Food Sci. Nutr. 2014, 65, 893–898. [Google Scholar]
- Xu, C.; Zeng, X.T.; Liu, T.Z.; Zhang, C.; Yang, Z.H.; Li, S.; Chen, X.Y. Fruits and vegetables intake and risk of bladder cancer: A prisma-compliant systematic review and dose-response meta-analysis of prospective cohort studies. Medicine 2015, 94, e759. [Google Scholar]
- Yao, B.; Yan, Y.; Ye, X.; Fang, H.; Xu, H.; Liu, Y.; Li, S.; Zhao, Y. Intake of fruit and vegetables and risk of bladder cancer: A dose-response meta-analysis of observational studies. Cancer Causes Control 2014, 25, 1645–1658. [Google Scholar]
- Wang, A.; Zhu, C.; Fu, L.; Wan, X.; Yang, X.; Zhang, H.; Miao, R.; He, L.; Sang, X.; Zhao, H. Citrus fruit intake substantially reduces the risk of esophageal cancer: A meta-analysis of epidemiologic studies. Medicine 2015, 94, e1390. [Google Scholar]
- Vingeliene, S.; Chan, D.S.; Aune, D.; Vieira, A.R.; Polemiti, E.; Stevens, C.; Abar, L.; Rosenblatt, D.N.; Greenwood, D.C.; Norat, T. An update of the WCRF/AICR systematic literature review on esophageal and gastric cancers and Citrus fruits intake. Cancer Causes Control 2016, 27, 837–851. [Google Scholar]
- Vrieling, A.; Verhage, B.A.; van Duijnhoven, F.J.; Jenab, M.; Overvad, K.; Tjonneland, A.; Olsen, A.; Clavel-Chapelon, F.; Boutron-Ruault, M.C.; Kaaks, R.; et al. Fruit and vegetable consumption and pancreatic cancer risk in the european prospective investigation into cancer and nutrition. Int. J. Cancer 2009, 124, 1926–1934. [Google Scholar]
- Ferlazzo, N.; Cirmi, S.; Calapai, G.; Ventura-Spagnolo, E.; Gangemi, S.; Navarra, M. Anti-inflammatory activity of Citrus bergamia derivatives: Where do we stand? Molecules 2016, 21, 1273. [Google Scholar] [CrossRef]
- Mannucci, C.; Navarra, M.; Calapai, F.; Squeri, R.; Gangemi, S.; Calapai, G. Clinical Pharmacology of Citrus bergamia: A Systematic Review. Phytother. Res. 2016. [Google Scholar] [CrossRef]
- Cirmi, S.; Ferlazzo, N.; Lombardo, G.E.; Ventura-Spagnolo, E.; Gangemi, S.; Calapai, G.; Navarra, M. Neurodegenerative diseases: Might Citrus flavonoids play a protective role? Molecules 2016, 21, 1312. [Google Scholar] [CrossRef]
- Citraro, R.; Navarra, M.; Leo, A.; Donato Di Paola, E.; Santangelo, E.; Lippiello, P.; Aiello, R.; Russo, E.; De Sarro, G. The anticonvulsant activity of a flavonoid-rich extract from orange juice involves both NMDA and GABA-benzodiazepine receptor complexes. Molecules 2016, 21. [Google Scholar] [CrossRef]
- Efferth, T.; Koch, E. Complex interactions between phytochemicals. The multi-target therapeutic concept of phytotherapy. Curr. Drug Targets 2011, 12, 122–132. [Google Scholar]
| Mechanism by Which Citrus Flavonoids May Fight against Cancer |
| Antioxidant activity, thus counteract oxidative stress |
| Anti-inflammatory effect |
| Phase II enzyme induction, hence enhancing detoxification |
| Phase I enzyme inhibition, thus stopping activation of carcinogens |
| Inhibition of cell proliferation |
| Inhibition of oncogene and/or induction of tumor suppressor gene |
| Induction of cell-cycle arrest |
| Induction of apoptosis |
| Inhibition of signal transduction pathways |
| Anti-angiogenic effect |
| Inhibition of cell adhesion, migration and invasion |
| Initiation Phase | |||
|---|---|---|---|
| Flavonoid | Concentration/Dose | Experimental Model | Reference |
| Quercetin | 0.1–5.0 μM | HgCl2/MeHg-treated HepG2 cells | [26] |
| Naringenin | 10–80 μM | Ferrous sulfate-exposed LNCaP cells | [27] |
| Naringenin | 200 mg/kg | NDEA-treated rats | [28] |
| Naringenin | 200 mg/kg | NDEA-treated rats | [29] |
| Naringin | 50–500 mg/kg | Ifos-treated mice | [30] |
| Naringin | 50–500 mg/kg | Dau-treated mice | [31] |
| Naringin | 10–200 mg/kg | DMH-injected rats | [32] |
| Hesperidin | 50–400 mg/kg | Cyclophosphamide-treated mice | [33] |
| Naringin, apigenin, hesperetin | 300 μg/plate | Aflatoxin B1-exposed Salmonella typhimurium TA100 | [34] |
| Diosmin, naringenin, naringin, rutin | 0.25–1.0 μM | Heterocyclic amines-exposed Salmonella typhimurium TA98 | [37] |
| Apigenin | 10–100 μM | 308 and HCT116 cells | [38] |
| Apigenin | 2.5 and 5 mg/kg | BP-treated mice | [39] |
| Quercetin, kaempferol, myricetin, apigenin | 5–25 μM | COS-1 cells | [40] |
| Apigenin | 1–50 μM | DMBA/TPA-exposed mice | [41] |
| Apigenin | 5 and 10 μmoles in 200 μL | UV-A/B-exposed SKH-1 mice | [42] |
| Apigenin, naringenin | 0.1% and 0.02% | AOM-treated rats | [43] |
| Hesperidin | 30 mg/kg | DMBA-treated rats | [44] |
| Hesperetin | 20 mg/kg | DMH-treated rats | [45] |
| Tangeretin | 50 mg/kg | DMBA-treated rats | [46] |
| Nobiletin | 160 and 320 nM | DMBA/TPA-exposed mice | [47] |
| Promotion Phase | |||
|---|---|---|---|
| Flavonoid | Concentration/Dose | Experimental Model | Reference |
| Quercetin, taxifolin, nobiletin, tangeretin | 2–8 μg/mL | HTB43 cells | [48] |
| Tangeretin | 50–100 μM | HL-60 cells | [49] |
| Tangeretin | 2.7–27 μM | HL-60 cells | [50] |
| Tangeretin, nobiletin | 54 μM (tangeretin) | MDA-MB-435, MCF-7, and HT-29 cells | [51] |
| 100–200 μM for MDA-MB-435 | |||
| 60 μM for MCF-7 | |||
| 200 μM for HT-29 (nobiletin) | |||
| Tangeretin | 10–50 μM | COLO 205 cells | [52] |
| Nobiletin | 20–200 μM | TMK-1, MKN-45, MKN-74, and KATO-III cells | [53] |
| Tangeretin | 10−7–10−4 M | T47D cells | [56] |
| Nobiletin | 20–30 μM | H2O2-treated SH-SY5Y cells | [57] |
| Tangeretin, nobiletin | IC50 4 mg/mL | Brain tumor cells | [58] |
| Tangeretin | 150 μM | A2780/CP70 and 2008/C13 cells | [59] |
| Tangeretin | 5–240 μM | AGS cells | [60] |
| Nobiletin | 1 × 10−7–5 × 10−4 mol/L | TRAP rats | [61] |
| Nobiletin | 0.05% | PhIP-treated rats | [62] |
| Nobiletin | 0.01%–0.05% | AOM-treated rats | [63] |
| Chrysin, quercetin, nobiletin | 100 ppm | AOM-treated mice | [64] |
| Nobiletin | 100 ppm | AOM/DSS-treated mice | [65] |
| Nobiletin | 1.25–80 μM | A549 cells | [66] |
| Nobiletin | 10−3 M | MH1C1 and HepG2 cells | [67] |
| Nobiletin | 10–100 μM | C6 cells | [68] |
| Nobiletin | 20–100 μM | U87 and Hs683 cells | [69] |
| Nobiletin | 0–200 μM | AGS, MKN-45, SNU-1, and SNU-16 cells | [70] |
| Nobiletin | 0–160 μM | HL-60, U937, THP-1, OCI-AML3, and MV4-11 cells | [71] |
| Nobiletin | 0.05 wt% | AOM/DSS-treated CD-1 mice | [72] |
| Apigenin | 1–100 μM | MDA-MB-453 cells | [73] |
| Apigenin | 0–40 μM | MCF-7, MCF-7 HER2, SK-BR-3 cells | [74] |
| Apigenin | 10–70 μM | MDA-MB-453, BT-474, SKBr-3, MCF-7, and HBL-100 cells | [75] |
| Apigenin | 0–60 μM | HT-29 and MG63 cells | [77] |
| Apigenin | 10–50 μM | HDF cells | [78] |
| Apigenin | IC50: 7.8 μg/mL for MCF-7 and 8.9 μg/mL for MDA-MB-468 cells | MCF-7 and MDA-MB-468 cells | [80] |
| Apigenin | 1–100 μM | BxPC-3 and MiaPaCa-2 cells | [81] |
| Apigenin | 6.25–100 μM | AsPC-1, CD18, MIA PaCa2, and S2-013 cells | [82] |
| Apigenin | 10–100 μM | BxPC-3 and PANC-1 cells | [83] |
| Apigenin | 10–80 μM | LNCaP cells | [84] |
| Apigenin | 1–20 μM | DU145 cells | [85] |
| Apigenin | 0–80 μM | SW480, HT-29, and Caco-2 cells | [86] |
| Apigenin | 10–10 μM | HCT-116, SW480, HT-29, and LoVo cells | [87] |
| Apigenin | 20–50 μg/mouse | 22Rv1 and PC-3 cells-implanted mice | [88] |
| Apigenin | 50 μM | SH-SY5Y cells | [89] |
| Apigenin | 15–60 μM and 25 mg/kg | NUB-7, LAN-5, and SK-N-BE cells and NUB-7 inoculated xenograft mice | [90] |
| Flavonids | 25–250 μM | HT-29, Caco-2, LLC-PK1, and MCF-7 cells | [92] |
| Diosmin | 0–120 μM and 15 mg/kg | HA22T cells and HA22T xenograft mice | [93] |
| Diosmin | 50–250 μM | DU145 cells | [94] |
| Diosmin, hesperidin | 1000 ppm | MNAN-injected rats | [95] |
| Diosmin, hesperidin | 1000 ppm | 4-NQO-exposed rats | [96] |
| Diosmin, hesperidin | 500–1000 ppm | OH-BBN-exposed rats | [97] |
| Diosmin, hesperidin | 1000 ppm | AOM-injected rats | [98] |
| 22 flavonoids | 0–10 μM | HL-60, A431, SK-OV-3, HeLa, HOS cells | [99] |
| Quercetin | 0–100 μM | Caco-2 and HT-29 and IEC-6 cells | [102] |
| Quercetin | 0–50 μM | Prostate and skin cells | [104] |
| Quercetin | 0–50 μM | MDA-MB-231, MDA-MB-453, AU565, BT483, BT474, and MCF-7 cells | [105] |
| Quercetin | 0–10 μM | SK-Br-3 and SK-Br-3-Lap R cells | [106] |
| Quercetin | 2.5–40 μM | MDA-MB-231, MCF-7, and MCF-10A cells | [107] |
| Quercetin | 1–10 μM | MCF-7ADR-resistant cells | [108] |
| Naringenin | 0–1 mM | HL-60 cells | [110] |
| Naringenin | 0.02–2.85 mmol | HT-29 cells | [112] |
| Naringenin | 10 μM | MCF-7 cells | [113] |
| Naringenin | 0–400 μM | THP-1 cells | [114] |
| Naringenin | 50–750 μM | HaCaT and A431 cells | [116] |
| Naringenin | 0.1–0.5 mM | HL-60 cells | [117] |
| Naringenin | 100 μM | A549, H460, and WI-38 cells | [118] |
| Naringenin, hesperetin, apigenin | 50 μM | MCF-7 and NCI-H460 cells | [119] |
| Naringenin, kaempferol | 25–100 μM | HK-2 cells | [121] |
| Naringenin | 10 mg/kg | Rats | [122] |
| Naringenin, naringin | 0.7 mg/kg (naringenin) and 2.4–9.4 mg/kg (naringin) | Rats | [123] |
| Naringenin | 100 μM | A549, MCF-7, HepG2, and MCF-7/DOX cells | [124] |
| Naringin, naringenin, quercetin | 50 mg/kg (naringin or naringenin) and 100 mg/kg (quercetin) | Rats | [125] |
| Naringenin | 200 mg/kg | MNNG-treated rats | [126] |
| Naringenin | 200 mg/kg | MNNG-treated rats | [127] |
| Naringenin | 50 mg/kg | C6 cells-injected rats | [128] |
| Naringin, naringenin | 2.5% | Hamsters | [129] |
| Naringin | 250–2000 μM | SiHa cells | [130] |
| Naringin | 1000 μmol/L | HeLa cells | [131] |
| Naringin | 0–3200 μM | HeLa and A549 cells | [132] |
| Naringin | 50–200 μM and 100 mg/kg | MDA-MB-231, MDA-MB-468, and BT-549 cells/MDA-MB-231 xenograft mice | [133] |
| Naringin | 0–150 μM | 5637 and T24 cells | [134] |
| Naringin | 1.2–3 mM | AGS cells | [137] |
| Naringin | 50–200 μM | MDA-MB-231, MDA-MB-468, and BT-549 cells | [138] |
| Naringin | 200 mg/kg | AOM-injected rats | [139] |
| Naringin | 10.25–35 mg/kg | W256 rats | [140] |
| Naringin | 150 mg/kg | Apc(Min/+) mice | [141] |
| Hesperetin, hesperidin, naringenin, naringin | 40–80 μM | HL-60, THP-1, and PMN cells | [143] |
| Hesperetin | 0–200 μM | MCF-7 cells | [144] |
| Hesperetin | 5–100 μM | HT-29 cells | [145] |
| Hesperetin | 0–125 μmol/L | BON cells | [146] |
| Hesperetin | 125–1000 μM | SiHa cells | [147] |
| Hesperetin | 0–600 μM and 10–40 mg/kg | HepG-2, SMMC-7721, and Huh-7/hepatocellular carcinoma xenograft mice | [148] |
| Hesperetin | 20 mg/kg | DMH-injected rats | [149] |
| Hesperidin, hesperitin, rutin, neohesperidin | 25–100 μg/mL | Panc-28 cells | [151] |
| Hesperidin | 1–100 μM | SNU-C4 cells | [152] |
| Hesperidin | 0–200 μM | HepG2 cells | [153] |
| Hesperidin | 0.1–2 mM | HepG2 cells | [154] |
| Hesperidin | 0–100 μM | Ramos cells | [155] |
| Hesperidin | 10–100 μM | NALM-6 cells | [156] |
| Hesperetin | 0–200 μM | MCF-7, MCF-10A, HMEC and MDA-MB-231 cells | [157] |
| Hesperidin | 20–100 μM | MCF-7 cells | [158] |
| Hesperidin | 0–100 μM | HeLa cells | [159] |
| Hesperidin | 0.32–32 μM | Caco-2, CCRF-CEM and CEM/ADR5000 cells | [160] |
| Hesperetin, quercetin | 30 μM | K562, K562/BCRP, MCF7/WT, and MCF7/MR cells | [161] |
| Hesperidin | 0–100 μM | MCF-7, LNCaP, PC-3 and DU-145 cells | [162] |
| Hesperidin | 500 ppm | 4-NQO-treated rats | [163] |
| Hesperidin | 1% | DMBA/TPA-treated mice | [164] |
| Hesperetin | 20 mg/kg | DMH-treated rats | [166] |
| Hesperetin | 10–50 mg/kg | DMBA-treated rats | [167] |
| Hesperidin | 25 mg/kg | BP-exposed mice | [168] |
| Hesperetin | 1000–5000 ppm | MCF-7 xenograft mice | [169] |
| Didymin | 0–20 μM | A549 and H460 cells | [170] |
| Poncirin | 50–200 μM | AGS cells | [171] |
| Progression Phase | |||
|---|---|---|---|
| Flavonoid | Concentration/Dose | Experimental Model | Reference |
| Flavonoids | 0.1–100 μmol/L | MDA, U343, and U118 cells | [173] |
| Rutin | 50–100 μM | GL-15 cells | [174] |
| Apigenin | 0–20 μM | A549 cells | [175] |
| Apigenin | 0–30 μM | PC-3, DU145, LNCaP, OVCAR-3, HCT-8, MCF-7 cells | [176] |
| Apigenin | 5 mg/L | HUVEC cells | [177] |
| Apigenin | 25 μM | HUVEC, HMVECs-d-Ad cells | [178] |
| Hesperetin and nobiletin | 0–100 μM and 30 μM | HUVECs cells and zebrafish | [179] |
| Nobiletin | 0–128 μM and 100 μg/egg | HUVEC and HDMEC cells and CAM | [180] |
| Nobiletin | 12.5–50 mg/kg | K562 cells xenograft mice | [181] |
| Quercetin | 0–100 μM and 50–100 nmol/10 μL/egg | HUVEC cells and CAM | [182] |
| Quercetin | 3.13–50 μg/mL | HUVEC cells | [183] |
| Naringin | 0–30 μM | JJ012 and SW1353 cells | [186] |
| Neringenin | 0–300 μM | TSGH-8301 cells | [187] |
| Tangeretin, rutin, and diosmin | 20 mg/animal | B16F10-inoculated mice | [188] |
| Naringenin and hesperitin | 10 μM/20 mg/g of pellets | B16-F10 cells/B16-F10-inoculated C57BL6/N mice | [189] |
| Naringenin | 0–200 μM and 100 mg/kg | 4T1 cells/4T1-injected BALB/c and C57BL/6 mice | [190] |
| Nobiletin | 64 μM | TPA-stimulated HT-1080 cells | [191] |
| Nobiletin | 0–64 μM | TPA-stimulated HT-1080 cells | [192] |
| Nobiletin | 0–100 μM | Caco-2, HT-29, Colo205, Colo320DM, LS174T, and LS180 cells | [193] |
| Nobiletin | 0–200 μM | MDA-MB-231 cells | [195] |
| Nobiletin | 0–256 μM/16–64 μM | TMK-1, MKN-45, and St-4 cell/TMK-1-injected mice | [196] |
| Nobiletin | 0–4.5 μM | HepG2, Caco-2, and AGS cells | [197] |
| Apigenin | 2.5–10 μg/mL | MDA-MB231 cells | [199] |
| Apigenin | 0–320 μM | MDA-MB-231, A549, SK-Hep1 cells | [200] |
| Apigenin | 0–50 μM | PC3-M, C4-2B, and DU145 cells | [201] |
| Apigenin | 20/40 μM | A2780 cells | [202] |
| Apigenin | 10–50 μM | HeLa cells | [203] |
| Apigenin | 0.75–1.5 mg/kg | AOM-treated rats | [204] |
| Apigenin | 5–20 μM | PMA-exposed SK-Hep1 and MDA-231 cells | [205] |
| Apigenin and quercetin | 1–10,000 nM/25–50 mg/kg | B16-BL6-injected mice | [206] |
| Quercetin | 80 μM | TPA-treated MCF-7 cells | [207] |
| Quercetin | 0–100 μmol/L | MDA-MB-231 cells | [208] |
| Quercetin | 50–100 μM | PC-3 cells | [209] |
| Quercetin | 25–125 mM | PC-3 cells | [210] |
| Quercetin | 50 μM | TPA-exposed U87 cells | [211] |
| Quercetin | 1–20 μM | HGF-exposed DAOY cells | [212] |
| Quercetin and luteolin | 10–20 μM | A431 cells | [213] |
| Quercetin | 20 to 80 μM/L | HeLa cells | [214] |
| Quercetin | 3.3 × 10−1 mM | B16-BL6 cells | [215] |
| Quercetin | 25 mg/kg | DMBA-treated rats | [216] |
| Citrus Juices and Extracts | Experimental Model | Reference |
|---|---|---|
| Citrus sinensis juice | DMBA-injected rats | [219] |
| Citrus sinensis juice | DMBA-injected rats | [220] |
| Citrus sinensis juice | AOM-injected rats | [221] |
| Citrus reticulata juice | AOM-injected rats | [222] |
| Citrus reticulata juice | NNK-injected mice | [223] |
| Citrus reticulata juice | AOM-injected rats | [225] |
| Citrus reticulata juice | CAL-62, C-643, 8505C cells | [226] |
| Lemon fruit extract | MCF-7 cells | [227] |
| Lemon seed extracts | MCF-7 cells | [228] |
| Citrus bergamia juice | SH-SY5Y cells | [229] |
| Citrus bergamia juice | HepG2 cells | [230] |
| Citrus bergamia juice | SK-N-SH/LAN-1 xenograft mice | [231] |
| Flavonoid-rich extract of bergamot juice | HT-29 cells | [232] |
| Citrus reticulata pericarpium extract | WEHI 3B cells | [244] |
| Citrus reticulata Blanco peel extract | SNU-668 cells | [245] |
| Citrus aurantium peel extract | A549 cells | [246] |
| Citrus aurantium peel extract | U937 cells | [247] |
| Orange peel extract | C57Bl/6 mice | [55] |
| Orange peel extract | Apc(Min/+) mice | [54] |
| Citrus aurantifolia peel extract | MCF-7 cells | [248] |
| Citrus paradis peel extract | Kasumi-1 cells | [249] |
| Citrus bergamia essential oil | SH-SY5Y cells | [250] |
| Citrus bergamia essential oil | SH-SY5Y cells | [251] |
| Study Design | Subjects | Reference |
|---|---|---|
| Case–control study | 935 nasopharyngeal carcinoma (NPC) patients aged 15 to 74 years and 1032 community controls | [252] |
| Case–control study | 304 esophagus squamous cell carcinoma patients and 743 hospital controls | [253] |
| Cohort study | 120,852 Dutch men and women aged 55–69 | [254] |
| Case–control study | 512 men and 86 women with cancer of the oral cavity and pharynx and 1008 men and 483 women controls | [255] |
| Case–control study | 527 incident, histologically confirmed cases and 1297 frequency-matched controls | [256] |
| Prospective study | 42,311 US men | [257] |
| Case–control study | 217 people with gastric cancer and 394 controls | [259] |
| Population-based case–control study | 1459 incident breast cancer cases and 1556 frequency-matched controls | [264] |
| Clinic-based case–control study | 384 cases of pancreatic cancer and 983 controls | [266] |
| Population-based case–control study | 532 cases of pancreatic cancer and 1701 controls | [267] |
| Case–control study | 130 incident patients with adenocarcinoma of the prostate and 274 controls | [268] |
| Hospital-based case–control study | 304 incident cases of cutaneous melanoma and 305 controls | [269] |
| Cohort Study | 42,470 Japanese adults with age ranging fron 40 to 79 years | [270] |
| Population-based case–control study | 876 male patients with laryngeal/hypopharyngeal carcinoma | [271] |
| Systematic review | Stomach cancer | [272] |
| Systematic review | Pancreatic cancer | [273] |
| Systematic review | Breast cancer | [274] |
| Meta-analysis | Bladder cancer | [275] |
| Systematic review and meta-analysis | Bladder cancer | [276] |
| Meta-analysis | Bladder cancer | [277] |
| Meta-analysis | Esophageal cancer | [278] |
| Systematic review | Esophageal and gastric cancers | [279] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cirmi, S.; Ferlazzo, N.; Lombardo, G.E.; Maugeri, A.; Calapai, G.; Gangemi, S.; Navarra, M. Chemopreventive Agents and Inhibitors of Cancer Hallmarks: May Citrus Offer New Perspectives? Nutrients 2016, 8, 698. https://doi.org/10.3390/nu8110698
Cirmi S, Ferlazzo N, Lombardo GE, Maugeri A, Calapai G, Gangemi S, Navarra M. Chemopreventive Agents and Inhibitors of Cancer Hallmarks: May Citrus Offer New Perspectives? Nutrients. 2016; 8(11):698. https://doi.org/10.3390/nu8110698
Chicago/Turabian StyleCirmi, Santa, Nadia Ferlazzo, Giovanni E. Lombardo, Alessandro Maugeri, Gioacchino Calapai, Sebastiano Gangemi, and Michele Navarra. 2016. "Chemopreventive Agents and Inhibitors of Cancer Hallmarks: May Citrus Offer New Perspectives?" Nutrients 8, no. 11: 698. https://doi.org/10.3390/nu8110698
APA StyleCirmi, S., Ferlazzo, N., Lombardo, G. E., Maugeri, A., Calapai, G., Gangemi, S., & Navarra, M. (2016). Chemopreventive Agents and Inhibitors of Cancer Hallmarks: May Citrus Offer New Perspectives? Nutrients, 8(11), 698. https://doi.org/10.3390/nu8110698










