Relationship between Added Sugars Consumption and Chronic Disease Risk Factors: Current Understanding
Abstract
:1. Introduction
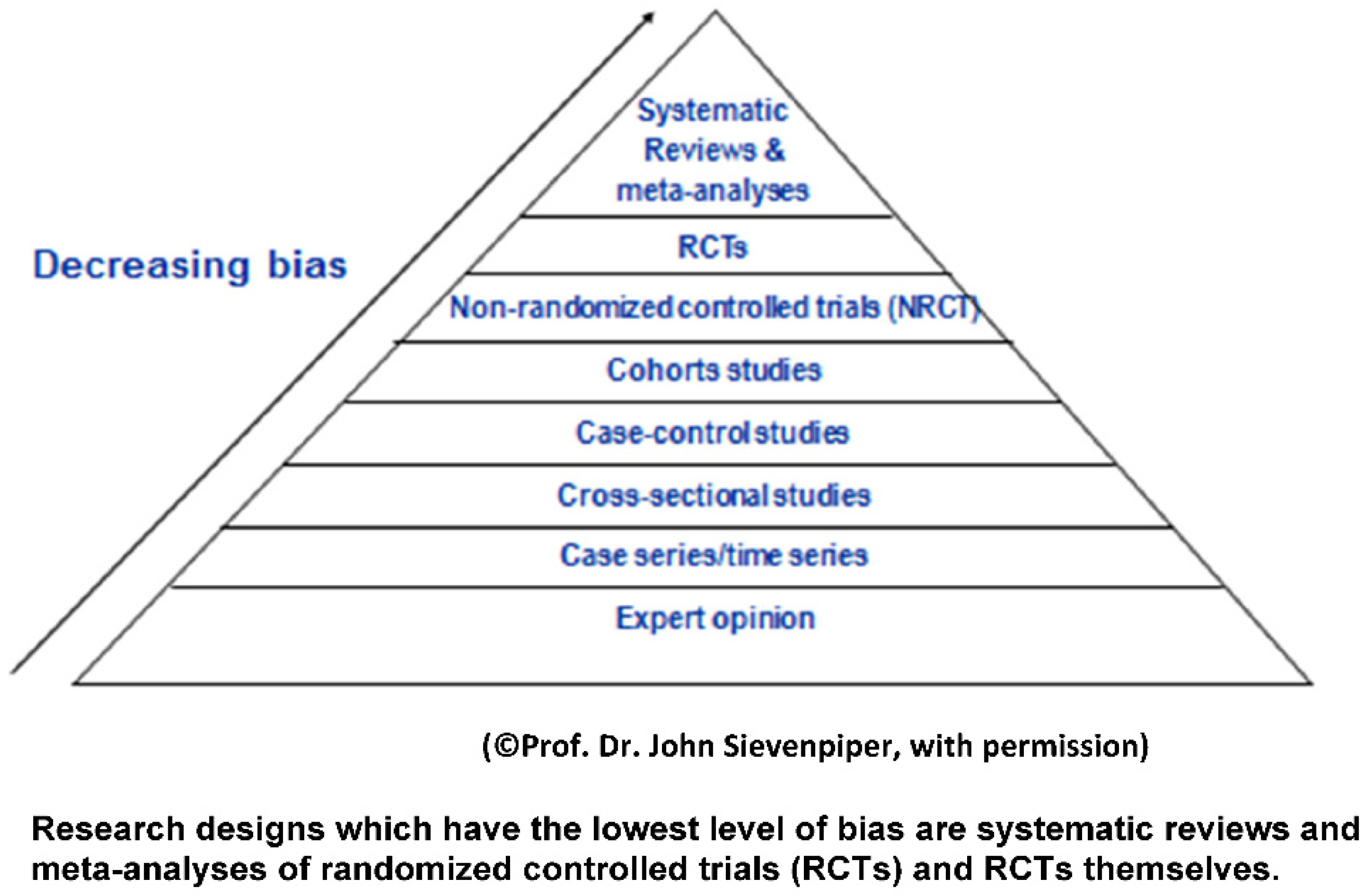
2. Levels of Evidence
3. Controversies Related to Metabolism of Fructose Containing Sugars
4. Effects of Sugars on Body Weight and Body Composition
5. Risk Factors for Diabetes
6. Risk Factors for Cardiovascular Disease
7. Effects of Sugars on the Brain
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AHA | American Heart Association |
| CVD | Cardiovascular Disease |
| fMRI | Functional MRI |
| HOMA | Homeostatic Model of Assessment |
| LDL | Low Density Lipoprotein |
| NAFLD | Non-Alcoholic Fatty Liver Disease |
| RCTs | Randomized Controlled Trials |
| SSB | Sugar Sweetened Beverages |
| NNS | Non-Nutritive Sweeteners |
References
- Gidding, S.S.; Lichtenstein, A.H.; Faith, M.S.; Karpyn, A.; Mennella, J.A.; Popkin, B.; Rowe, J.; Van Horn, L.; Whitsel, L. Implementing american heart association pediatric and adult nutrition guidelines: A scientific statement from the american heart association nutrition committee of the council on nutrition, physical activity and metabolism, council on cardiovascular disease in the young, council on arteriosclerosis, thrombosis and vascular biology, council on cardiovascular nursing, council on epidemiology and prevention, and council for high blood pressure research. Circulation 2009, 119, 1161–1175. [Google Scholar] [PubMed]
- Thomas, M.C.; Moran, J.; Forsblom, C.; Harjutsalo, V.; Thorn, L.; Ahola, A.; Waden, J.; Tolonen, N.; Saraheimo, M.; Gordin, D.; et al. The association between dietary sodium intake, esrd, and all-cause mortality in patients with type 1 diabetes. Diabetes Care 2011, 34, 861–866. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M.; Mente, A.; Rangarajan, S.; McQueen, M.J.; Wang, X.; Liu, L.; Yan, H.; Lee, S.F.; Mony, P.; Devanath, A.; et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N. Engl. J. Med. 2014, 371, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Okamura, T.; Tamaki, S.; Kadowaki, T.; Hayakawa, T.; Kita, Y.; Okayama, A.; Ueshima, H.; Group, N.D.R. Egg consumption, serum cholesterol, and cause-specific and all-cause mortality: The national integrated project for prospective observation of non-communicable disease and its trends in the aged, 1980 (nippon data80). Am. J. Clin. Nutr. 2004, 80, 58–63. [Google Scholar] [PubMed]
- Van Buul, V.J.; Tappy, L.; Brouns, F.J. Misconceptions about fructose-containing sugars and their role in the obesity epidemic. Nutr. Res. Rev. 2014, 27, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Tappy, L.; Lê, K.-A. Health effects of fructose and fructose-containing caloric sweeteners: Where do we stand 10 years after the initial whistle blowings? Curr. Diab. Rep. 2015, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kahn, R.; Sievenpiper, J.L. Dietary sugar and body weight: Have we reached a crisis in the epidemic of obesity and diabetes?: We have, but the pox on sugar is overwrought and overworked. Diabetes Care 2014, 37, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Rippe, J. Fructose, High Fructose Corn Syrup, Sucrose and Health; Humana Press: New York, NY, USA, 2014. [Google Scholar]
- Rippe, J. The metabolic and endocrine response and health implications of consuming sweetened beverages: Findings from recent, randomized, controlled trials. Adv. Nutr. 2013, 4, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Rippe, J.M.; Angelopoulos, T.J. Sucrose, high-fructose corn syrup, and fructose, their metabolism and potential health effects: What do we really know? Adv. Nutr. 2013, 4, 236–245. [Google Scholar] [CrossRef] [PubMed]
- White, J. Straight talk about high-fructose corn syrup: What it is and what it ain’t. Am. J. Clin. Nutr. 2008, 88, 1716S–1721S. [Google Scholar] [CrossRef] [PubMed]
- White, J.S. Challenging the fructose hypothesis: New perspectives on fructose consumption and metabolism. Adv. Nutr. 2013, 4, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Rippe, J.M.; Angelopoulos, T.J. Fructose-containing sugars and cardiovascular disease. Adv. Nutr. 2015, 6, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Popkin, B.M. Dietary sugar and body weight: Have we reached a crisis in the epidemic of obesity and diabetes?: Health be damned! Pour on the sugar. Diabetes Care 2014, 37, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Lustig, R.H. Fructose: Metabolic, hedonic, and societal parallels with ethanol. J. Am. Diet. Assoc. 2010, 110, 1307–1321. [Google Scholar] [CrossRef] [PubMed]
- Rippe, J.M.; Angelopoulos, T.J. Sugars and health controversies: What does the science say? Adv. Nutr. 2015, 6, 493S–503S. [Google Scholar] [CrossRef]
- Klurfeld, D.M.; Foreyt, J.; Angelopoulos, T.J.; Rippe, J.M. Lack of evidence for high fructose corn syrup as the cause of the obesity epidemic. Int. J. Obes. (Lond.) 2012, 27, 771–773. [Google Scholar] [CrossRef] [PubMed]
- American Dietetic Association. Position of the American dietetic association: Use of nutritive and nonnutritive sweeteners. J. Am. Diet. Assoc. 2004, 104, 255–275. [Google Scholar]
- Popkin, B.M. The World Is Fat: The Fads, Trends, Policies, and Products That Are Fattening the Human Race; Penguin Group: New York, NY, USA, 2008. [Google Scholar]
- DiNicolantonio, J.J.; Lucan, S.C. The wrong white crystals: Not salt but sugar as aetiological in hypertension and cardiometabolic disease. Open Heart 2014, 1, e000167. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; O’Keefe, J.H.; Lucan, S.C. Added fructose: A principal driver of type 2 diabetes mellitus and its consequences. Mayo Clin. Proc. 2015, 90, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Lucan, S.C.; DiNicolantonio, J.J. How calorie-focused thinking about obesity and related diseases may mislead and harm public health. An alternative. Public Health Nutr. 2015, 18, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Olsen, N.J.; Heitmann, B.L. Intake of calorically sweetened beverages and obesity. Obes. Rev. 2009, 10, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Schulze, M.B.; Hu, F.B. Intake of sugar-sweetened beverages and weight gain: A systematic review. Am. J. Clin. Nutr. 2006, 84, 274–288. [Google Scholar] [PubMed]
- Malik, V.S.; Popkin, B.M.; Bray, G.A.; Després, J.-P.; Hu, F.B. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation 2010, 121, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A. Fructose and risk of cardiometabolic disease. Curr. Atheroscler. Rep. 2012, 14, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Marckmann, P. Dietary treatment of thrombogenic disorders related to the metabolic syndrome. Br. J. Nutr. 2000, 83 (Suppl. 1), S121–S126. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Popkin, B.M. Calorie-sweetened beverages and fructose: What have we learned 10 years later. Pediatr. Obes. 2013, 8, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Feig, D.; Soletsky, B.; Johnson, R. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension. J. Am. Med. Assoc. 2008, 300, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.; Choi, H.K.; Lustig, R.H.; Hsu, C.Y. Sugar-sweetened beverages, serum uric acid, and blood pressure in adolescents. J. Pediatr. 2009, 154, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Yoffe, P.; Hills, N.; Lustig, R.H. The relationship of sugar to population-level diabetes prevalence: An econometric analysis of repeated cross-sectional data. PLoS ONE 2013, 8, e57873. [Google Scholar] [CrossRef] [PubMed]
- Goran, M.I.; Ulijaszek, S.J.; Ventura, E.E. High fructose corn syrup and diabetes prevalence: A global perspective. Glob. Public Health 2013, 8, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.M. The epidemiology of nonalcoholic fatty liver disease in adults. J. Clin. Gastroenterol. 2006, 40, S5–S10. [Google Scholar] [CrossRef] [PubMed]
- McCullough, A. Update on nonalcoholic fatty liver disease. J. Clin. Gastroenterol. 2002, 34, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Stephan, B.C.; Wells, J.C.; Brayne, C.; Albanese, E.; Siervo, M. Increased fructose intake as a risk factor for dementia. J. Gerontol. A. Biol. Sci. Med. Sci. 2010, 65, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Cantley, L.C. Cancer, metabolism, fructose, artificial sweeteners, and going cold turkey on sugar. BMC Biol. 2014, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Bartrina, J.A.; Rodrigo, C.P. Aassociation between sucrose intake and cancer: A review of the evidence. Nutr. Hosp. 2013, 4, 95–105. [Google Scholar]
- Stanhope, K.; Griffen, S.; Bair, B.; Swarbrick, M.; Kelm, N.; Havel, P. Twenty four hour endocrine and metabolic profiles following consumption of high-fructose corn syrup-, sucrose-, fructose-, and glucose-sweetened beverages with meals. Am. J. Clin. Nutr. 2008, 87, 1194–1203. [Google Scholar] [PubMed]
- Cox, C.L.; Stanhope, K.L.; Schwarz, J.M.; Graham, J.L.; Hatcher, B.; Griffen, S.C.; Bremer, A.A.; Berglund, L.; McGahan, J.P.; Havel, P.J.; et al. Consumption of fructose-sweetened beverages for 10 weeks reduces net fat oxidation and energy expenditure in overweight/obese men and women. Eur. J. Clin. Nutr. 2012, 66, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Stice, E.; Burger, K.S.; Yokum, S. Relative ability of fat and sugar tastes to activate reward, gustatory, and somatosensory regions. Am. J. Clin. Nutr. 2013, 98, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, A.; Mohapel, P.; Bouter, B.; Frielingsdorf, H.; Pizzo, D.; Brundin, P.; Erlanson-Albertsson, C. High-fat diet impairs hippocampal neurogenesis in male rats. Eur. J. Neurol. 2006, 13, 1385–1388. [Google Scholar] [CrossRef] [PubMed]
- Funari, V.A.; Herrera, V.L.; Freeman, D.; Tolan, D.R. Genes required for fructose metabolism are expressed in purkinje cells in the cerebellum. Brain Res. Mol. Brain Res. 2005, 142, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.C.; Martin, R.J.; Whitney, M.L.; Edwards, G.L. Intracerebroventricular injection of fructose stimulates feeding in rats. Nutr. Neurosci. 2002, 5, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.J.; Isenberg, K.; Cormier, R.J.; Benz, A.; Zorumski, C.F. Expression of fructose sensitive glucose transporter in the brains of fructose-fed rats. Neuroscience 2006, 140, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Bray, G. Energy and fructose from beverages sweetened with sugar or high-fructose corn syrup pose a health risk for some people. Adv. Nutr. 2013, 4, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Te Morenga, L.; Mallard, S.; Mann, J. Dietary sugars and body weight: Systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ 2013, 346, e7492. [Google Scholar] [CrossRef] [PubMed]
- Scientific Advisory Committee on Nutrition (SACN). Carbohydrates and Health Report; The Stationery Office: London, UK, 1 September 2014.
- Advisory Report to the Secretary of Health and Human Services and the Secretary of Agriculture. In Scientific Report of the 2015 Dietary Guidelines Advisory Committee; USDA: Washington, DC, USA, 2015.
- Johnson, R.; Appel, L.; Brands, M.; Howard, B.; Lefevre, M.; Lustig, R.; Sacks, F.; Steffen, L.; Wylie-Rosett, J.; American Heart Association Nutrition Committee of the Council on Nutrition, Physical Activity, and Metabolism and the Council on Epidemiology and Prevention. Dietary sugars intake and cardiovascular health: A scientific statement from the american heart association. Circulation 2009, 120, 1011–1020. [Google Scholar] [PubMed]
- Scottish Intercollegiate Guidelines Network (SIGN). Available online: http://www.Sign.Ac.Uk/guidelines/fulltext/50/ (accessed on 17 August 2016).
- Lowndes, J.; Sinnett, S.; Pardo, S.; Nguyen, V.T.; Melanson, K.J.; Yu, Z.; Lowther, B.E.; Rippe, J.M. The effect of normally consumed amounts of sucrose or high fructose corn syrup on lipid profiles, body composition and related parameters in overweight/obese subjects. Nutrients 2014, 6, 1128–1144. [Google Scholar] [CrossRef] [PubMed]
- Lowndes, J.; Kawiecki, D.; Pardo, S.; Nguyen, V.; Melanson, K.J.; Yu, Z.; Rippe, J.M. The effects of four hypocaloric diets containing different levels of sucrose or high fructose corn syrup on weight loss and related parameters. Nutr. J. 2012, 11, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Lowndes, J.; Sinnett, S.; Yu, Z.; Rippe, J. The effects of fructose-containing sugars on weight, body composition and cardiometabolic risk factors when consumed at up to the 90th percentile population consumption level for fructose. Nutrients 2014, 6, 3153–3168. [Google Scholar] [CrossRef] [PubMed]
- Antar, M.A.; Little, J.A.; Lucas, C.; Buckley, G.C.; Csima, A. Interrelationship between the kinds of dietary carbohydrate and fat in hyperlipoproteinemic patients. 3. Synergistic effect of sucrose and animal fat on serum lipids. Atherosclerosis 1970, 11, 191–201. [Google Scholar] [CrossRef]
- Bantle, J.P.; Swanson, J.E.; Thomas, W.; Laine, D.C. Metabolic effects of dietary sucrose in type ii diabetic subjects. Diabetes Care 1993, 16, 1301–1305. [Google Scholar] [CrossRef] [PubMed]
- Black, R.N.; Spence, M.; McMahon, R.O.; Cuskelly, G.J.; Ennis, C.N.; McCance, D.R.; Young, I.S.; Bell, P.M.; Hunter, S.J. Effect of eucaloric high- and low-sucrose diets with identical macronutrient profile on insulin resistance and vascular risk: A randomized controlled trial. Diabetes 2006, 55, 3566–3572. [Google Scholar] [CrossRef] [PubMed]
- Cooper, P.L.; Wahlqvist, M.L.; Simpson, R.W. Sucrose versus saccharin as an added sweetener in non-insulin-dependent diabetes: Short- and medium-term metabolic effects. Diabet. Med. 1988, 5, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Groen, J.J.; Balogh, M.; Yaron, E.; Cohen, A.M. Effect of interchanging bread and sucrose as main source of carbohydrate in a low fat diet on the serum cholesterol levels of healthy volunteer subjects. Am. J. Clin. Nutr. 1966, 19, 46–58. [Google Scholar] [PubMed]
- Marckmann, P.; Raben, A.; Astrup, A. Ad libitum intake of low-fat diets rich in either starchy foods or sucrose: Effects on blood lipids, factor vii coagulant activity, and fibrinogen. Metab. Clin. Exp. 2000, 49, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, L.B.; Raben, A.; Stender, S.; Astrup, A. Effect of sucrose on inflammatory markers in overweight humans. Am. J. Clin. Nutr. 2005, 82, 421–427. [Google Scholar] [PubMed]
- Stanhope, K.L.; Bremer, A.A.; Medici, V.; Nakajima, K.; Ito, Y.; Nakano, T.; Chen, G.; Fong, T.H.; Lee, V.; Menorca, R.I.; et al. Consumption of fructose and high fructose corn syrup increase postprandial triglycerides, LDL-cholesterol, and apolipoprotein-b in young men and women. J. Clin. Endocrinol. Metab. 2011, 96, E1596–E1605. [Google Scholar] [CrossRef] [PubMed]
- Tappy, L.; Le, K. Metabolic effects of fructose and the worldwide increase in obesity. Physiol. Rev. 2010, 90, 23–46. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.Z.; Empie, M.W. Fructose metabolism in humans—What isotopic tracer studies tell us. Nutr. Metab. (Lond.) 2012, 9, 89. [Google Scholar] [CrossRef] [PubMed]
- Hellerstein, M.K.; Schwarz, J.M.; Neese, R.A. Regulation of hepatic de novo lipogenesis in humans. Annu. Rev. Nutr. 1996, 16, 523–557. [Google Scholar] [CrossRef] [PubMed]
- Hellerstein, M.K. No common energy currency: De novo lipogenesis as the road less traveled. Am. J. Clin. Nutr. 2001, 74, 707–708. [Google Scholar] [PubMed]
- Faeh, D.; Minehira, K.; Schwarz, J.M.; Periasamy, R.; Park, S.; Tappy, L. Effect of fructose overfeeding and fish oil administration on hepatic de novo lipogenesis and insulin sensitivity in healthy men. Diabetes 2005, 54, 1907–1913. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.M.; Noworolski, S.M.; Wen, M.J.; Dyachenko, A.; Prior, J.L.; Weinberg, M.E.; Herraiz, L.A.; Tai, V.W.; Bergeron, N.; Bersot, T.P.; et al. Effect of a high-fructose weight-maintaining diet on lipogenesis and liver fat. J. Clin. Endocrinol. Metab. 2015, 100, 2434–2442. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.-M.; Noworolski, S.M.; Wen, M.J.; Jones, G.M.; Sinclair, E.; Dyachenco, A.; Tai, V.; Alin, M.V.; Erkin-Cakmak, A.; Gugliucci, A.; et al. Isocaloric fructose restriction for 10 days reduces hepatic de novo lipogenesis and liver fat in obese Latino and African American children. In Proceedings of the Endocrine Society’s 97th Annual Meeting and Expo, San Diego, CA, USA, 5–8 March 2015.
- Sievenpiper, J.L.; de Souza, R.J.; Kendall, C.W.; Jenkins, D.J. Is fructose a story of mice but not men? J. Am. Diet. Assoc. 2011, 111, 219–220; author reply 220–212. [Google Scholar] [CrossRef] [PubMed]
- Melanson, K.J.; Summers, A.; Nguyen, V.; Brosnahan, J.; Lowndes, J.; Angelopoulos, T.J.; Rippe, J.M. Body composition, dietary composition, and components of metabolic syndrome in overweight and obese adults after a 12-week trial on dietary treatments focused on portion control, energy density, or glycemic index. Nutr. J. 2012, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.; Havel, P. Endocrine and metabolic effects of consuming beverages sweetened with fructose, glucose, sucrose or high-fructose corn syrup. Am. J. Clin. Nutr. 2008, 88, 1733S–1737S. [Google Scholar] [CrossRef] [PubMed]
- Soenen, S.; Westerterp-Plantenga, M.S. No differences in satiety or energy intake after high fructose corn syrup, sucrose, or milk preloads. Am. J. Clin. Nutr. 2007, 86, 1586–1594. [Google Scholar] [PubMed]
- Barclay, A.; Brand-Miller, J. The Australian paradox: A substantial decline in sugars intake over the same timeframe that overweight and obesity have increased. Nutrients 2014, 6, 663–664. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Hao, T.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Changes in diet and lifestyle and long-term weight gain in women and men. N. Engl. J. Med. 2011, 364, 2392–2404. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, K.A.; Shikany, J.M.; Keating, K.D.; Allison, D.B. Will reducing sugar-sweetened beverage consumption reduce obesity? Evidence supporting conjecture is strong, but evidence when testing effect is weak. Obes. Rev. 2013, 14, 620–633. [Google Scholar] [CrossRef] [PubMed]
- Sievenpiper, J.L.; de Souza, R.J.; Mirrahimi, A.; Yu, M.E.; Carleton, A.J.; Beyene, J.; Chiavaroli, L.; Di Buono, M.; Jenkins, A.L.; Leiter, L.A.; et al. Effect of fructose on body weight in controlled feeding trials: A systematic review and meta-analysis. Ann. Intern. Med. 2012, 156, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Pan, A.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages and weight gain in children and adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2013, 98, 1084–1102. [Google Scholar] [CrossRef] [PubMed]
- Dolan, L.C.; Potter, S.M.; Burdock, G.A. Evidence-based review on the effect of normal dietary consumption of fructose on development of hyperlipidemia and obesity in healthy, normal weight individuals. Crit. Rev. Food Sci. Nutr. 2010, 50, 53–84. [Google Scholar] [CrossRef] [PubMed]
- Dolan, L.C.; Potter, S.M.; Burdock, G.A. Evidence-based review on the effect of normal dietary consumption of fructose on blood lipids and body weight of overweight and obese individuals. Crit. Rev. Food Sci. Nutr. 2010, 50, 889–918. [Google Scholar] [CrossRef] [PubMed]
- Maersk, M.; Belza, A.; Stødkilde-Jørgensen, H.; Ringgaard, S.; Chabanova, E.; Thomsen, H.; Pedersen, S.B.; Astrup, A.; Richelsen, B. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: A 6-mo randomized intervention study. Am. J. Clin. Nutr. 2012, 95, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.; Schwarz, J.; Keim, N.; Griffen, S.; Bremer, A.; Graham, J.; Hatcher, B.; Cox, C.; Dyachenko, A.; Zhang, W.; et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J. Clin. Investig. 2009, 119, 1322–1334. [Google Scholar] [CrossRef] [PubMed]
- Cozma, A.I.; Sievenpiper, J.L.; de Souza, R.J.; Chiavaroli, L.; Ha, V.; Wang, D.D.; Mirrahimi, A.; Yu, M.E.; Carleton, A.J.; Di Buono, M.; et al. Effect of fructose on glycemic control in diabetes: A systematic review and meta-analysis of controlled feeding trials. Diabetes Care 2012, 35, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- Ha, V.; Sievenpiper, J.L.; de Souza, R.J.; Chiavaroli, L.; Wang, D.D.; Cozma, A.I.; Mirrahimi, A.; Matthew, E.Y.; Carleton, A.J.; Dibuono, M. Effect of fructose on blood pressure a systematic review and meta-analysis of controlled feeding trials. Hypertension 2012, 59, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Saris, W.H.; Astrup, A.; Prentice, A.M.; Zunft, H.J.; Formiguera, X.; Verboeket-van de Venne, W.P.; Raben, A.; Poppitt, S.D.; Seppelt, B.; Johnston, S.; et al. Randomized controlled trial of changes in dietary carbohydrate/fat ratio and simple vs complex carbohydrates on body weight and blood lipids: The carmen study. The carbohydrate ratio management in european national diets. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D.; Heymsfield, S.B.; Kemnitz, J.W.; Klein, S.; Schoeller, D.A.; Speakman, J.R. Energy balance and its components: Implications for body weight regulation. Am. J. Clin. Nutr. 2012, 95, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Swinburn, B.; Sacks, G.; Ravussin, E. Increased food energy supply is more than sufficient to explain the us epidemic of obesity. Am. J. Clin. Nutr. 2009, 90, 1453–1456. [Google Scholar] [CrossRef] [PubMed]
- Rippe, J.M.; Dysinger, W.S.; Rust, R.; Frank, A.; Blair, S.N.; Parkinson, M. American college of lifestyle medicine expert panel discussion: The treat the cause movement. Am. J. Lifestyle Med. 2014, 8, 291–300. [Google Scholar] [CrossRef]
- United States Department of Agriculture-Economic Research Service. Calories average daily per capita calories from the US food supply, adjusted for spoilage and other waste. In Loss-Adjusted Food Availability Data Series; USDA: Washington, DC, USA, 2013. [Google Scholar]
- Sievenpiper, J.L.; Tappy, L.; Brouns, F. Fructose as a driver of diabetes: An incomplete view of the evidence. Mayo Clin. Proc. 2015, 90, 984–988. [Google Scholar] [CrossRef] [PubMed]
- Xi, B.; Li, S.; Liu, Z.; Tian, H.; Yin, X.; Huai, P.; Tang, W.; Zhou, D.; Steffen, L.M. Intake of fruit juice and incidence of type 2 diabetes: A systematic review and meta-analysis. PLoS ONE 2014, 9, e93471. [Google Scholar] [CrossRef] [PubMed]
- Tsilas, C.S.; de Souza, R.J.; Tawfik, R.; Blanco-Mejia, S.; Cozma, A.I.; Mirrahimi, A.; Jayalath, V.; Ha, V.; Beyene, J.; Kendall, C.W.C.; et al. No relation between total sugars intake and incident diabetes: A systematic review and meta-anaylsis of cohorts. In Proceedings of the 32nd International Symposium on Diabetes and Nutrition, Reykjavik, Iceland, 25–27 June 2014.
- The Interact Consortium, Consumption of sweet beverages and type 2 diabetes incidence in European adults: Results from epic-interact. Diabetologia 2013, 56, 1520–1530.
- Janket, S.-J.; Manson, J.E.; Sesso, H.; Buring, J.E.; Liu, S. A prospective study of sugar intake and risk of type 2 diabetes in women. Diabetes Care 2003, 26, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A.M.; English, D.R.; O’Dea, K.; Giles, G.G. Glycemic index and dietary fiber and the risk of type 2 diabetes. Diabetes Care 2004, 27, 2701–2706. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.A.; Kushi, L.H.; Jacobs, D.R., Jr.; Slavin, J.; Sellers, T.A.; Folsom, A.R. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am. J. Clin. Nutr. 2000, 71, 921–930. [Google Scholar] [PubMed]
- Colditz, G.A.; Manson, J.E.; Stampfer, M.J.; Rosner, B.; Willett, W.C.; Speizer, F.E. Diet and risk of clinical diabetes in women. Am. J. Clin. Nutr. 1992, 55, 1018–1023. [Google Scholar] [PubMed]
- Aeberli, I.; Gerber, P.A.; Hochuli, M.; Kohler, S.; Haile, S.R.; Gouni-Berthold, I.; Berthold, H.K.; Spinas, G.A.; Berneis, K. Low to moderate sugar-sweetened beverage consumption impairs glucose and lipid metabolism and promotes inflammation in healthy young men: A randomized controlled trial. Am. J. Clin. Nutr. 2011, 94, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Aeberli, I.; Hochuli, M.; Gerber, P.A.; Sze, L.; Murer, S.B.; Tappy, L.; Spinas, G.A.; Berneis, K. Moderate amounts of fructose consumption impair insulin sensitivity in healthy young men: A randomized controlled trial. Diabetes Care 2013, 36, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.C.; Davis, S.N.; Mann, S.L.; Cherrington, A.D. Acute fructose administration improves oral glucose tolerance in adults with type 2 diabetes. Diabetes Care 2001, 24, 1882–1887. [Google Scholar] [CrossRef] [PubMed]
- Lowndes, J.; Sinnett, S.; Rippe, J. No change in oral glucose tolerance tests as a result of ten weeks of consumption of various fructose containing sugars or glucose. In Proceedings of the Endocrine Society’s 96th Annual Meeting and Expo, Chicago, IL, USA, 21–24 June 2014.
- Johnson, R.K.; Appel, L.J.; Brands, M.; Howard, B.V.; Lefevre, M.; Lustig, R.H.; Sacks, F.; Steffen, L.M.; Wylie-Rosett, J. Dietary sugars intake and cardiovascular health: A scientific statement from the American heart association. Circulation 2009, 120, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Stone, N.; Ballantye, C.; Bttiner, V.; Criqui, M.; Ginsberg, H.; Goldberg, H.; Howard, W.; Jacobson, M.; Kris Etherton, P.; et al. Triglycerides and cardiovascular disease: A scientific statement from the American heart association. Circulation 2011, 123, 2292–2333. [Google Scholar] [CrossRef] [PubMed]
- Livesey, G.; Taylor, R. Fructose consumption and consequences for glycation, plasma triacylglycerol, and body weight: Meta-analyses and meta-regression models of intervention studies. Am. J. Clin. Nutr. 2008, 88, 1419–1437. [Google Scholar] [PubMed]
- Wang, D.D.; Sievenpiper, J.L.; de Souza, R.J.; Cozma, A.I.; Chiavaroli, L.; Ha, V.; Mirrahimi, A.; Carleton, A.J.; Di Buono, M.; Jenkins, A.L.; et al. Effect of fructose on postprandial triglycerides: A systematic review and meta-analysis of controlled feeding trials. Atherosclerosis 2014, 232, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Archer, E.; Pavela, G.; Lavie, C.J. The inadmissibility of what we eat in America and nhanes dietary data in nutrition and obesity research and the scientific formulation of national dietary guidelines. Mayo Clin. Proc. 2015, 90, 911–926. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, Z.; Gregg, E.W.; Flanders, W.D.; Merritt, R.; Hu, F.B. Added sugar intake and cardiovascular diseases mortality among us adults. JAMA Intern. Med. 2014, 174, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L.; Medici, V.; Bremer, A.A.; Lee, V.; Lam, H.D.; Nunez, M.V.; Chen, G.X.; Keim, N.L.; Havel, P.J. A dose-response study of consuming high-fructose corn syrup–sweetened beverages on lipid/lipoprotein risk factors for cardiovascular disease in young adults. Am. J. Clin. Nutr. 2015. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L.; Havel, P.J. Fructose consumption: Potential mechanisms for its effects to increase visceral adiposity and induce dyslipidemia and insulin resistance. Curr. Opin. Lipidol. 2008, 19, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Te Morenga, L.A.; Howatson, A.J.; Jones, R.M.; Mann, J. Dietary sugars and cardiometabolic risk: Systematic review and meta-analyses of randomized controlled trials of the effects on blood pressure and lipids. Am. J. Clin. Nutr. 2014, 100, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Bremer, A.A.; Auinger, P.; Byrd, R.S. Relationship between insulin resistance-associated metabolic parameters and anthropometric measurements with sugar-sweetened beverage intake and physical activity levels in us adolescents: Findings from the 1999–2004 national health and nutrition examination survey. Arch. Pediatr. Adolesc. Med. 2009, 163, 328–335. [Google Scholar] [PubMed]
- Dhingra, R.; Sullivan, L.; Jacques, P.F.; Wang, T.J.; Fox, C.S.; Meigs, J.B.; D’Agostino, R.B.; Gaziano, J.M.; Vasan, R.S. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation 2007, 116, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, M.; Serre, F.; Cantin, L.; Ahmed, S.H. Intense sweetness surpasses cocaine reward. PLoS ONE 2007, 2, e698. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.P.; Bartness, T.J.; Mielke, J.G.; Parent, M.B. A high fructose diet impairs spatial memory in male rats. Neurobiol. Learn. Mem. 2009, 92, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Messier, C.; Whately, K.; Liang, J.; Du, L.; Puissant, D. The effects of a high-fat, high-fructose, and combination diet on learning, weight, and glucose regulation in c57bl/6 mice. Behav. Brain Res. 2007, 178, 139–145. [Google Scholar] [CrossRef] [PubMed]
- De Silva, A.; Salem, V.; Matthews, P.M.; Dhillo, W.S. The use of functional MRI to study appetite control in the CNS. Exp. Diabetes Res. 2012, 2012, 764017. [Google Scholar] [CrossRef] [PubMed]
- Premack, D. Human and animal cognition: Continuity and discontinuity. Proc. Natl. Acad. Sci. USA 2007, 104, 13861–13867. [Google Scholar] [CrossRef] [PubMed]
- Sarter, M. Animal cognition: Defining the issues. Neurosci. Biobehav. Rev. 2004, 28, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Page, K.A.; Luo, S.; Romero, A.; Adam, T.C.; Hu, H.; Monterosso, J. Fructose compared to glucose ingestion preferentially activates brain reward regions in response to high-calorie food cues in young, obese hispanic females. Endocrinol. Rev. 2012, 33, 1666. [Google Scholar]
- Purnell, J.Q.; Klopfenstein, B.A.; Stevens, A.A.; Havel, P.J.; Adams, S.H.; Dunn, T.N.; Krisky, C.; Rooney, W.D. Brain functional magnetic resonance imaging response to glucose and fructose infusions in humans. Diabetes Obes. Metab. 2011, 13, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Pena-Gomez, C.; Alonso-Alonso, M.; Bravo, S.; Magerowski, G.; Sinnett, S.; Blackburn, G.; Rippe, J. Hypothalamic fmri responses to different sugars under normal intake conditions: A pilot study. In Proceedings of the Obesity Society 32nd Annual Scientific Meeting (NAASO), Atlanta, GA, USA, 11–17 November 2013.
- Wang, G.J.; Volkow, N.D.; Fowler, J.S. The role of dopamine in motivation for food in humans: Implications for obesity. Expert Opin. Ther. Targets 2002, 6, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Blum, K.; Braverman, E.R.; Holder, J.M.; Lubar, J.F.; Monastra, V.J.; Miller, D.; Lubar, J.O.; Chen, T.J.; Comings, D.E. Reward deficiency syndrome: A biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J. Psychoact. Drugs 2000, 32 (Suppl. i–iv), 1–112. [Google Scholar] [CrossRef]
- Lowndes, J.; Rippe, J.M. No effect of sugar sweetened milk on performance of a battery of cognitive assessment tests. FASEB J. 2016, 30, 1160–1163. [Google Scholar]
- Lowndes, J.; Angelopoulos, T.J.; Rippe, J.M. No effect of sugar sweetened or diet beverages on performance of a battery of cognitive assessment tests. FASEB J. 2016, 30, 1160–1164. [Google Scholar]
- Lustig, R.H.; Schmidt, L.A.; Brindis, C.D. Public health: The toxic truth about sugar. Nature 2012, 482, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Bocarsly, M.E.; Powell, E.S.; Avena, N.M.; Hoebel, B.G. High-fructose corn syrup causes characteristics of obesity in rats: Increased body weight, body fat and triglyceride levels. Pharmacol. Biochem. Behav. 2010, 97, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Avena, N.M.; Hoebel, B.G. Bingeing, withdrawal and craving: An animal model of sugar addiction. In Food and Addiction: A Comprehension Handbook; Gold, M., Brownell, K., Eds.; Oxford University Press: New York, NY, USA, 2012. [Google Scholar]
- Ziauddeen, H.; Farooqi, I.; Fletcher, P. Obesity and the brain: How convincing is the addiction model? Nat. Rev. 2012, 13, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Benton, D. The plausibility of sugar addiction and its role in obesity and eating disorders. Clin. Nutr. 2010, 29, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Corwin, R.L.W.; Hayes, J.E. Are sugars addictive? Perspectives for practitioners. In Fructose, High Fructose Corn Syrup, Sucrose and Health; Rippe, J., Ed.; Springer: New York, NY, USA, 2014; pp. 199–215. [Google Scholar]
- Slavin, J. Two more pieces to the 1000-piece carbohydrate puzzle. Am. J. Clin. Nutr. 2014, 100, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am. J. Clin. Nutr. 2010, 91, 535–546. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Otto, M.C.; Mozaffarian, D.; Kromhout, D.; Bertoni, A.G.; Sibley, C.T.; Jacobs, D.R., Jr.; Nettleton, J.A. Dietary intake of saturated fat by food source and incident cardiovascular disease: The multi-ethnic study of atherosclerosis. Am. J. Clin. Nutr. 2012, 96, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Astrup, A. A changing view on saturated fatty acids and dairy: From enemy to friend. Am. J. Clin. Nutr. 2014, 100, 1407–1408. [Google Scholar] [CrossRef] [PubMed]
- Andon, B.; Anderson, J. The oatmeal-cholesterol connection: 10 years later. Am. J. Lifestyle Med. 2008, 2, 51–57. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture; U.S. Department of Health and Human Services. Report of the Advisory Committee on the Dietary Guidelines for Americans 2010, 7th ed.U.S. Government Printing Office: Washington, DC, USA, 2010.
| Type of Analysis | Findings | |
|---|---|---|
| Lowndes et al. [51] | 50th percentile consumption of fructose containing sugars | No increase in body weight over 10 weeks and no increase in triglycerides. No increase in risk factors for diabetes |
| Lowndes et al. [52] | Comparison between 10 and 20 percent of calories from either HFCS or sucrose in hypocaloric diets | Significant weight loss occurred in all groups |
| Lowndes et al. [53] | RCT 355 men and women consuming 8%, 18% or 30% of kcals per days either sucrose or HFCS | Average weight gain over 2 pounds over 10 week period. Mostly driven by 30% kcal per day group. No increased risk factors for diabetes. 10% increase in triglycerides confounded by 2 pound weight gain. |
| Antar et al. [54] | Randomized Control Trial | Increase in fasting triglycerides from various levels of added sugar consumption |
| Bantle et al. [55] | Randomized Control Trial | Increase in fasting triglycerides from various levels of added sugar consumption |
| Black et al. [56] | Randomized Control Trial | Increase in fasting triglycerides from various levels of added sugar consumption |
| Cooper et al. [57] | Randomized Control Trial | Increase in fasting triglycerides from various levels of added sugar consumption |
| Groen et al. [58] | Randomized Control Trial | Increase in fasting triglycerides from various levels of added sugar consumption |
| Marckmann et al. [59] | Randomized Control Trial | Increase in fasting triglycerides from various levels of added sugar consumption |
| Sorensen et al. [60] | Randomized Control Trial | Increase in fasting triglycerides from various levels of added sugar consumption |
| Stanhope et al. [61] | Randomized Control Trial | Increase in fasting triglycerides from various levels of added sugar consumption |
| Type of Analysis | Findings | |
|---|---|---|
| Sievenpiper et al. [76] | Aggregated randomized control trials looking at isocaloric exchange of either sugar or fructose with other macronutrients to assess effects on body weight in adults | No significant effect of either sugar or fructose on body weight |
| Te Morenga et al. [46] | Aggregated randomized control trials looking at isocaloric exchange of either sugar or fructose with other macronutrients to assess effects on body weight in adults | No significant effect of either sugar or fructose on body weight |
| Malik et al. [77] | Meta-analysis of 5 trials | 2 of 5 trials resulted in significant weight loss from reducing sugar calories in one model but not another |
| Dolan et al. [78] | Normal weight individuals. Interventional Studies utilizing the FDA guidance for evidence based reviews | No difference with regard to obesity from fructose consumption in normal weight individuals |
| Dolan et al. [79] | Obese individuals. Interventional Studies utilizing the FDA guidance for evidence based reviews | No difference with regard to obesity from fructose consumption in obese individuals |
| Cozma et al. [82] | Systematic review and meta-analysis of 18 RCTs | Decrease in risk factors for diabetes such as glycosylated proteins |
| Malik et al. [24] | Meta-analysis of 8 cohort studies | 4 did not find a significant effect of SSB on incidence of diabetes and 5 did not adjust findings for energy intake and body weight |
| Ha et al. [83] | 15 studies involving 355 individuals | Slight decreases in diastolic and mean blood pressure and isocaloric substitution or hypercaloric trials |
| Type of Analysis | Findings | |
|---|---|---|
| Hodge et al. [94] | Cohort Study | No significant association between sugar intake and diabetes |
| Meyer et al. [95] | Cohort Study in Older women | Significant negative association between sugar intake and diabetes |
| Colditz et al. [96] | Cohort Study in women | No association between sugar intake and diabetes |
| Interact [92] | Cohort Study in European Adults | No increase in diabetes risk with added sugars |
| Archer et al. [105] | NHANES data analysis | Individuals who consumed 25% or more of calories from added sugars experienced an increase associated risk of cardiovascular disease compared to individuals who consumed less than 10% of calories from added sugars |
| Yang et al. [106] | NHANES data analysis | CVD risk increased to 1.30 for individuals who consumed 10 to 24.9% of calories and 2.75 for those who consumed 25% or more calories for added sugars compared to individuals who consumed less than 10% of calories from added sugars |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rippe, J.M.; Angelopoulos, T.J. Relationship between Added Sugars Consumption and Chronic Disease Risk Factors: Current Understanding. Nutrients 2016, 8, 697. https://doi.org/10.3390/nu8110697
Rippe JM, Angelopoulos TJ. Relationship between Added Sugars Consumption and Chronic Disease Risk Factors: Current Understanding. Nutrients. 2016; 8(11):697. https://doi.org/10.3390/nu8110697
Chicago/Turabian StyleRippe, James M., and Theodore J. Angelopoulos. 2016. "Relationship between Added Sugars Consumption and Chronic Disease Risk Factors: Current Understanding" Nutrients 8, no. 11: 697. https://doi.org/10.3390/nu8110697
APA StyleRippe, J. M., & Angelopoulos, T. J. (2016). Relationship between Added Sugars Consumption and Chronic Disease Risk Factors: Current Understanding. Nutrients, 8(11), 697. https://doi.org/10.3390/nu8110697




