The Effect of Zinc and Selenium Supplementation Mode on Their Bioavailability in the Rat Prostate. Should Administration Be Joint or Separate?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
- Zn: Zinc gluconate (C12H22O14Zn·xH2O), 5.0 mgZn/kg b.w.
- Se: Sodium selenite (Na2SeO3), 2.8 μgSe/kg b.w.
- SeMet: Selenomethionine (CH3SeCH2CH2CH(NH2)COOH), 2.8 μgSe/kg b.w.
- Zn + Se: Zinc gluconate, 5.0 mgZn/kg b.w. and sodium selenite, 2.8 μgSe/kg b.w.
- Zn + SeMet: Zinc gluconate, 5.0 mgZn/kg b.w. and selenomethionine, 2.8 μgSe/kg b.w.
2.2. Element Determinations
2.3. Metallothionein-Like Proteins Determinations
2.4. Biochemical Parameters
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer statistics. CA Cancer J. Clin. 2010, 60, 277–300. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Prostate Cancer. Available online: http//:www.cancer.org/acs/groups/cid/documents/webcontent/003134-pdf.pdf (accessed on 23 June 2016).
- Costello, L.C.; Liu, Y.; Franklin, R.B.; Kennedy, M.C. Zinc inhibition of mitochondrial aconitase and its importance in citrate metabolism of prostate epithelial cells. J. Biol. Chem. 1997, 272, 28875–28881. [Google Scholar] [CrossRef] [PubMed]
- Costello, L.C.; Franklin, R.B.; Liu, Y.; Kennedy, M.C. Zinc causes a shift toward citrate at equilibrium of the m-aconitase reaction of prostate mitochondria. J. Inorg. Biochem. 2000, 78, 161–165. [Google Scholar] [CrossRef]
- Costello, L.C.; Franklin, R.B. The intermediary metabolism of the prostate: A key to understanding the pathogenesis and progression of prostate malignancy. Oncology 2000, 59, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Liang, J.Y.; Li, T.L.; Guan, Z.X.; Zou, J.; Franklin, R.; Costello, L.C. Zinc induces mitochondria apoptogenesis in prostate cells. Mol. Urol. 2000, 4, 31–36. [Google Scholar] [PubMed]
- Costello, L.C.; Franklin, R.B.; Feng, P. Mitochondrial function, zinc, and intermediary metabolism relationships in normal prostate and prostate cancer. Mitochondrion 2005, 5, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Dakubo, G.D.; Parr, R.L.; Costello, L.C.; Franklin, R.B.; Thayer, R.E. Altered metabolism and mitochondrial genome in prostate cancer. J. Clin. Pathol. 2006, 59, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Kanoni, S.; Dedoussis, G.V.; Herbein, G.; Fulop, T.; Varin, A.; Jajte, J.; Rink, L.; Monti, D.; Mariani, E.; Malavolta, M.; et al. Assessment of gene-nutrient interactions on inflammatory status of the elderly with the use of a zinc diet score-ZINCAGE study. J. Nutr. Biochem. 2010, 21, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Dedoussis, G.V.; Kanoni, S.; Mariani, E.; Cattini, L.; Herbein, G.; Fulop, T.; Varin, A.; Rink, L.; Jajte, J.; Monti, D.; et al. Mediterranean diet and plasma concentration of inflammatory markers in old and very old subjects in the ZINCAGE population study. Clin. Chem. Lab. Med. 2008, 46, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.B.; Winkleby, M.A.; Radimer, K.L. Dietary intakes and serum nutrients differ between adults from food-insufficient and food-sufficient families: Third National Health and Nutrition Examination Survey, 1988–1994. J. Nutr. 2001, 131, 1232–1246. [Google Scholar] [PubMed]
- Gonzalez, A.; Peters, U.; Lampe, J.W.; White, E. Zinc intake from supplements and diet and prostate cancer. Nutr. Cancer 2009, 61, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S.; Beck, F.W.; Bao, B.; Fitzgerald, J.T.; Snell, D.C.; Steinberg, J.D.; Cardozo, L.J. Zinc supplementation decreases incidence of infections in the elderly: Effect of zinc on generation of cytokines and oxidative stress. Am. J. Clin. Nutr. 2007, 85, 837–844. [Google Scholar] [PubMed]
- Barnett, J.B.; Hamer, D.H.; Meydani, S.N. Low zinc status: A new risk factor for pneumonia in the elderly? Nutr. Rev. 2010, 68, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Sapota, A.; Daragó, A.; Skrzypińska-Gawrysiak, M.; Nasiadek, M.; Klimczak, M.; Kilanowicz, A. The bioavailability of different zinc compounds used as human dietary supplements in rat prostate: A comparative study. Biometals 2014, 27, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Wegmüller, R.; Tay, F.; Zeder, C.; Brnic, M.; Hurrell, R.F. Zinc absorption by young adults from supplemental zinc citrate is comparable with that from zinc gluconate and higher than from zinc oxide. J. Nutr. 2014, 144, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium in cancer prevention: A review of the evidence and mechanism of action. Proc. Nutr. Soc. 2005, 64, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Santhosh, K.B.; Priyadarsini, K.I. Selenium nutrition: How important is it? Biomed. Prev. Nutr. 2014, 4, 333–341. [Google Scholar] [CrossRef]
- World Health Organization. International program on chemical safety. In Selenium; WHO Press: Geneva, Switzerland, 1987; p. 58. [Google Scholar]
- Yang, H.; Jia, X. Safety evaluation of Se-methylselenocysteine as nutritional selenium supplement: Acute toxicity, genotoxicity and subchronic toxicity. Regul. Toxicol. Pharmacol. 2014, 70, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. The function of zinc metallothionein: A link between cellular zinc and redox state. J. Nutr. 2000, 130, 1455S–1458S. [Google Scholar] [PubMed]
- Danch, A.; Drozdz, M. A simplified technique of fluorometric selenium assay in biological material. Diagn. Lab. 1996, 32, 529–534. [Google Scholar]
- Eaton, D.L.; Cherian, M.G. Determination of metallothionein in tissues by cadmium-hemoglobin affinity assay. Methods Enzymol. 1991, 205, 83–88. [Google Scholar] [PubMed]
- Haase, H.; Rink, L. The immune system and the impact of zinc during aging. Immun. Ageing 2009, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, Á.J. Zinc, aging, and immunosenescence: An overview. Pathobiol. Aging Age Relat. Dis. 2015, 5, 25592. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.D.; Metter, E.J.; Chan, D.W.; Sokoll, L.J.; Landis, P.; Nelson, W.G.; Muller, D.; Andres, R.; Carter, H.B. Plasma selenium level before diagnosis and the risk of prostate cancer development. J. Urol. 2001, 166, 2034–2038. [Google Scholar] [CrossRef]
- Costello, L.C.; Franklin, R.B. The clinical relevance of the metabolism of prostate cancer; zinc and tumor suppression: Connecting the dots. Mol. Cancer 2006, 5, 17. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Costello, L.C.; Franklin, R.B. Zinc is decreased in prostate cancer: An established relationship of prostate cancer! J. Biol. Inorg. Chem. 2011, 16, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Costello, L.C.; Franklin, R.B. Novel role of zinc in the regulation of prostate citrate metabolism and its implications in prostate cancer. Prostate 1998, 35, 285–296. [Google Scholar] [CrossRef]
- Elzanaty, S. Association between age and epididymal and accessory sex gland function and their relation to sperm motility. Arch. Androl. 2007, 53, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Zaichick, V.Y.; Sviridova, T.V.; Zaichick, S.V. Zinc in the human prostate gland: Normal, hyperplastic and cancerous. Int. Urol. Nephrol. 1997, 29, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Sapota, A.; Darago, A.; Taczalski, J.; Kilanowicz, A. Disturbed homeostasis of zinc and other essential elements in the prostate gland dependent on the character of pathological lesions. Biometals 2009, 22, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, S.L.; McCormick, N.H.; Velasquez, V.; Lopez, V. Zinc in specialized secretory tissues: Roles in the pancreas, prostate, and mammary gland. Adv. Nutr. 2011, 2, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Costello, L.C.; Franklin, R.B.; Tan, M.T. A Critical Assessment of Epidemiology Studies Regarding Dietary/Supplemental Zinc and Prostate Cancer Risk. Open. Urol. Nephrol. J. 2008, 1. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.B.; Costello, L.C. Zinc as an anti-tumor agent in prostate cancer and in other cancers. Arch. Biochem. Biophys. 2007, 463, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.P.; Dwivedi, S.; Dhakad, U.; Murthy, R.C.; Choubey, V.K.; Goel, A.; Sankhwar, S.N. Status and Interrelationship of Zinc, Copper, Iron, Calcium and Selenium in Prostate Cancer. Indian. J. Clin. Biochem. 2016, 31, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Platz, E.A.; Helzlsouer, K.J. Selenium, zinc, and prostate cancer. Epidemiol. Rev. 2001, 23, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Hurst, R.; Hooper, L.; Norat, T.; Lau, R.; Aune, D.; Greenwood, D.C.; Vieira, R.; Collings, R.; Harvey, L.J.; Sterne, J.A.; et al. Selenium and prostate cancer: Systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 96, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Dennert, G.; Zwahlen, M.; Brinkman, M.; Vinceti, M.; Zeegers, M.P.; Horneber, M. Selenium for preventing cancer. Cochrane Database Syst. Rev. 2011, 11, CD005195. [Google Scholar]
- Meyer, F.; Galan, P.; Douville, P.; Bairati, I.; Kegle, P.; Bertrais, S.; Estaquio, C.; Hercberg, S. Antioxidant vitamin and mineral supplementation and prostate cancer prevention in the SU.VI.MAX trial. Int. J. Cancer 2005, 116, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Metallothionein redox biology in the cytoprotective and cytotoxic functions of zinc. Exp. Gerontol. 2008, 43, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Iwai, N.; Watanabe, C.; Suzuki, T.; Suzuki, K.T.; Tohyama, C. Metallothionein induction by sodium selenite at two different ambient temperatures in mice. Arch. Toxicol. 1988, 62, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.G.; Vallee, B.L. The metallothionein/thionein system: An oxidoreductive metabolic zinc link. Chembiochem 2009, 10, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Mocchegiani, E.; Malavolta, M.; Muti, E.; Costarelli, L.; Cipriano, C.; Piacenza, F.; Tesei, S.; Giacconi, R.; Lattanzio, F. Zinc, metallothioneins and longevity: Interrelationships with niacin and selenium. Curr. Pharm. Des. 2008, 14, 2719–2732. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, K.; Morihara, N.; Usui, S.; Hayama, M.; Sugimura, Y.; Hirano, K. Castration- and aging-induced changes in the expression of zinc transporter and metallothionein in rat prostate. J. Androl. 2011, 32, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Celec, P. Benign prostatic hyperplasia. In Handbook of Models for Human Aging; Conn, P.M., Ed.; Elsevier: San Diego, CA, USA, 2006; pp. 641–649. [Google Scholar]
- Behne, D.; Kyriakopoulos, A.; Kalcklösch, M.; Weiss-Nowak, C.; Pfeifer, H.; Gessner, H.; Hammel, C. Two new selenoproteins found in the prostatic glandular epithelium and in the spermatid nuclei. Biomed. Environ. Sci. 1997, 10, 340–345. [Google Scholar] [PubMed]
- Hmielnicka, J.; Zareba, G.; Witasik, M.; Brzeźnicka, E. Zinc-selenium interaction in the rat. Biol. Trace Elem. Res. 1988, 15, 267–276. [Google Scholar] [CrossRef]
- Thomson, C.D.; Robinson, M.F.; Butler, J.A.; Whanger, P.D. Long-term supplementation with selenate and selenomethionine: Selenium and glutathione peroxidase (EC 1.11 1.9) in blood components of New Zealand women. Br. J. Nutr. 1993, 69, 577–588. [Google Scholar] [CrossRef] [PubMed]
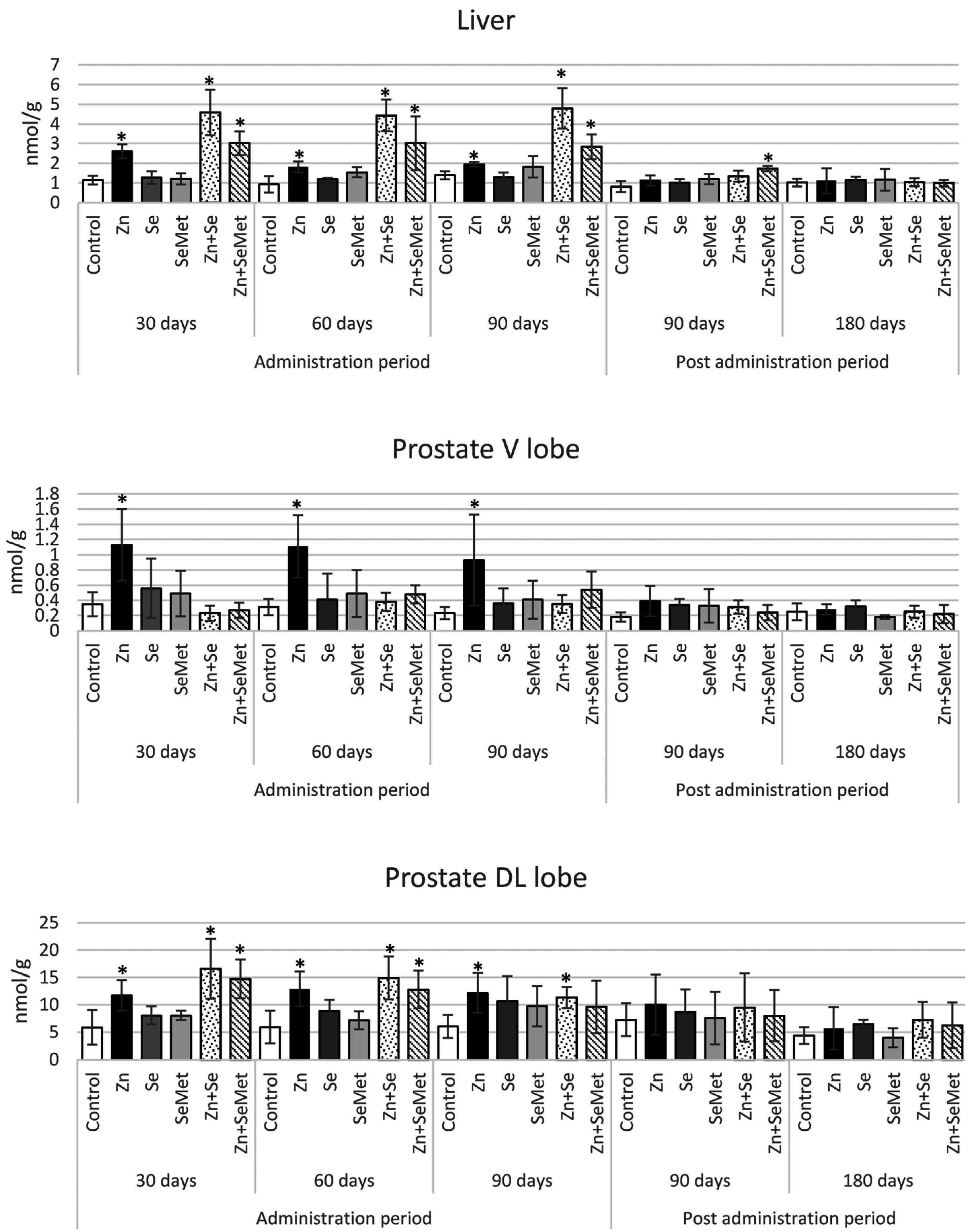
| Blood (μg/mL) | Liver (μg/g w.t.) | Prostate (μg/g w.t.) | ||
|---|---|---|---|---|
| V Lobe | DL Lobe | |||
| Administration Period | ||||
| 30-day | ||||
| Control | 5.67 ± 0.18 | 38.77 ± 1.73 | 28.03 ± 7.21 | 240.27 ± 66.58 |
| Zn | 5.83 ± 0.21 | 42.80 ± 1.82 * | 31.89 ± 3.14 | 396.77 ± 63.18 * |
| Se | 5.63 ± 0.14 | 41.69 ± 1.73 | 21.01 ± 6.45 | 298.33 ± 22.99 |
| SeMet | 5.97 ± 0.22 | 41.07 ± 2.39 | 20.98 ± 5.55 | 238.22 ± 42.61 |
| Zn + Se | 5.98 ± 0.20 | 52.89 ± 2.57 * | 21.54 ± 6.21 | 338.97 ± 87.62 |
| Zn + SeMet | 5.67 ± 0.31 | 50.53 ± 5.40 * | 21.54 ± 4.98 | 309.13 ± 82.32 |
| 60-day | ||||
| Control | 5.83 ± 0.34 | 37.23 ± 2.84 | 25.63 ± 4.21 | 256.33 ± 40.15 |
| Zn | 5.43 ± 0.21 | 43.70 ± 3.78 * | 29.56 ± 3.11 | 382.21 ± 61.22 * |
| Se | 5.83 ± 0.14 | 38.95 ± 4.64 | 24.39 ± 2.66 | 284.33 ± 39.21 |
| SeMet | 5.37 ± 0.25 | 38.02 ± 4.60 | 20.48 ± 3.74 | 231.22 ± 48.71 |
| Zn + Se | 5.98 ± 0.33 | 44.72 ± 4.01 * | 20.14 ± 4.15 | 341.11 ± 70.55 |
| Zn + SeMet | 5.77 ± 0.24 | 44.90 ± 2.36 * | 22.65 ± 3.11 | 298.77 ± 41.20 |
| 90-day | ||||
| Control | 5.43 ± 0.24 | 34.94 ± 2.68 | 20.34 ± 2.83 | 244.81 ± 28.11 |
| Zn | 5.43 ± 0.19 | 43.50 ± 3.77 * | 29.39 ± 5.38 * | 374.86 ± 38.35 * |
| Se | 5.16 ± 0.27 | 37.04 ± 1.56 | 23.67 ± 6.31 | 285.77 ± 49.42 |
| SeMet | 5.83 ± 0.24 | 40.67 ± 3.44 | 19.74 ± 4.57 | 225.20 ± 68.75 |
| Zn + Se | 5.64 ± 0.18 | 44.13 ± 4.10 * | 16.83 ± 7.28 | 306.08 ± 49.00 |
| Zn + SeMet | 5.76 ± 0.36 | 43.52 ± 3.17 * | 21.73 ± 11.23 | 301.98 ± 46.35 |
| Post Administration Period | ||||
| 90-day | ||||
| Control | 5.25 ± 0.31 | 35.42 ± 2.68 | 15.25 ± 4.10 | 275.23 ± 44.29 |
| Zn | 5.87 ± 0.36 | 41.29 ± 4.19 | 10.53 ± 6.32 | 375.18 ± 64.09 * |
| Se | 5.16 ± 0.22 | 37.19 ± 3.71 | 13.75 ± 6.02 | 232.16 ± 26.50 |
| SeMet | 5.76 ± 0.38 | 35.48 ± 4.14 | 14.69 ± 4.32 | 263.68 ± 51.97 |
| Zn + Se | 5.44 ± 0.26 | 38.08 ± 3.16 | 10.32 ± 3.06 | 267.30 ± 75.21 |
| Zn + SeMet | 5.91 ± 0.41 | 38.91 ± 2.83 | 13.25 ± 3.37 | 275.65 ± 37.11 |
| 180-day | ||||
| Control | 5.33 ± 0.35 | 36.41 ± 3.85 | 14.81 ± 5.54 | 261.56 ± 69.86 |
| Zn | 5.04 ± 0.28 | 40.08 ± 1.81 | 11.89 ± 1.88 | 303.30 ± 91.57 |
| Se | 4.98 ± 0.24 | 35.95 ± 2.53 | 12.36 ± 4.07 | 282.10 ± 8.64 |
| SeMet | 5.49 ± 0.28 | 39.56 ± 5.57 | 11.91 ± 3.24 | 193.87 ± 74.09 |
| Zn + Se | 4.87 ± 0.31 | 35.96 ± 1.78 | 13.38 ± 7.02 | 258.20 ± 64.57 |
| Zn + SeMet | 5.88 ± 0.42 | 38.13 ± 1.88 | 10.52 ± 2.94 | 237.64 ± 50.23 |
| Blood (μg/mL) | Liver (μg/g w.t.) | Prostate (μg/g w.t.) | ||
|---|---|---|---|---|
| V Lobe | DL Lobe | |||
| Administration Period | ||||
| 30-day | ||||
| Control | 0.51 ± 0.03 | 1.18 ± 0.02 | 0.41 ± 0.05 | 0.23 ± 0.03 |
| Zn | 0.53 ± 0.02 | 1.17 ± 0.07 | 0.37 ± 0.05 | 0.28 ± 0.06 |
| Se | 0.56 ± 0.03 | 1.24 ± 0.02 * | 0.40 ± 0.08 | 0.30 ± 0.03 * |
| SeMet | 0.56 ± 0.03 | 1.27 ± 0.03 * | 0.52 ± 0.05 * | 0.31 ± 0.02 * |
| Zn + Se | 0.52 ± 0.04 | 1.24 ± 0.05 * | 0.41 ± 0.04 | 0.32 ± 0.02 * |
| Zn + SeMet | 0.50 ± 0.02 | 1.26 ± 0.05 * | 0.51 ± 0.05 * | 0.31 ± 0.02 * |
| 60-day | ||||
| Control | 0.53 ± 0.02 | 1.22 ± 0.02 | 0.41 ± 0.07 | 0.24 ± 0.04 |
| Zn | 0.51 ± 0.02 | 1.28 ± 0.09 | 0.42 ± 0.04 | 0.27 ± 0.02 |
| Se | 0.58 ± 0.02 * | 1.41 ± 0.09 * | 0.48 ± 0.06 | 0.36 ± 0.06 * |
| SeMet | 0.56 ± 0.01 * | 1.40 ± 0.12 * | 0.63 ± 0.08 * | 0.38 ± 0.04 * |
| Zn + Se | 0.55 ± 0.02 | 1.31 ± 0.06 * | 0.50 ± 0.06 | 0.34 ± 0.05 * |
| Zn + SeMet | 0.52 ± 0.03 | 1.31 ± 0.06 * | 0.62 ± 0.07 * | 0.36 ± 0.04 * |
| 90-day | ||||
| Control | 0.51 ± 0.03 | 1.21 ± 0.08 | 0.45 ± 0.07 | 0.28 ± 0.02 |
| Zn | 0.52 ± 0.02 | 1.27 ± 0.09 | 0.49 ± 0.05 | 0.27 ± 0.03 |
| Se | 0.59 ± 0.03 * | 1.41 ± 0.04 * | 0.49 ± 0.04 | 0.42 ± 0.04 * |
| SeMet | 0.58 ± 0.03 * | 1.39 ± 0.04 * | 0.69 ± 0.09 * | 0.39 ± 0.02 * |
| Zn + Se | 0.51 ± 0.04 | 1.36 ± 0.05 * | 0.52 ± 0.04 | 0.37 ± 0.07 * |
| Zn + SeMet | 0.50 ± 0.04 | 1.41 ± 0.06 * | 0.62 ± 0.05 * | 0.39 ± 0.06 * |
| Post Administration Period | ||||
| 90-day | ||||
| Control | 0.51 ± 0.03 | 1.13 ± 0.10 | 0.36 ± 0.04 | 0.26 ± 0.03 |
| Zn | 0.52 ± 0.02 | 1.20 ± 0.07 | 0.36 ± 0.07 | 0.27 ± 0.02 |
| Se | 0.52 ± 0.02 | 1.24 ± 0.02 * | 0.30 ± 0.03 | 0.32 ± 0.02 * |
| SeMet | 0.56 ± 0.03 | 1.37 ± 0.12 * | 0.39 ± 0.03 | 0.31 ± 0.01 * |
| Zn + Se | 0.50 ± 0.02 | 1.24 ± 0.08 * | 0.44 ± 0.05 | 0.33 ± 0.04 * |
| Zn + SeMet | 0.55 ± 0.04 | 1.24 ± 0.07 * | 0.42 ± 0.07 | 0.37 ± 0.05 * |
| 180-day | ||||
| Control | 0.51 ± 0.04 | 1.09 ± 0.09 | 0.30 ± 0.02 | 0.26 ± 0.02 |
| Zn | 0.51 ± 0.02 | 1.18 ± 0.05 | 0.32 ± 0.05 | 0.30 ± 0.03 |
| Se | 0.50 ± 0.02 | 1.26 ± 0.08 | 0.38 ± 0.08 | 0.33 ± 0.04 * |
| SeMet | 0.54 ± 0.05 | 1.21 ± 0.05 | 0.32 ± 0.03 | 0.30 ± 0.01 * |
| Zn + Se | 0.50 ± 0.03 | 1.09 ± 0.08 | 0.31 ± 0.08 | 0.31 ± 0.03 * |
| Zn + SeMet | 0.55 ± 0.05 | 1.27 ± 0.13 | 0.31 ± 0.07 | 0.36 ± 0.04 * |
| TAS (mM/L Plasma) | ESOD (U/g Hb) | GPx (U/g Hb) | |
|---|---|---|---|
| Administration Period | |||
| 30-day | |||
| Control | 1.07 ± 0.13 | 3282.5 ± 423.6 | 21.85 ± 1.34 |
| Zn | 1.13 ± 0.14 | 3690.0 ± 440.3 | 21.79 ± 2.37 |
| Se | 1.20 ± 0.16 | 3614.0 ± 164.8 | 24.18 ± 2.33 |
| SeMet | 1.06 ± 0.20 | 3384.0 ± 262.3 | 22.03 ± 1.12 |
| Zn + Se | 1.16 ± 0.16 | 3614.0 ± 589.2 | 22.61 ± 1.39 |
| Zn + SeMet | 1.16 ± 0.15 | 3512.0 ± 234.0 | 21.89 ± 1.54 |
| 60-day | |||
| Control | 1.06 ± 0.09 | 3393.4 ± 388.8 | 21.89 ± 1.88 |
| Zn | 1.06 ± 0.10 | 3802.5 ± 393.3 | 21.98 ± 2.21 |
| Se | 1.01 ± 0.06 | 3420.0 ± 191.1 | 24.81 ± 1.94 |
| SeMet | 1.02 ± 0.07 | 3280.0 ± 362.2 | 23.05 ± 2.59 |
| Zn + Se | 0.99 ± 0.11 | 3724.1 ± 423.1 | 23.22 ± 1.99 |
| Zn + SeMet | 0.96 ± 0.09 | 3632.2 ± 352.3 | 22.33 ± 2.19 |
| 90-day | |||
| Control | 0.99 ± 0.03 | 3803.3 ± 192.9 | 21.64 ± 1.70 |
| Zn | 0.95 ± 0.11 | 4220.0 ± 624.1 | 22.94 ± 3.00 |
| Se | 0.90 ± 0.10 | 3470.0 ± 435.6 | 25.35 ± 4.53 |
| SeMet | 0.99 ± 0.12 | 3520.0 ± 449.2 | 22.65 ± 2.89 |
| Zn + Se | 0.91 ± 0.07 | 3970.0 ± 701.2 | 23.11 ± 2.96 |
| Zn + SeMet | 0.94 ± 0.05 | 3828.0 ± 877.7 | 21.83 ± 1.31 |
| Post Administration Period | |||
| 90-day | |||
| Control | 0.76 ± 0.03 | 3230.0 ± 372.4 | 20.62 ± 1.58 |
| Zn | 076 ± 0.08 | 3542.5 ± 352.8 | 21.75 ± 1.51 |
| Se | 0.76 ± 0.09 | 3438.0 ± 222.1 | 24.31 ± 2.49 |
| SeMet | 0.83 ± 0.05 | 3102.0 ± 368.8 | 21.32 ± 1.56 |
| Zn + Se | 0.77 ± 0.10 | 3530.0 ± 593.5 | 21.38 ± 1.47 |
| Zn + SeMet | 0.73 ± 0.15 | 3556.0 ± 421.0 | 20.83 ± 1.70 |
| 180-day | |||
| Control | 0.74 ± 0.07 | 3443.1 ± 325.6 | 20.68 ± 2.09 |
| Zn | 0.91 ± 0.11 | 3802.0 ± 419.7 | 20.02 ± 1.33 |
| Se | 0.89 ± 0.09 | 3490.0 ± 310.4 | 22.37 ± 1.78 |
| SeMet | 0.89 ± 0.10 | 3676.2 ± 290.0 | 20.68 ± 1.03 |
| Zn + Se | 0.91 ± 0.10 | 3381.0 ± 361.9 | 21.93 ± 1.57 |
| Zn + SeMet | 0.89 ± 0.16 | 3880.2 ± 411.1 | 20.39 ± 1.79 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daragó, A.; Sapota, A.; Nasiadek, M.; Klimczak, M.; Kilanowicz, A. The Effect of Zinc and Selenium Supplementation Mode on Their Bioavailability in the Rat Prostate. Should Administration Be Joint or Separate? Nutrients 2016, 8, 601. https://doi.org/10.3390/nu8100601
Daragó A, Sapota A, Nasiadek M, Klimczak M, Kilanowicz A. The Effect of Zinc and Selenium Supplementation Mode on Their Bioavailability in the Rat Prostate. Should Administration Be Joint or Separate? Nutrients. 2016; 8(10):601. https://doi.org/10.3390/nu8100601
Chicago/Turabian StyleDaragó, Adam, Andrzej Sapota, Marzenna Nasiadek, Michał Klimczak, and Anna Kilanowicz. 2016. "The Effect of Zinc and Selenium Supplementation Mode on Their Bioavailability in the Rat Prostate. Should Administration Be Joint or Separate?" Nutrients 8, no. 10: 601. https://doi.org/10.3390/nu8100601
APA StyleDaragó, A., Sapota, A., Nasiadek, M., Klimczak, M., & Kilanowicz, A. (2016). The Effect of Zinc and Selenium Supplementation Mode on Their Bioavailability in the Rat Prostate. Should Administration Be Joint or Separate? Nutrients, 8(10), 601. https://doi.org/10.3390/nu8100601






