Tauroursodeoxycholic Acid Attenuates Renal Tubular Injury in a Mouse Model of Type 2 Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Grouping
2.2. Physical and Biochemical Analysis
2.3. Histology Analysis
2.4. Terminal Deoxynucleotidyl Transferase (TdT)-Mediated dUTP Nick-End-Labeling (TUNEL) Assay
2.5. RNA Extraction and Real-Time PCR
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
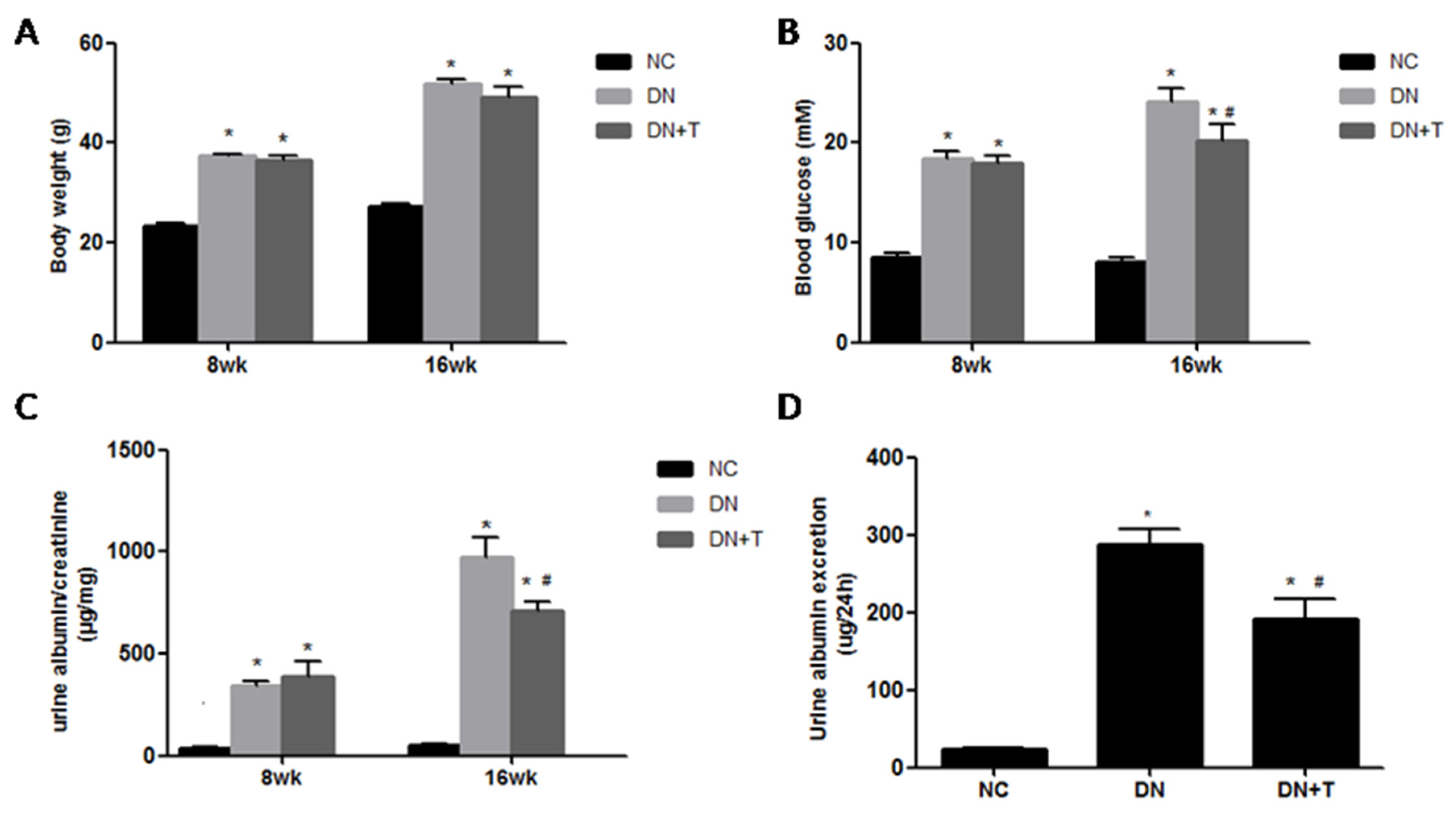
3.1. Effects of TUDCA on Biochemical Markers in the Different Groups
3.2. TUDCA Improves the Renal Morphologyin db/db Mice
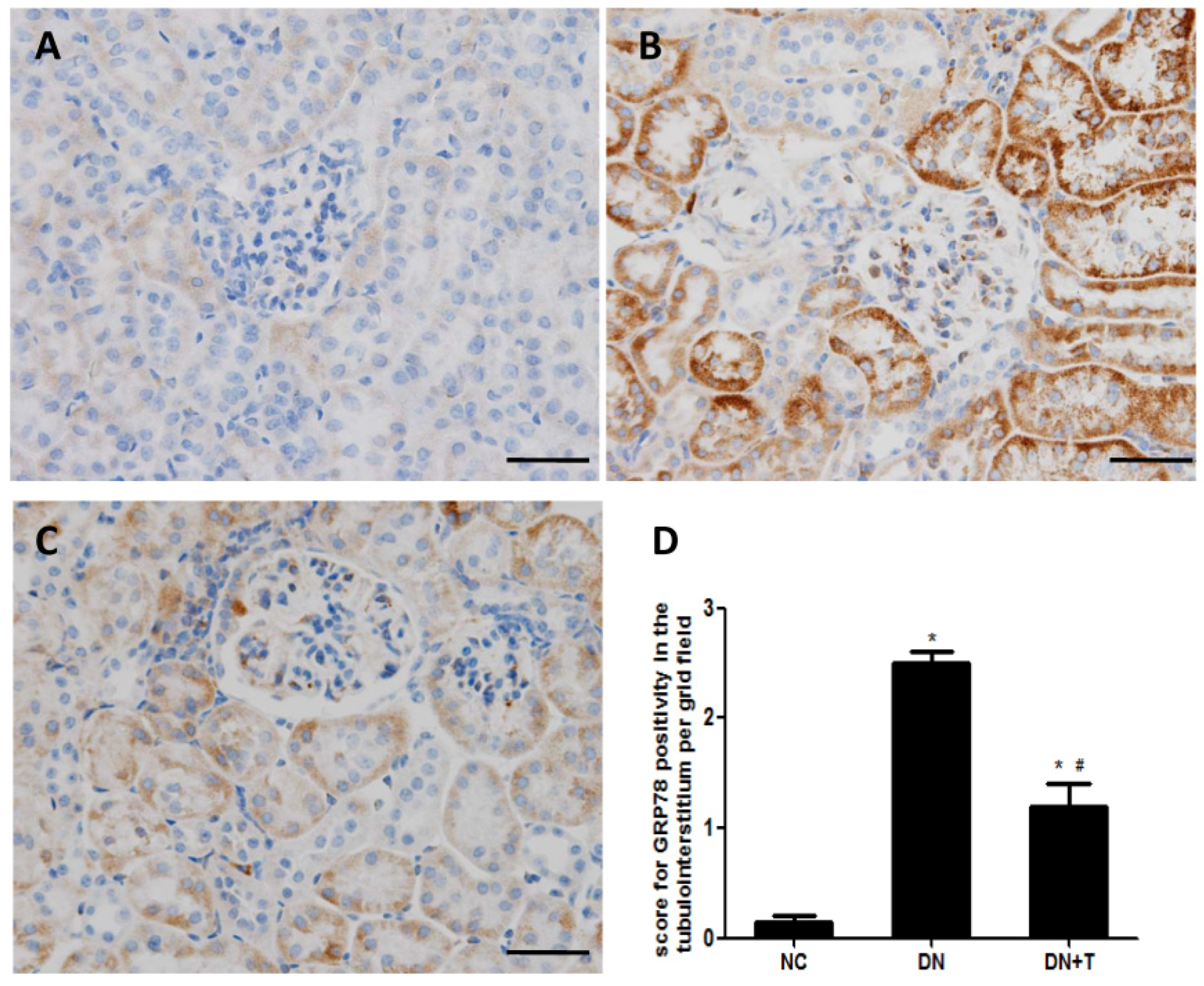
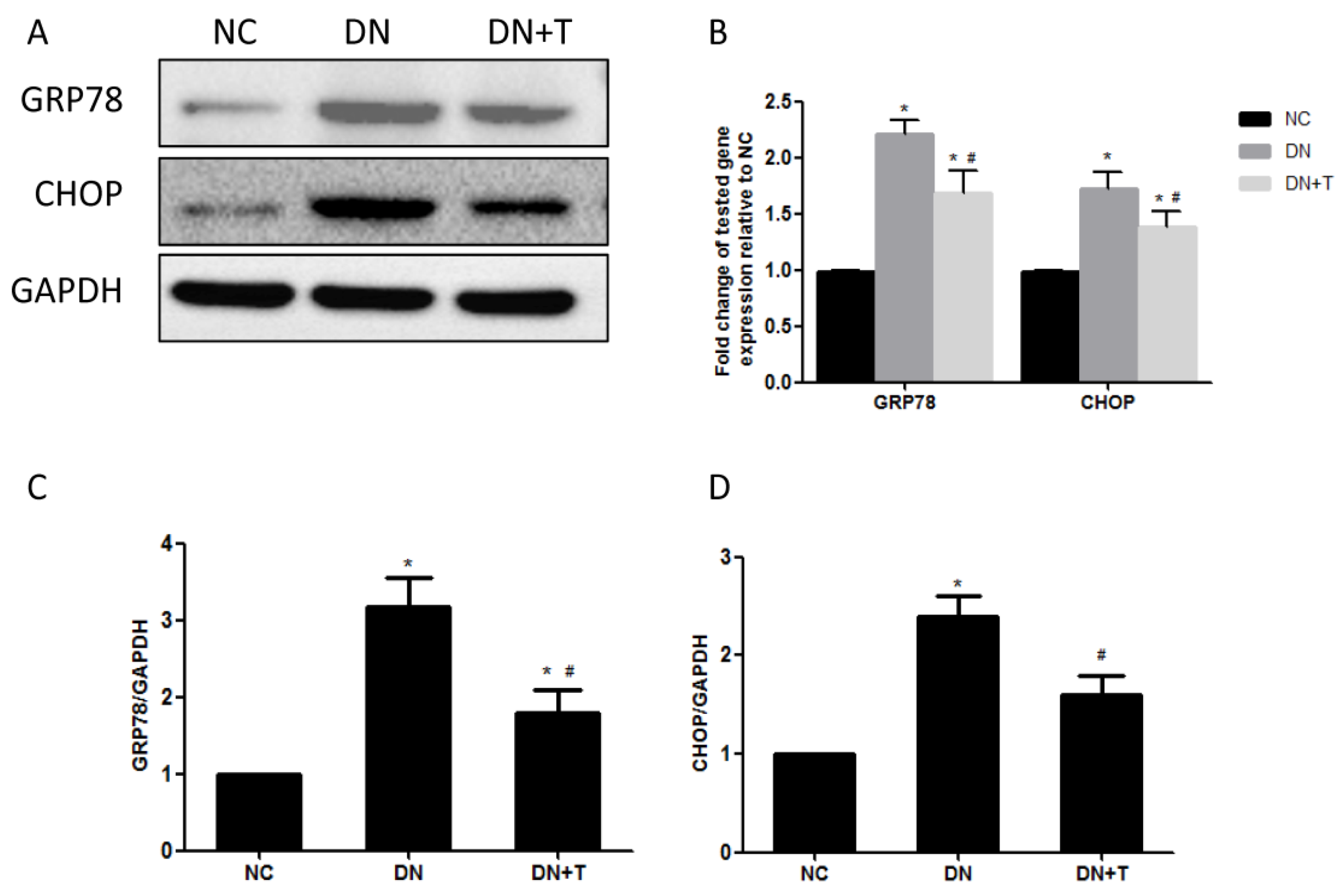
3.3. TUDCA Inhibits ER Stress Induced by Diabetes in the Kidneys of db/db Mice
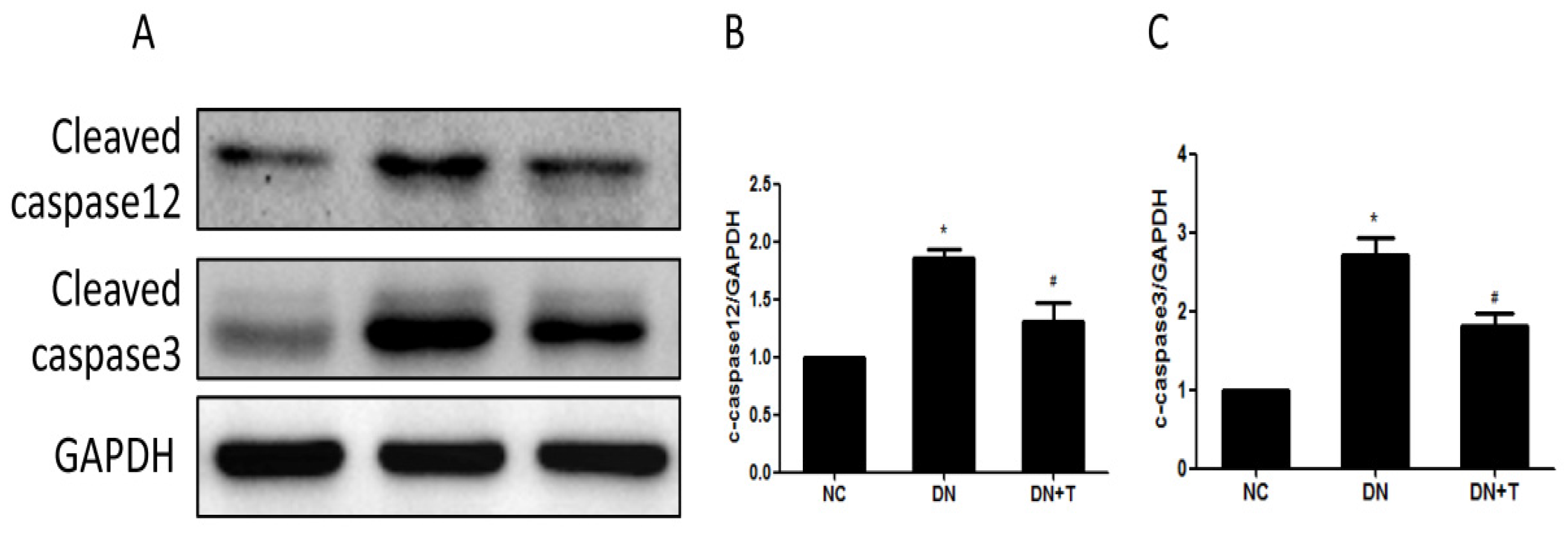
3.4. TUDCA Inhibits ER Stress–Associated Apoptosis Pathways
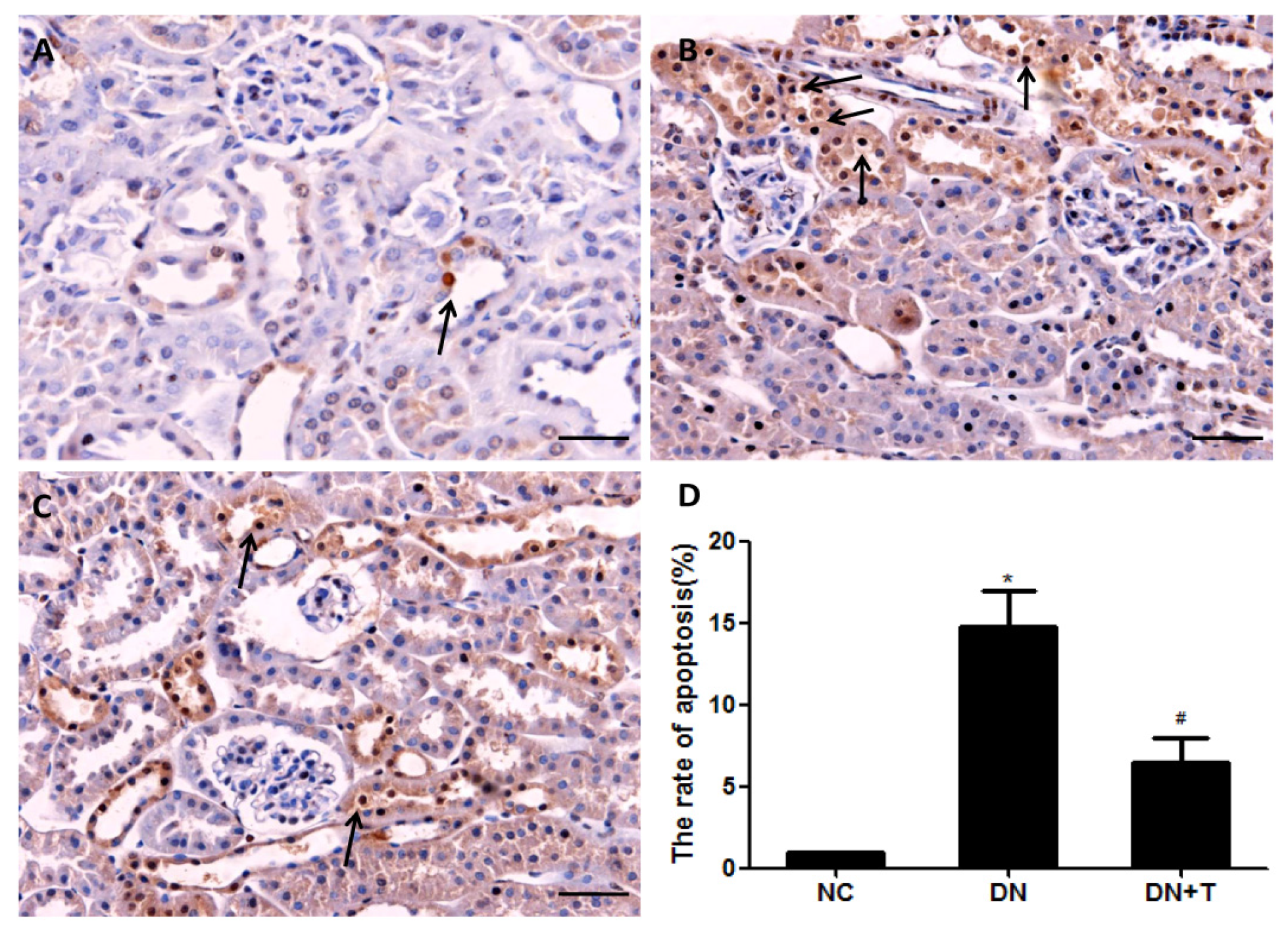
3.5. TUDCA Reduces the Apoptosis of Tubular Cell in db/db Mice
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Collins, A.J.; Foley, R.N.; Herzog, C.; Chavers, B.; Gilbertson, D.; Herzog, C.; Ishani, A.; Johansen, K.; Kasiske, B.; Kutner, N.; et al. Us renal data system 2012 annual data report. Am. J. Kidney Dis. 2013, 61, e1-476. [Google Scholar] [CrossRef] [PubMed]
- Atkins, R.C.; Zimmet, P. Diabetic kidney disease: Act now or pay later. Nephrol. Dial. Transplant. 2010, 25, 331–333. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, Y.S.; Sun, L.; Xie, P.; Liu, F.Y.; Chen, S. A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu. Rev. Pathol. 2011, 6, 395–423. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R.E.; Cooper, M.E. The tubulointerstitium in progressive diabetic kidney disease: More than an aftermath of glomerular injury? Kidney Int. 1999, 56, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Jenkin, K.A.; McAinch, A.J.; Zhang, Y.; Kelly, D.J.; Hryciw, D.H. Elevated cannabinoid receptor 1 and G protein-coupled receptor 55 expression in proximal tubule cells and whole kidney exposed to diabetic conditions. Clin. Exp. Pharmacol. Physiol. 2015, 42, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Brezniceanu, M.L.; Lau, C.J.; Godin, N.; Chenier, I.; Duclos, A.; Ethier, J.; Filep, J.G.; Ingelfinger, J.R.; Zhang, S.L.; Chan, J.S. Reactive oxygen species promote caspase-12 expression and tubular apoptosis in diabetic nephropathy. J. Am. Soc. Nephrol. 2010, 21, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Brezniceanu, M.L.; Liu, F.; Wei, C.C.; Chenier, I.; Godin, N.; Zhang, S.L.; Filep, J.G.; Ingelfinger, J.R.; Chan, J.S. Attenuation of interstitial fibrosis and tubular apoptosis in db/db transgenic mice overexpressing catalase in renal proximal tubular cells. Diabetes 2008, 57, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Robertson, S.; Burns, K.D. Evidence of apoptosis in human diabetic kidney. Mol. Cell. Biochem. 2004, 259, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Pallepati, P.; Averill-Bates, D.A. Activation of ER stress and apoptosis by hydrogen peroxide in hela cells: Protective role of mild heat preconditioning at 40 degrees C. Biochim. Biophys. Acta 2011, 1813, 1987–1999. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Olberding, K.E.; White, C.; Li, C. Bcl-2 proteins regulate er membrane permeability to luminal proteins during ER stress-induced apoptosis. Cell Death Differ. 2011, 18, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H. ER stress and diseases. FEBS J. 2007, 274, 630–658. [Google Scholar] [CrossRef] [PubMed]
- Cybulsky, A.V. The intersecting roles of endoplasmic reticulum stress, ubiquitin- proteasome system, and autophagy in the pathogenesis of proteinuric kidney disease. Kidney Int. 2013, 84, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Yoshida, H. Endoplasmic reticulum stress in kidney function and disease. Curr. Opin. Nephrol. Hypertens. 2015, 24, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Xiao, W.; Li, Z.; Li, X.; Chuang, P.Y.; Jim, B.; Zhang, W.; Wei, C.; Wang, N.; Jia, W.; et al. RTN1 mediates progression of kidney disease by inducing ER stress. Nat. Commun. 2015, 6, 7841. [Google Scholar] [CrossRef] [PubMed]
- Baban, B.; Liu, J.Y.; Mozaffari, M.S. Endoplasmic reticulum stress response and inflammatory cytokines in type 2 diabetic nephropathy: Role of indoleamine 2,3-dioxygenase and programmed death-1. Exp. Mol. Pathol. 2013, 94, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Inagi, R. Endoplasmic reticulum stress as a progression factor for kidney injury. Curr. Opin. Pharmacol. 2010, 10, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, U.; Yilmaz, E.; Ozcan, L.; Furuhashi, M.; Vaillancourt, E.; Smith, R.O.; Gorgun, C.Z.; Hotamisligil, G.S. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 2006, 313, 1137–1140. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, Z.; Zhao, S.; Xiang, R. Chemical chaperones reduce ER stress and adipose tissue inflammation in high fat diet-induced mouse model of obesity. Sci. Rep. 2016, 6, 27486. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.F.; Yuan, J.R.; Qin, D.; Gu, J.F.; Zhao, B.J.; Zhang, L.; Zhao, D.; Chen, J.; Hou, X.F.; Yang, N.; et al. Protection of tauroursodeoxycholic acid on high glucose-induced human retinal microvascular endothelial cells dysfunction and streptozotocin-induced diabetic retinopathy rats. J. Ethnopharmacol. 2016, 185, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.L.; Wang, L.; Chen, X.; Wang, Y.M.; Guo, H.J.; Chu, S.; Liu, C.; Zhang, X.M.; Peng, W. Ursodeoxycholic acid and 4-phenylbutyrate prevent endoplasmic reticulum stress-induced podocyte apoptosis in diabetic nephropathy. Lab. Investig. 2016, 96, 610–622. [Google Scholar] [CrossRef] [PubMed]
- Drummond, K.; Mauer, M.; International Diabetic Nephropathy Study Group. The early natural history of nephropathy in type 1 diabetes: II. Early renal structural changes in type 1 diabetes. Diabetes 2002, 51, 1580–1587. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.L.; Sun, L.; Li, Y.Y.; Shao, M.M.; Cheng, X.Y.; Ge, N.; Lu, J.D.; Li, S.M. Ace-inhibitor suppresses the apoptosis induced by endoplasmic reticulum stress in renal tubular in experimental diabetic rats. Exp. Clin. Endocrinol. Diabetes 2009, 117, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Docherty, N.G.; O’Sullivan, O.E.; Healy, D.A.; Fitzpatrick, J.M.; Watson, R.W. Evidence that inhibition of tubular cell apoptosis protects against renal damage and development of fibrosis following ureteric obstruction. Am. J. Phys. Ren. Physiol. 2006, 290, F4–F13. [Google Scholar] [CrossRef] [PubMed]
- Asmellash, S.; Stevens, J.L.; Ichimura, T. Modulating the endoplasmic reticulum stress response with trans-4,5-dihydroxy-1,2-dithiane prevents chemically induced renal injuryin vivo. Toxicol. Sci. 2005, 88, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Schroder, M.; Kaufman, R.J. ER stress and the unfolded protein response. Mutat. Res. 2005, 569, 29–63. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, S.J.; Yun, C.Y.; Oyadomari, S.; Novoa, I.; Zhang, Y.; Jungreis, R.; Nagata, K.; Harding, H.P.; Ron, D. Chop induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004, 18, 3066–3077. [Google Scholar] [CrossRef] [PubMed]
- Jing, G.; Wang, J.J.; Zhang, S.X. ER stress and apoptosis: A new mechanism for retinal cell death. Exp. Diabetes Res. 2012, 2012, 589589. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, R.; Torreggiani, M.; Ting, A.; Xiong, H.; Striker, G.E.; Vlassara, H.; Zheng, F. Induction of diabetes in aged C57B6 mice results in severe nephropathy: An association with oxidative stress, endoplasmic reticulum stress, and inflammation. Am. J. Pathol. 2010, 176, 2163–2176. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Yuan, J. Cross-talk between two cysteine protease families activation of caspase-12 by calpain in apoptosis. J. Cell Biol. 2000, 150, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Zhu, H.; Morishima, N.; Li, E.; Xu, J.; Yankner, B.A.; Yuan, J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 2000, 403, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Hitomi, J.; Katayama, T.; Taniguchi, M.; Honda, A.; Imaizumi, K.; Tohyama, M. Apoptosis induced by endoplasmic reticulum stress depends on activation of caspase-3 via caspase-12. Neurosci. Lett. 2004, 357, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Burrows, J.A.; Willis, L.K.; Perlmutter, D.H. Chemical chaperones mediate increased secretion of mutant alpha 1-antitrypsin (alpha 1-AT) Z: A potential pharmacological strategy for prevention of liver injury and emphysema in alpha 1-AT deficiency. Proc. Natl. Acad. Sci. USA 2000, 97, 1796–1801. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, L.; Ergin, A.S.; Lu, A.; Chung, J.; Sarkar, S.; Nie, D.; Myers, M.G., Jr.; Ozcan, U. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009, 9, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, R.E.; Brimble, E.; Werner, K.E.; Cruz, G.L.; Ask, K.; Ingram, A.J.; Dickhout, J.G. 4-phenylbutyrate inhibits tunicamycin-induced acute kidney injury via chop/gadd153 repression. PLoS ONE 2014, 9, e84663. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Fu, L.; Xiao, M.; Xu, C.; Sun, L.; Zhang, T.; Zheng, F.; Mei, C. The nephroprotective effect of tauroursodeoxycholic acid on ischaemia/reperfusion-induced acute kidney injury by inhibiting endoplasmic reticulum stress. Basic Clin. Pharmacol. Toxicol. 2012, 111, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Xie, D.; Wu, X.; Cao, H.; Su, W.; Yang, J. Involvement of endoplasmic reticulum stress in albuminuria induced inflammasome activation in renal proximal tubular cells. PLoS ONE 2013, 8, e72344. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, C.P.; Xu, K.F.; Mao, X.D.; Lu, Y.B.; Fang, L.; Yang, J.W.; Liu, C. Effect of taurine-conjugated ursodeoxycholic acid on endoplasmic reticulum stress and apoptosis induced by advanced glycation end products in cultured mouse podocytes. Am. J. Nephrol. 2008, 28, 1014–1022. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Fan, Y.; Zeng, C.; He, L.; Wang, N. Tauroursodeoxycholic Acid Attenuates Renal Tubular Injury in a Mouse Model of Type 2 Diabetes. Nutrients 2016, 8, 589. https://doi.org/10.3390/nu8100589
Zhang J, Fan Y, Zeng C, He L, Wang N. Tauroursodeoxycholic Acid Attenuates Renal Tubular Injury in a Mouse Model of Type 2 Diabetes. Nutrients. 2016; 8(10):589. https://doi.org/10.3390/nu8100589
Chicago/Turabian StyleZhang, Jing, Ying Fan, Chuchu Zeng, Li He, and Niansong Wang. 2016. "Tauroursodeoxycholic Acid Attenuates Renal Tubular Injury in a Mouse Model of Type 2 Diabetes" Nutrients 8, no. 10: 589. https://doi.org/10.3390/nu8100589
APA StyleZhang, J., Fan, Y., Zeng, C., He, L., & Wang, N. (2016). Tauroursodeoxycholic Acid Attenuates Renal Tubular Injury in a Mouse Model of Type 2 Diabetes. Nutrients, 8(10), 589. https://doi.org/10.3390/nu8100589



