Peroxisome Proliferator-Activated Receptor Activation is Associated with Altered Plasma One-Carbon Metabolites and B-Vitamin Status in Rats
Abstract
:1. Introduction
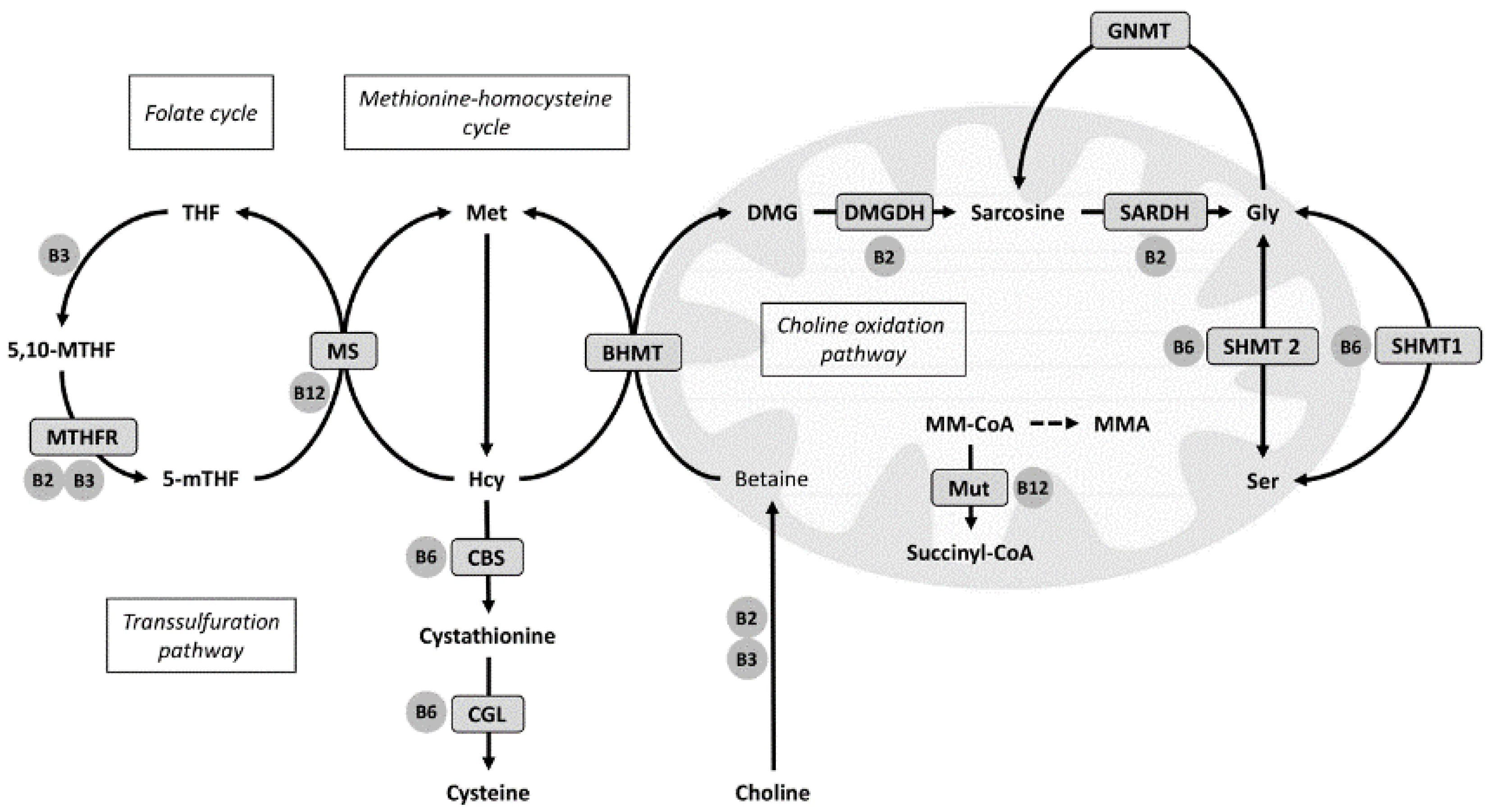
2. Materials and Methods
2.1. Animals and Diets
2.2. Ethics Statement
2.3. Biochemical Analyses
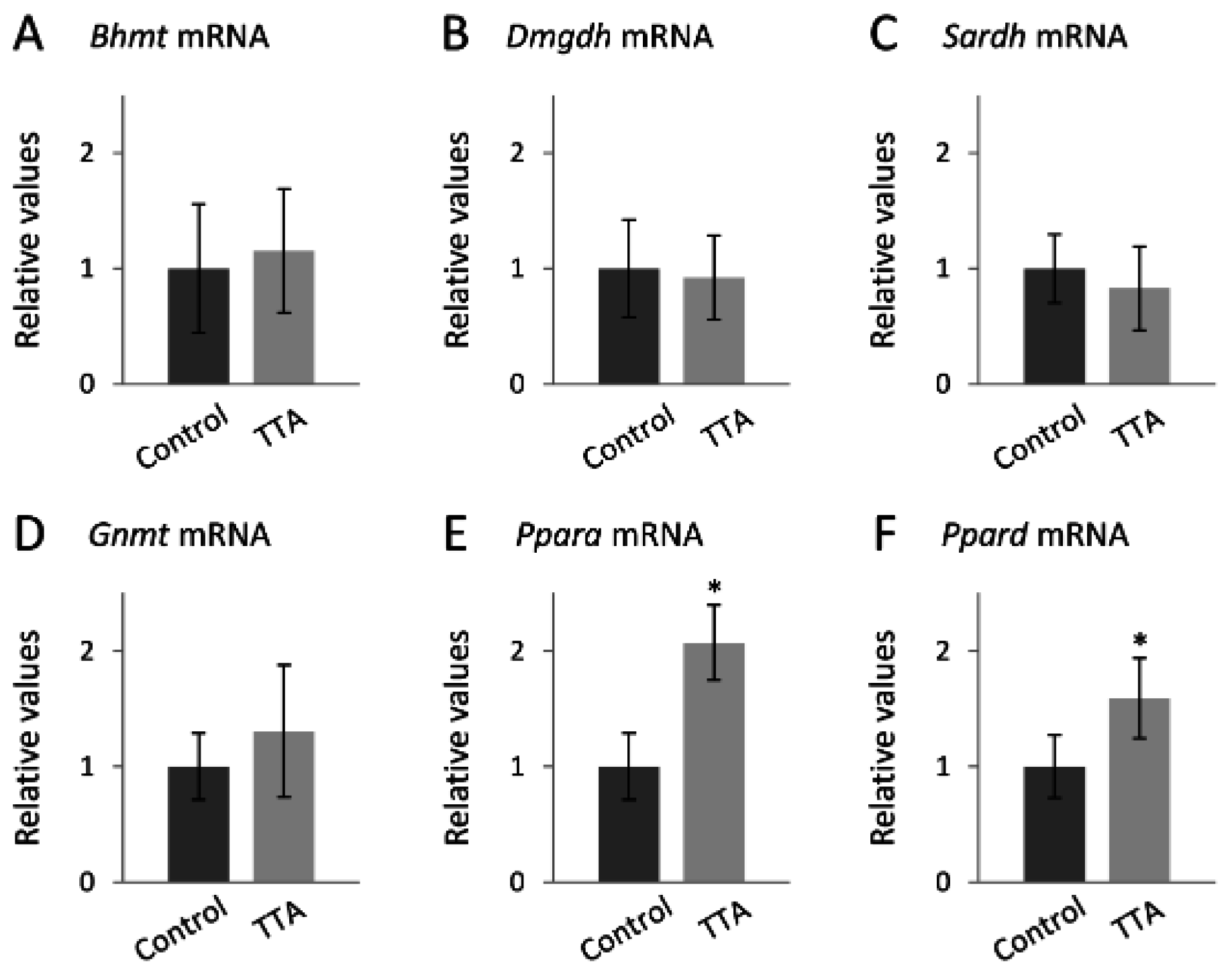
2.4. Gene Expression Analysis
2.5. Statistical Analyses and Presentation of Data
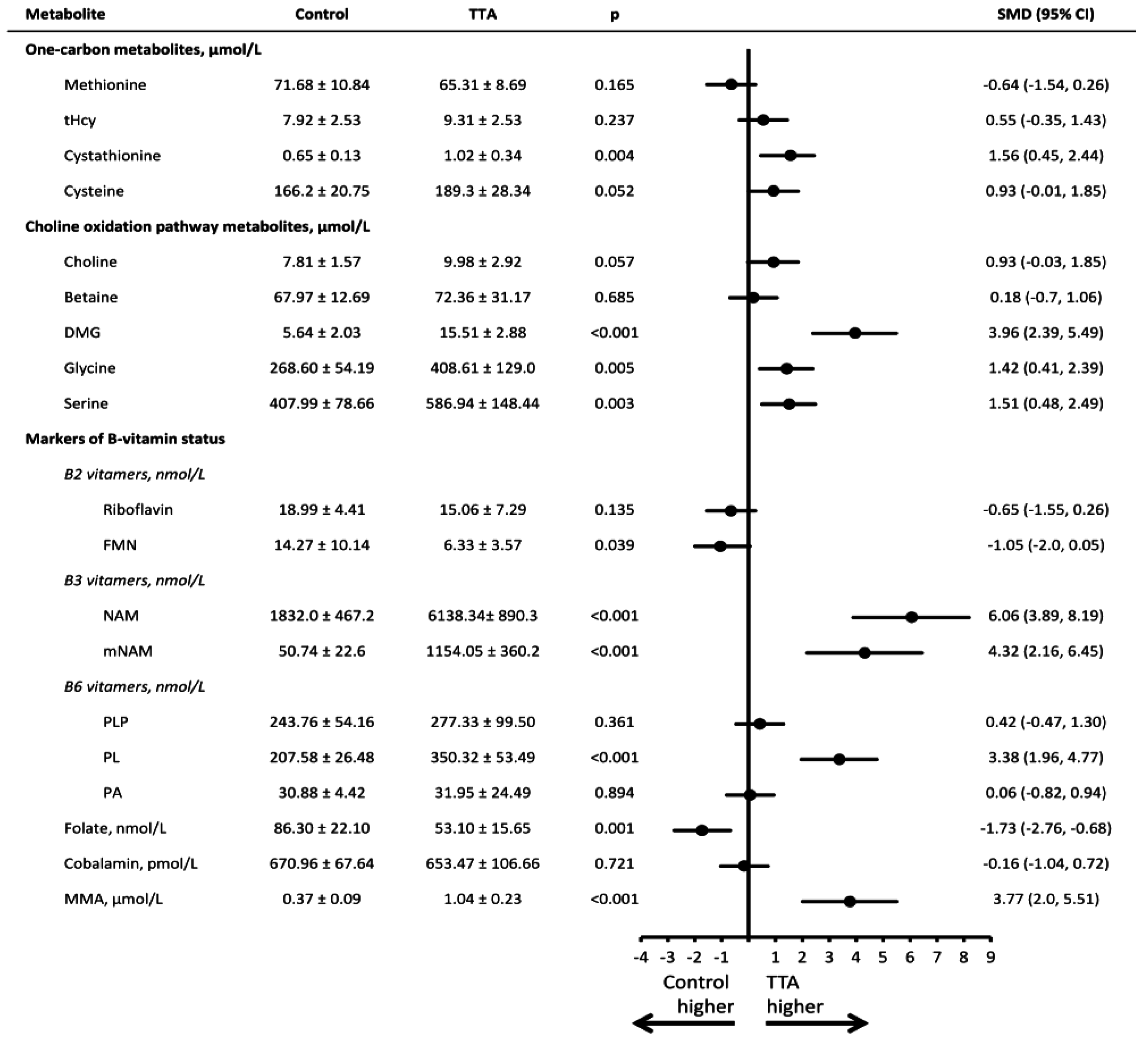
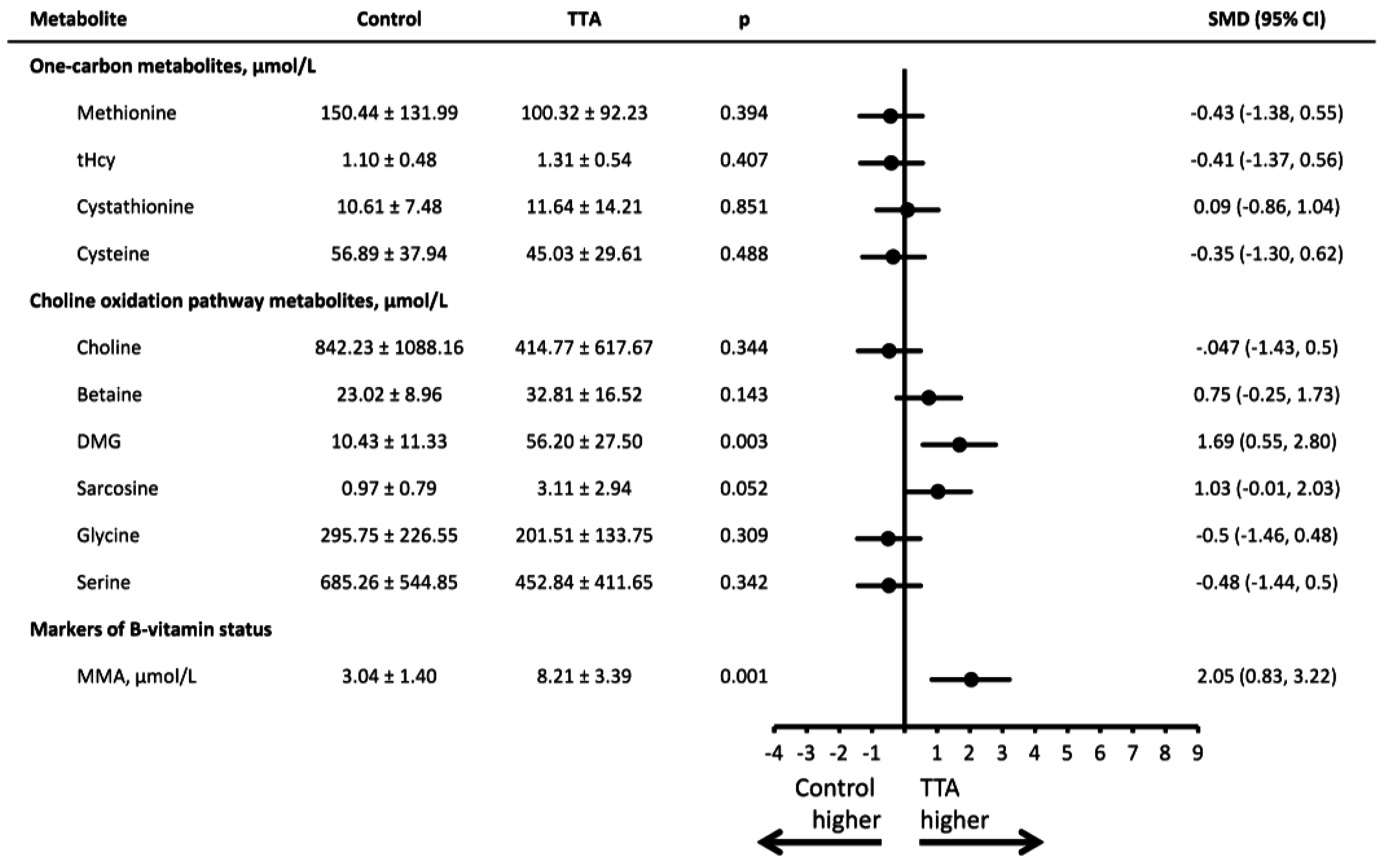
3. Results
| r (95% CI) | P | |
|---|---|---|
| Methionine | −0.02 | 0.93 |
| tHcy | −0.10 | 0.71 |
| Cystathionine | 0.25 | 0.33 |
| Cysteine | 0.09 | 0.73 |
| Choline | 0.08 | 0.93 |
| Betaine | 0.62 | 0.008 |
| DMG | 0.79 | <0.001 |
| Glycine | −0.13 | 0.63 |
| Serine | −0.17 | 0.51 |
| MMA | 0.73 | <0.001 |
4. Discussion
4.1. Principal Findings
4.2. Possible Mechanisms
4.2.1. TTA Treatment and the Choline Oxidation Pathway
4.2.2. TTA Treatment and Vitamin B3
4.2.3. TTA Treatment and Vitamin B6
4.2.4. TTA Treatment and Vitamin B12 Status
4.3. Strengths and Limitations
4.4. Clinical Application
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Refsum, H.; Ueland, P.M.; Nygard, O.; Vollset, S.E. Homocysteine and cardiovascular disease. Annu. Rev. Med. 1998, 49, 31–62. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.; Halsey, J.; Lewington, S.; Lonn, E.; Armitage, J.; Manson, J.E.; Bonaa, K.H.; Spence, J.D.; Nygard, O.; Jamison, R.; et al. Effects of lowering homocysteine levels with B vitamins on cardiovascular disease, cancer, and cause-specific mortality: Meta-analysis of 8 randomized trials involving 37 485 individuals. Arch. Intern. Med. 2010, 170, 1622–1631. [Google Scholar] [PubMed]
- Joseph, J.; Handy, D.E.; Loscalzo, J. Quo vadis: Whither homocysteine research? Cardiovasc. Toxicol. 2009, 9, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Ueland, P.M. Choline and betaine in health and disease. J. Inherit. Metab. Dis. 2011, 34, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Schartum-Hansen, H.; Pedersen, E.R.; Svingen, G.F.; Ueland, P.M.; Seifert, R.; Ebbing, M.; Strand, E.; Bleie, O.; Nygard, O. Plasma choline, smoking, and long-term prognosis in patients with stable angina pectoris. Eur. J. Prev. Cardiol. 2014, 22, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Svingen, G.F.; Ueland, P.M.; Pedersen, E.K.; Schartum-Hansen, H.; Seifert, R.; Ebbing, M.; Loland, K.H.; Tell, G.S.; Nygard, O. Plasma dimethylglycine and risk of incident acute myocardial infarction in patients with stable angina pectoris. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2041–2048. [Google Scholar] [CrossRef] [PubMed]
- Svingen, G.F.; Schartum-Hansen, H.; Ueland, P.M.; Pedersen, E.R.; Seifert, R.; Ebbing, M.; Bonaa, K.H.; Mellgren, G.; Nilsen, D.W.; Nordrehaug, J.E.; et al. Elevated plasma dimethylglycine is a risk marker of mortality in patients with coronary heart disease. Eur. J. Prev. Cardiol. 2014, 22, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Lever, M.; George, P.M.; Elmslie, J.L.; Atkinson, W.; Slow, S.; Molyneux, S.L.; Troughton, R.W.; Richards, A.M.; Frampton, C.M.; Chambers, S.T. Betaine and secondary events in an acute coronary syndrome cohort. PLoS ONE 2012. [Google Scholar] [CrossRef] [PubMed]
- Selhub, J. Homocysteine metabolism. Annu. Rev. Nutr. 1999, 19, 217–246. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.D.; Harris, B.J.; Kyle, W.E. Methionine metabolism in mammals: Kinetic study of betaine-homocysteine methyltransferase. Arch. Biochem. Biophys. 1972, 153, 320–324. [Google Scholar] [CrossRef]
- Porter, D.H.; Cook, R.J.; Wagner, C. Enzymatic properties of dimethylglycine dehydrogenase and sarcosine dehydrogenase from rat liver. Arch. Biochem. Biophys. 1985, 243, 396–407. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Tan, Y.; Wei, J.; Chang, Y.; Jin, T.; Zhu, H. Betaine supplement alleviates hepatic triglyceride accumulation of apolipoprotein e deficient mice via reducing methylation of peroxisomal proliferator-activated receptor alpha promoter. Lipids Health Dis. 2013, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, K.; Camejo, G.; Lanne, B.; Halvarsson, T.; Landergren, M.R.; Oakes, N.D. Beyond lipids, pharmacological pparalpha activation has important effects on amino acid metabolism as studied in the rat. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1157–E1165. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.V.; Torres, N.; Tovar, A.R. Ppar-alpha as a key nutritional and environmental sensor for metabolic adaptation. Adv. Nutr. 2013, 4, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Rakhshandehroo, M.; Knoch, B.; Muller, M.; Kersten, S. Peroxisome proliferator-activated receptor alpha target genes. PPAR Res. 2010. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, M.V.; Pan, Z.; Zhu, Y.; Tordjman, K.; Schneider, J.G.; Coleman, T.; Turk, J.; Semenkovich, C.F. “New” hepatic fat activates pparalpha to maintain glucose, lipid, and cholesterol homeostasis. Cell. Metab. 2005, 1, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Forman, B.M.; Chen, J.; Evans, R.M. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc. Natl. Acad. Sci. USA 1997, 94, 4312–4317. [Google Scholar] [CrossRef] [PubMed]
- Vigerust, N.F.; Cacabelos, D.; Burri, L.; Berge, K.; Wergedahl, H.; Christensen, B.; Portero-Otin, M.; Viste, A.; Pamplona, R.; Berge, R.K.; et al. Fish oil and 3-thia fatty acid have additive effects on lipid metabolism but antagonistic effects on oxidative damage when fed to rats for 50 weeks. J. Nutr. Biochem. 2012, 23, 1384–1393. [Google Scholar] [CrossRef] [PubMed]
- Kersten, S.; Mandard, S.; Escher, P.; Gonzalez, F.J.; Tafuri, S.; Desvergne, B.; Wahli, W. The peroxisome proliferator-activated receptor alpha regulates amino acid metabolism. FASEB J. 2001, 15, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Aleman, G.; Ortiz, V.; Contreras, A.V.; Quiroz, G.; Ordaz-Nava, G.; Langley, E.; Torres, N.; Tovar, A.R. Hepatic amino acid-degrading enzyme expression is downregulated by natural and synthetic ligands of pparalpha in rats. J. Nutr. 2013, 143, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Ntaios, G.; Savopoulos, C.; Chatzopoulos, S.; Mikhailidis, D.; Hatzitolios, A. Iatrogenic hyperhomocysteinemia in patients with metabolic syndrome: A systematic review and metaanalysis. Atherosclerosis 2011, 214, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Lever, M.; McEntyre, C.J.; George, P.M.; Slow, S.; Chambers, S.T.; Foucher, C. Fenofibrate causes elevation of betaine excretion but not excretion of other osmolytes by healthy adults. J. Clin. Lipidol. 2014, 8, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Lever, M.; McEntyre, C.J.; George, P.M.; Slow, S.; Elmslie, J.L.; Lunt, H.; Chambers, S.T.; Parry-Strong, A.; Krebs, J.D. Extreme urinary betaine losses in type 2 diabetes combined with bezafibrate treatment are associated with losses of dimethylglycine and choline but not with increased losses of other osmolytes. Cardiovasc. Drugs. Ther. 2014, 28, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Chu, R.; Lim, H.; Brumfield, L.; Liu, H.; Herring, C.; Ulintz, P.; Reddy, J.K.; Davison, M. Protein profiling of mouse livers with peroxisome proliferator-activated receptor alpha activation. Mol. Cell. Biol. 2004, 24, 6288–6297. [Google Scholar] [CrossRef] [PubMed]
- Wrzesinski, K.; León, I.R.; Kulej, K.; Sprenger, R.R.; Bjorndal, B.; Christensen, B.J.; Berge, R.K.; Ole, N.J.; Rogowska-Wrzesinska, A. Proteomics identifies molecular networks affected by tetradecylthioacetic acid and fish oil supplemented diets. J. Proteom. 2013, 84, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Ohnishi, M.; Iguchi, S.; Sano, K.; Umezawa, C. Peroxisome-proliferator regulates key enzymes of the tryptophan-nad+ pathway. Toxicol. Appl. Pharmacol. 1999, 158, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Christensen, B.J.; Berge, K.; Wergedahl, H.; Bohov, P.; Berge, R.K.; Svendsen, E.; Viste, A. Bioactive fatty acids reduce development of gastric cancer following duodenogastric reflux in rats. Surg. Sci. 2012, 3, 34–42. [Google Scholar] [CrossRef]
- Bjorndal, B.; Brattelid, T.; Strand, E.; Vigerust, N.F.; Svingen, G.F.; Svardal, A.; Nygard, O.; Berge, R.K. Fish oil and the pan-ppar agonist tetradecylthioacetic acid affect the amino acid and carnitine metabolism in rats. PLoS ONE 2013. [Google Scholar] [CrossRef] [PubMed]
- Ueland, P.M.; Midttun, O.; Windelberg, A.; Svardal, A.; Skalevik, R.; Hustad, S. Quantitative profiling of folate and one-carbon metabolism in large-scale epidemiological studies by mass spectrometry. Clin. Chem. Lab. Med. 2007, 45, 1737–1745. [Google Scholar] [CrossRef] [PubMed]
- Midttun, O.; Kvalheim, G.; Ueland, P.M. High-throughput, low-volume, multianalyte quantification of plasma metabolites related to one-carbon metabolism using hplc-ms/ms. Anal. Bioanal. Chem. 2013, 405, 2009–2017. [Google Scholar] [CrossRef] [PubMed]
- Midttun, O.; Hustad, S.; Ueland, P.M. Quantitative profiling of biomarkers related to b-vitamin status, tryptophan metabolism and inflammation in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass. Spectrom. 2009, 23, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, B.P.; Broin, S.D. Microbiological assay for vitamin b12 performed in 96-well microtitre plates. J. Clin. Pathol. 1991, 44, 592–595. [Google Scholar] [CrossRef] [PubMed]
- Molloy, A.M.; Scott, J.M. Microbiological assay for serum, plasma, and red cell folate using cryopreserved, microtiter plate method. Methods Enzymol. 1997, 281, 43–53. [Google Scholar] [PubMed]
- Lysne, V.; Bjorndal, B.; Vik, R.; Nordrehaug, J.E.; Skorve, J.; Nygard, O.; Berge, R.K. A protein extract from chicken reduces plasma homocysteine in rats. Nutrients 2015, 7, 4498–4511. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.L.; Jensen, J.L.; Orntoft, T.F. Normalization of real-time quantitative reverse transcription-pcr data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate—A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B-Methodol. 1995, 57, 289–300. [Google Scholar]
- Cook, R.J.; Misono, K.S.; Wagner, C. Identification of the covalently bound flavin of dimethylglycine dehydrogenase and sarcosine dehydrogenase from rat liver mitochondria. J. Biol. Chem. 1984, 259, 12475–12480. [Google Scholar]
- Kutzbach, C.; Stokstad, E.L. Mammalian methylenetetrahydrofolate reductase. Partial purification, properties, and inhibition by s-adenosylmethionine. Biochim. Biophys. Acta 1971, 250, 459–477. [Google Scholar] [CrossRef]
- Leclerc, D.; Wilson, A.; Dumas, R.; Gafuik, C.; Song, D.; Watkins, D.; Heng, H.H.; Rommens, J.M.; Scherer, S.W.; Rosenblatt, D.S.; et al. Cloning and mapping of a cdna for methionine synthase reductase, a flavoprotein defective in patients with homocystinuria. Proc. Natl. Acad. Sci. USA 1998, 95, 3059–3064. [Google Scholar] [CrossRef] [PubMed]
- Herbert, V.; Larrabee, A.R.; Buchanan, J.M. Studies on identification of a folate compound of human serum. J. Clin. Investig. 1962, 41, 1134–1138. [Google Scholar] [CrossRef] [PubMed]
- Ericsson, A.; Turner, N.; Hansson, G.I.; Wallenius, K.; Oakes, N.D. Pharmacological pparalpha activation markedly alters plasma turnover of the amino acids glycine, serine and arginine in the rat. PLoS ONE 2014. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, Z.; Dai, Z.; Yang, Y.; Wang, J.; Wu, G. Glycine metabolism in animals and humans: Implications for nutrition and health. J. Amino Acids 2013, 45, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Van Vlies, N.; Ferdinandusse, S.; Turkenburg, M.; Wanders, R.J.; Vaz, F.M. Ppar alpha-activation results in enhanced carnitine biosynthesis and octn2-mediated hepatic carnitine accumulation. Biochim. Biophys. Acta 2007, 1767, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Bremer, J. Carnitine--metabolism and functions. Physiol. Rev. 1983, 63, 1420–1480. [Google Scholar] [PubMed]
- Penberthy, T.; Kirkland, J. Niacin. In Present Knowledge in Nutrition, 10th ed.; Erdman, J.W., Macdonald, I.A., Zeisel, S.H., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 293–306. [Google Scholar]
- Vaz, F.M.; Wanders, R.J. Carnitine biosynthesis in mammals. Biochem. J. 2002, 361, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Blusztajn, J.K.; Zeisel, S.H. Measurement of the formation of betaine aldehyde and betaine in rat-liver mitochondria by a high-pressure liquid-chromatography radioenzymatic-assay. Biochim. Biophys. Acta 1992, 1117, 333–339. [Google Scholar] [CrossRef]
- Shin, M.; Kim, I.; Inoue, Y.; Kimura, S.; Gonzalez, F.J. Regulation of mouse hepatic alpha-amino-beta-carboxymuconate-epsilon-semialdehyde decarboxylase, a key enzyme in the tryptophan-nicotinamide adenine dinucleotide pathway, by hepatocyte nuclear factor 4alpha and peroxisome proliferator-activated receptor alpha. Mol. Pharmacol. 2006, 70, 1281–1290. [Google Scholar] [PubMed]
- Zhen, Y.; Krausz, K.W.; Chen, C.; Idle, J.R.; Gonzalez, F.J. Metabolomic and genetic analysis of biomarkers for peroxisome proliferator-activated receptor alpha expression and activation. Mol. Endocrinol. 2007, 21, 2136–2151. [Google Scholar] [CrossRef] [PubMed]
- Montanez, J.E.; Peters, J.M.; Correll, J.B.; Gonzalez, F.J.; Patterson, A.D. Metabolomics: An essential tool to understand the function of peroxisome proliferator-activated receptor alpha. Toxicol. Pathol. 2013, 41, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Pyper, S.R.; Viswakarma, N.; Yu, S.; Reddy, J.K. Ppar Alpha: Energy combustion, hypolipidemia, inflammation and cancer. Nucl. Recept. Signal. 2010, 8, e002. [Google Scholar] [CrossRef] [PubMed]
- Mosharov, E.; Cranford, M.R.; Banerjee, R. The quantitatively important relationship between homocysteine metabolism and glutathione synthesis by the transsulfuration pathway and its regulation by redox changes. Biochemistry 2000, 39, 13005–13011. [Google Scholar] [CrossRef] [PubMed]
- Taysi, S. Oxidant/antioxidant status in liver tissue of vitamin b6 deficient rats. Clin. Nutr. 2005, 24, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Paul, L.; Ueland, P.M.; Selhub, J. Mechanistic perspective on the relationship between pyridoxal 5'-phosphate and inflammation. Nutr. Rev. 2013, 71, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Ueland, P.M.; Ulvik, A.; Rios-Avila, L.; Midttun, O.; Gregory, J.F. Direct and functional biomarkers of vitamin b6 status. Annu. Rev. Nutr. 2015, 35, 33–70. [Google Scholar] [CrossRef] [PubMed]
- Dyroy, E.; Yndestad, A.; Ueland, T.; Halvorsen, B.; Damas, J.K.; Aukrust, P.; Berge, R.K. Antiinflammatory effects of tetradecylthioacetic acid involve both peroxisome proliferator-activated receptor alpha-dependent and -independent pathways. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1364–1369. [Google Scholar] [CrossRef] [PubMed]
- Syversen, U.; Stunes, A.K.; Gustafsson, B.I.; Obrant, K.J.; Nordsletten, L.; Berge, R.; Thommesen, L.; Reseland, J.E. Different skeletal effects of the peroxisome proliferator activated receptor (ppar)alpha agonist fenofibrate and the ppargamma agonist pioglitazone. BMC Endocr. Disord. 2009, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Di Salvo, M.L.; Contestabile, R.; Safo, M.K. Vitamin b(6) salvage enzymes: Mechanism, structure and regulation. Biochim. Biophys. Acta 2011, 1814, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Devalia, V.; Hamilton, M.S.; Molloy, A.M.; the British Committee for Standards in Haematology. Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br. J. Haematol. 2014, 166, 496–513. [Google Scholar] [CrossRef] [PubMed]
- Hannibal, L.; DiBello, P.M.; Jacobsen, D.W. Proteomics of vitamin b12 processing. Clin. Chem. Lab. Med. 2013, 51, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hannibal, L.; Gherasim, C.; Jacobsen, D.W.; Banerjee, R. A human vitamin b12 trafficking protein uses glutathione transferase activity for processing alkylcobalamins. J. Biol. Chem. 2009, 284, 33418–33424. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.; Loscalzo, J. Methoxistasis: Integrating the roles of homocysteine and folic acid in cardiovascular pathobiology. Nutrients 2013, 5, 3235–3256. [Google Scholar] [CrossRef] [PubMed]
- Dahlhoff, C.; Desmarchelier, C.; Sailer, M.; Furst, R.W.; Haag, A.; Ulbrich, S.E.; Hummel, B.; Obeid, R.; Geisel, J.; Bader, B.L.; et al. Hepatic methionine homeostasis is conserved in c57bl/6n mice on high-fat diet despite major changes in hepatic one-carbon metabolism. PLoS ONE 2013. [Google Scholar] [CrossRef]
- Lima, C.P.; Davis, S.R.; Mackey, A.D.; Scheer, J.B.; Williamson, J.; Gregory, J.F., 3rd. Vitamin b-6 deficiency suppresses the hepatic transsulfuration pathway but increases glutathione concentration in rats fed ain-76a or ain-93g diets. J. Nutr. 2006, 136, 2141–2147. [Google Scholar] [PubMed]
- Hannibal, L.; DiBello, P.M.; Yu, M.; Miller, A.; Wang, S.; Willard, B.; Rosenblatt, D.S.; Jacobsen, D.W. The mmachc proteome: Hallmarks of functional cobalamin deficiency in humans. Mol. Genet. Metab. 2011, 103, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, R.; Murakami, T.; Obayashi, M.; Nakai, N.; Jaskiewicz, J.; Fujiwara, Y.; Shimomura, Y.; Harris, R.A. Clofibric acid stimulates branched-chain amino acid catabolism by three mechanisms. Arch. Biochem. Biophys. 2002, 407, 231–240. [Google Scholar] [CrossRef]
- Duval, C.; Muller, M.; Kersten, S. Par alpha and dyslipidemia. Biochim. Biophys. Acta 2007, 1771, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.M.; Cheung, C.; Gonzalez, F.J. Peroxisome proliferator-activated receptor-alpha and liver cancer: Where do we stand? J. Mol. Med. 2005, 83, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Duncan, T.M.; Reed, M.C.; Nijhout, H.F. A population model of folate-mediated one-carbon metabolism. Nutrients 2013, 5, 2457–2474. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lysne, V.; Strand, E.; Svingen, G.F.T.; Bjørndal, B.; Pedersen, E.R.; Midttun, Ø.; Olsen, T.; Ueland, P.M.; Berge, R.K.; Nygård, O. Peroxisome Proliferator-Activated Receptor Activation is Associated with Altered Plasma One-Carbon Metabolites and B-Vitamin Status in Rats. Nutrients 2016, 8, 26. https://doi.org/10.3390/nu8010026
Lysne V, Strand E, Svingen GFT, Bjørndal B, Pedersen ER, Midttun Ø, Olsen T, Ueland PM, Berge RK, Nygård O. Peroxisome Proliferator-Activated Receptor Activation is Associated with Altered Plasma One-Carbon Metabolites and B-Vitamin Status in Rats. Nutrients. 2016; 8(1):26. https://doi.org/10.3390/nu8010026
Chicago/Turabian StyleLysne, Vegard, Elin Strand, Gard F. T. Svingen, Bodil Bjørndal, Eva R. Pedersen, Øivind Midttun, Thomas Olsen, Per M. Ueland, Rolf K. Berge, and Ottar Nygård. 2016. "Peroxisome Proliferator-Activated Receptor Activation is Associated with Altered Plasma One-Carbon Metabolites and B-Vitamin Status in Rats" Nutrients 8, no. 1: 26. https://doi.org/10.3390/nu8010026
APA StyleLysne, V., Strand, E., Svingen, G. F. T., Bjørndal, B., Pedersen, E. R., Midttun, Ø., Olsen, T., Ueland, P. M., Berge, R. K., & Nygård, O. (2016). Peroxisome Proliferator-Activated Receptor Activation is Associated with Altered Plasma One-Carbon Metabolites and B-Vitamin Status in Rats. Nutrients, 8(1), 26. https://doi.org/10.3390/nu8010026





