The Effect of Changing Serum 25-Hydroxyvitamin D Concentrations on Metabolic Syndrome: A Longitudinal Analysis of Participants of a Preventive Health Program
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Measurements for Serum 25(OH)D and Other Biomarkers
2.3. Metabolic Syndrome Determination
2.4. Assessment for Other Variables
2.5. Statistical Analyses
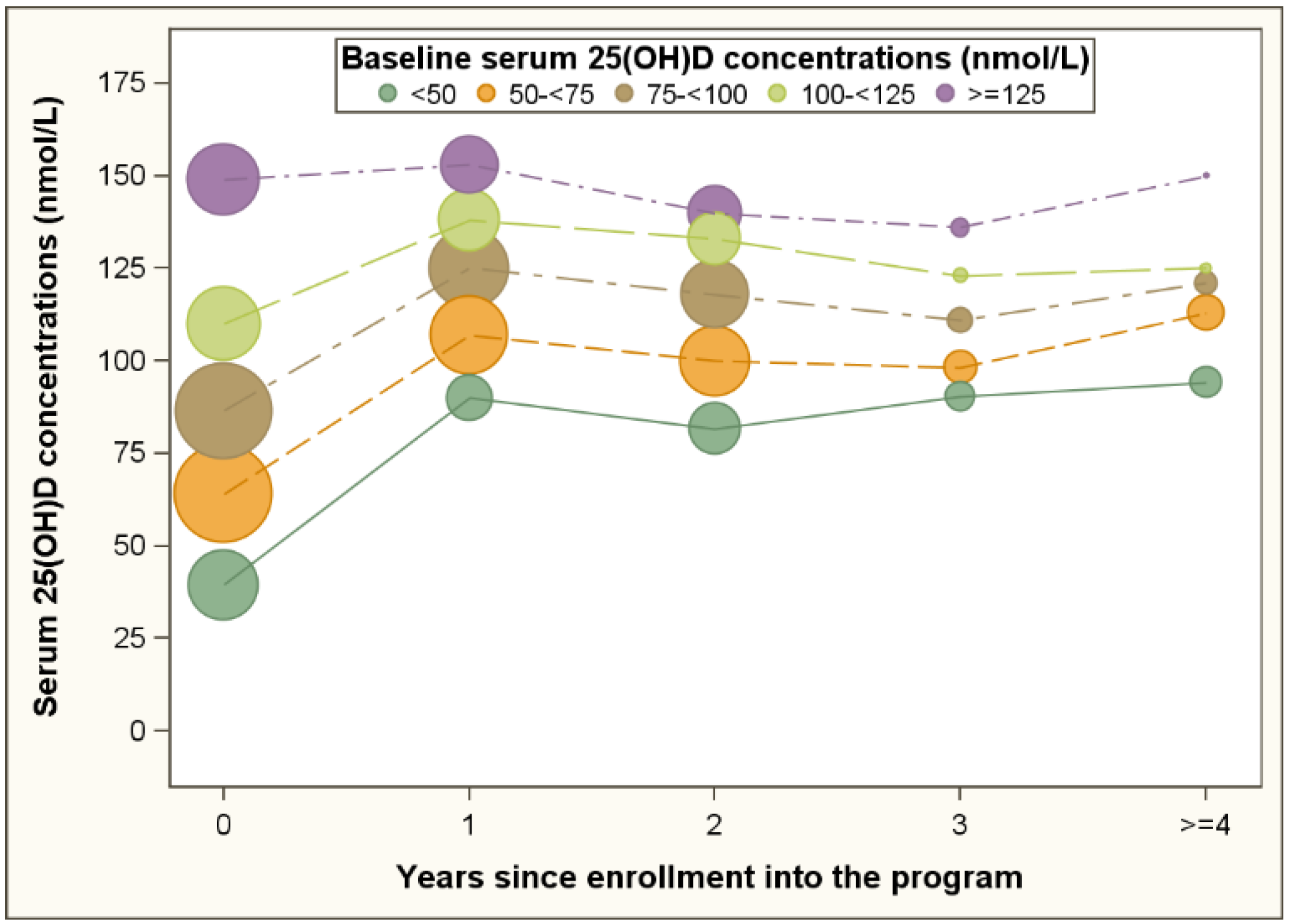
3. Results
| Baseline | Follow Up | |
|---|---|---|
| Serum 25(OH)D, nmol/L | ||
| Mean (SD) | 89 (42) | 121 (46) |
| Median (IQR) | 82 (61–108) | 115 (89–147) |
| Metabolic syndrome, n (%) | 1172 (18) | 1393 (21) |
| Metabolic syndrome components, n (%) | ||
| Elevated waist circumference | 2469 (37) | 2433 (36) |
| Elevated blood pressure | 2442 (37) | 2367 (35) |
| Elevated triglycerides | 1290 (19) | 1460 (22) |
| Elevated fasting glucose | 1149 (17) | 1417 (21) |
| Reduced HDL-cholesterol | 1160 (17) | 1482 (22) |
| Women, n (%) | 3526 (53) | 3526 (53) |
| Age, mean (SD) | 51 (15) | 52 (15) |
| Season, n (%) | ||
| Summer | 1235 (19) | 954 (14) |
| Fall | 1015 (15) | 966 (15) |
| Winter | 2665 (40) | 2753 (41) |
| Spring | 1767 (26) | 2009 (30) |
| Tobacco smoking status, n (%) a | ||
| Never smoker | 2680 (56) | 1964 (57) |
| Quit smoker | 1505 (31) | 1021 (30) |
| Current smoker | 605 (13) | 458 (13) |
| Missing | 1892 | 3239 |
| Alcohol drinking status, n (%) a | ||
| Non-drinker | 1744 (38) | 1891 (40) |
| Drinker | 2850 (62) | 2857 (60) |
| Missing | 2088 | 1934 |
| Physical activity, n (%) a | ||
| Low | 1932 (40) | 1806 (37) |
| Moderate | 1471 (31) | 1549 (31) |
| High | 1418 (29) | 1586 (32) |
| Missing | 1861 | 1741 |
| Univariate Analysis | Multivariable Analysis a | ||||
|---|---|---|---|---|---|
| # visits | OR (95% CI) | p | OR (95% CI) | p | |
| Baseline 25(OHD), nmol/L | |||||
| <50 | 1556 | Reference | Reference | ||
| 50–<75 | 2775 | 0.88 (0.74–1.05) | 0.17 | 0.81 (0.65–1.00) | 0.06 |
| 75–<100 | 2535 | 0.54 (0.45–0.65) | <0.01 | 0.51 (0.40–0.64) | <0.01 |
| 100–<125 | 1609 | 0.39 (0.31–0.49) | <0.01 | 0.40 (0.30–0.53) | <0.01 |
| ≥125 | 1544 | 0.32 (0.25–0.40) | <0.01 | 0.27 (0.20–0.36) | <0.01 |
| Changes in 25(OH)D during follow up compared with baseline, nmol/L | |||||
| No improvement | 1980 | Reference | Reference | ||
| Increase of <25 | 3087 | 1.27 (1.08–1.50) | <0.01 | 0.76 (0.62–0.93) | 0.01 |
| Increase of 25–<50 | 1994 | 1.22 (1.03–1.45) | 0.02 | 0.64 (0.52–0.80) | <0.01 |
| Increase of 50–<75 | 1069 | 1.20 (0.98–1.47) | 0.06 | 0.59 (0.46–0.77) | <0.01 |
| Increase of ≥75 | 1889 | 1.23 (1.03–1.46) | 0.02 | 0.56 (0.44–0.70) | <0.01 |
| Baseline metabolic syndrome | 10019 | 19.7 (17.0–22.8) | <0.01 | 16.7 (14.3–19.4) | <0.01 |
| Men vs. women | 10019 | 1.34 (1.19–1.51) | <0.01 | 1.24 (1.07–1.43) | <0.01 |
| Age at baseline | 10019 | 1.02 (1.02–1.03) | <0.01 | 1.03 (1.03–1.04) | <0.01 |
| Season at baseline | |||||
| Summer | 1717 | Reference | Reference | ||
| Fall | 1422 | 1.12 (0.91–1.39) | 0.29 | 0.88 (0.68–1.13) | 0.31 |
| Winter | 4244 | 1.19 (1.00–1.42) | 0.05 | 0.98 (0.79–1.20) | 0.83 |
| Spring | 2636 | 0.99 (0.82–1.19) | 0.91 | 0.84 (0.67–1.05) | 0.12 |
| Season at follow up | |||||
| Summer | 1707 | Reference | Reference | ||
| Fall | 1530 | 1.03 (0.87–1.22) | 0.69 | 0.97 (0.78–1.20) | 0.77 |
| Winter | 3554 | 0.99 (0.86–1.14) | 0.95 | 0.96 (0.80–1.14) | 0.64 |
| Spring | 3228 | 0.94 (0.81–1.09) | 0.42 | 0.93 (0.77–1.12) | 0.42 |
| Tobacco smoking status b | |||||
| Never smoker | 2742 | Reference | Reference | ||
| Quit smoker | 1402 | 1.33 (1.11–1.60) | <0.01 | 1.09 (0.86–1.37) | 0.48 |
| Current smoker | 684 | 1.21 (0.95–1.54) | 0.12 | 0.80 (0.61–1.07) | 0.13 |
| Alcohol drinking status b | |||||
| Non-drinker | 2569 | Reference | Reference | ||
| Drinker | 4496 | 0.67 (0.59–0.76) | <0.01 | 0.77 (0.66–0.91) | <0.01 |
| Physical activity b at baseline | |||||
| Low | 2629 | Reference | Reference | ||
| Moderate | 2004 | 0.60 (0.51–0.70) | <0.01 | 0.79 (0.65–0.97) | 0.02 |
| High | 1917 | 0.35 (0.29–0.43) | <0.01 | 0.57 (0.45–0.71) | <0.01 |
| Physical activity change b during follow up | |||||
| No improvement | 2437 | Reference | Reference | ||
| Moderate improvement | 1588 | 1.09 (0.93–1.28) | 0.28 | 0.93 (0.75–1.14) | 0.46 |
| High improvement | 920 | 0.90 (0.74–1.09) | 0.29 | 0.82 (0.63–1.07) | 0.14 |
| Participants without Metabolic Syndrome at Baseline (n = 5510) | Participants without Metabolic Syndrome and Naïve to Vitamin D Supplementation at Baseline (n = 1930) | |||||
|---|---|---|---|---|---|---|
| # visits | OR (95% CI) a | p | # visits | OR (95% CI) a | p | |
| Baseline 25(OHD), nmol/L | ||||||
| <50 | 1140 | Reference | 459 | Reference | ||
| 50–<75 | 2144 | 0.78 (0.60–1.01) | 0.06 | 910 | 0.83 (0.57–1.21) | 0.32 |
| 75–<100 | 2159 | 0.49 (0.37–0.64) | <0.01 | 694 | 0.59 (0.38–0.91) | 0.02 |
| 100–<125 | 1447 | 0.37 (0.27–0.52) | <0.01 | 287 | 0.57 (0.32–1.03) | 0.06 |
| ≥125 | 1375 | 0.24 (0.16–0.34) | <0.01 | 205 | 0.17 (0.07–0.43) | <0.01 |
| Changes in 25(OH)D during follow up compared with baseline, nmol/L | ||||||
| No improvement | 1680 | Reference | 421 | Reference | ||
| Increase of <25 | 2506 | 0.77 (0.60–0.98) | 0.04 | 661 | 0.63 (0.39–1.00) | 0.05 |
| Increase of 25–<50 | 1644 | 0.62 (0.47–0.81) | <0.01 | 518 | 0.73 (0.46–1.17) | 0.19 |
| Increase of 50–<75 | 884 | 0.51 (0.37–0.71) | <0.01 | 339 | 0.56 (0.32–0.96) | 0.03 |
| Increase of ≥75 | 1551 | 0.60 (0.45–0.79) | <0.01 | 616 | 0.58 (0.36–0.93) | 0.02 |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed]
- Wilson, P.W.; D’Agostino, R.B.; Parise, H.; Sullivan, L.; Meigs, J.B. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 2005, 112, 3066–3072. [Google Scholar] [CrossRef] [PubMed]
- Mottillo, S.; Filion, K.B.; Genest, J.; Joseph, L.; Pilote, L.; Poirier, P.; Rinfret, S.; Schiffrin, E.L.; Eisenberg, M.J. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 56, 1113–1132. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Metabolic syndrome pandemic. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Riediger, N.D.; Clara, I. Prevalence of metabolic syndrome in the Canadian adult population. CMAJ 2011, 183, E1127–E1134. [Google Scholar] [CrossRef] [PubMed]
- Sheets, D.J.; Gallagher, E.M. Aging in Canada: State of the art and science. Gerontologist 2013, 53, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D status: Measurement, interpretation, and clinical application. Ann. Epidemiol. 2009, 19, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A. Optimal serum 25-hydroxyvitamin D levels for multiple health outcomes. Adv. Exp. Med. Biol. 2008, 624, 55–71. [Google Scholar] [PubMed]
- Giovannucci, E.; Liu, Y.; Rimm, E.B.; Hollis, B.W.; Fuchs, C.S.; Stampfer, M.J.; Willett, W.C. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J. Natl. Cancer Inst. 2006, 98, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Pencina, M.J.; Booth, S.L.; Jacques, P.F.; Ingelsson, E.; Lanier, K.; Benjamin, E.J.; D’Agostino, R.B.; Wolf, M.; Vasan, R.S. Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008, 117, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Kunutsor, S.; Vitezova, A.; Oliver-Williams, C.; Chowdhury, S.; Kiefte-de-Jong, J.C.; Khan, H.; Baena, C.P.; Prabhakaran, D.; Hoshen, M.B.; et al. Vitamin D and risk of cause specific death: Systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ 2014, 348, g1903. [Google Scholar] [CrossRef] [PubMed]
- Vitezova, A.; Zillikens, M.C.; van Herpt, T.T.; Sijbrands, E.J.; Hofman, A.; Uitterlinden, A.G.; Franco, O.H.; Kiefte-de Jong, J.C. Vitamin D status and metabolic syndrome in the elderly: The Rotterdam Study. Eur. J. Endocrinol. 2015, 172, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Ajani, U.A.; McGuire, L.C.; Liu, S. Concentrations of serum vitamin D and the metabolic syndrome among, U.S. adults. Diabetes Care 2005, 28, 1228–1230. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.R.; Arora, P.; Garcia-Bailo, B.; Wolever, T.M.; Morrison, H.; El-Sohemy, A.; Karmali, M.; Badawi, A. Plasma vitamin D levels and risk of metabolic syndrome in Canadians. Clin. Investig. Med. 2011, 34, 377–384. [Google Scholar]
- Kayaniyil, S.; Vieth, R.; Harris, S.B.; Retnakaran, R.; Knight, J.A.; Gerstein, H.C.; Perkins, B.A.; Zinman, B.; Hanley, A.J. Association of 25(OH)D and PTH with metabolic syndrome and its traditional and nontraditional components. J. Clin. Endocrinol. Metab. 2011, 96, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Il Kang, M.; Won Oh, K.; Kwon, H.S.; Lee, J.H.; Lee, W.C.; Yoon, K.H.; Son, H.Y. The association of serum vitamin D level with presence of metabolic syndrome and hypertension in middle-aged Korean subjects. Clin. Endocrinol. (Oxf.) 2010, 73, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Hypponen, E.; Boucher, B.J.; Berry, D.J.; Power, C. 25-hydroxyvitamin D, IGF-1, and metabolic syndrome at 45 years of age: A cross-sectional study in the 1958 British Birth Cohort. Diabetes 2008, 57, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.Y.; Jeong, H.S.; Kim do, H. Blood vitamin D status and metabolic syndrome in the general adult population: A dose-response meta-analysis. J. Clin. Endocrinol. Metab. 2014, 99, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, C.; Lu, Z.X.; Magliano, D.J.; Dunstan, D.W.; Shaw, J.E.; Zimmet, P.Z.; Sikaris, K.; Ebeling, P.R.; Daly, R.M. Low serum 25-hydroxyvitamin D is associated with increased risk of the development of the metabolic syndrome at five years: Results from a national, population-based prospective study (The Australian Diabetes, Obesity and Lifestyle Study: AusDiab). J. Clin. Endocrinol. Metab. 2012, 97, 1953–1961. [Google Scholar] [CrossRef] [PubMed]
- Kayaniyil, S.; Harris, S.B.; Retnakaran, R.; Vieth, R.; Knight, J.A.; Gerstein, H.C.; Perkins, B.A.; Zinman, B.; Hanley, A.J. Prospective association of 25(OH)D with metabolic syndrome. Clin. Endocrinol. (Oxf.) 2014, 80, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Skaaby, T.; Husemoen, L.L.; Pisinger, C.; Jorgensen, T.; Thuesen, B.H.; Fenger, M.; Linneberg, A. Vitamin D status and changes in cardiovascular risk factors: A prospective study of a general population. Cardiology 2012, 123, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Al-Daghri, N.M.; Alkharfy, K.M.; Al-Saleh, Y.; Al-Attas, O.S.; Alokail, M.S.; Al-Othman, A.; Moharram, O.; El-Kholie, E.; Sabico, S.; Kumar, S.; et al. Modest reversal of metabolic syndrome manifestations with vitamin D status correction: A 12-month prospective study. Metabolism 2012, 61, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Ekwaru, J.P.; Zwicker, J.D.; Holick, M.F.; Giovannucci, E.; Veugelers, P.J. The importance of body weight for the dose response relationship of oral vitamin D supplementation and serum 25-hydroxyvitamin d in healthy volunteers. PLoS ONE 2014, 9, e111265. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P.; French, C.B.; Nguyen, S.; Ferreira, M.; Baggerly, L.L.; Brunel, L.; Veugelers, P. A novel approach localizes the association of vitamin D status with insulin resistance to one region of the 25-hydroxyvitamin D continuum. Adv. Nutr. 2013, 4, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P. Toward a physiological referent for the vitamin D requirement. J. Endocrinol. Investig. 2014, 37, 1127–1130. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Langlois, K.; Greene-Finestone, L.; Little, J.; Hidiroglou, N.; Whiting, S. Vitamin D status of Canadians as measured in the 2007 to 2009 Canadian Health Measures Survey. Health Rep. 2010, 21, 47–55. [Google Scholar] [PubMed]
- Janz, T.; Pearson, C. Vitamin D Blood Levels of Canadians. In Health at a Glance; Statistics Canada: Ottawa, ON, Canada, 2013; (Catalogue No 82-624-X); Available online: http://www.statcan.gc.ca/pub/82-624-x/2013001/article/11727-eng.pdf (accessed on 4 August 2015).
- Rucker, D.; Allan, J.A.; Fick, G.H.; Hanley, D.A. Vitamin D insufficiency in a population of healthy western Canadians. CMAJ 2002, 166, 1517–1524. [Google Scholar] [PubMed]
- Lipscombe, L.L.; Hux, J.E. Trends in diabetes prevalence, incidence, and mortality in Ontario, Canada 1995–2005: A population-based study. Lancet 2007, 369, 750–756. [Google Scholar] [CrossRef]
- Lau, R.S.; Ohinmaa, A.; Johnson, J. Predicting the Future Burden of Diabetes in Alberta from 2008 to 2035. Can. J. Diabetes 2011, 35, 274–281. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, T.-M.; Ekwaru, J.P.; Setayeshgar, S.; Veugelers, P.J. The Effect of Changing Serum 25-Hydroxyvitamin D Concentrations on Metabolic Syndrome: A Longitudinal Analysis of Participants of a Preventive Health Program. Nutrients 2015, 7, 7271-7284. https://doi.org/10.3390/nu7095338
Pham T-M, Ekwaru JP, Setayeshgar S, Veugelers PJ. The Effect of Changing Serum 25-Hydroxyvitamin D Concentrations on Metabolic Syndrome: A Longitudinal Analysis of Participants of a Preventive Health Program. Nutrients. 2015; 7(9):7271-7284. https://doi.org/10.3390/nu7095338
Chicago/Turabian StylePham, Truong-Minh, John Paul Ekwaru, Solmaz Setayeshgar, and Paul J. Veugelers. 2015. "The Effect of Changing Serum 25-Hydroxyvitamin D Concentrations on Metabolic Syndrome: A Longitudinal Analysis of Participants of a Preventive Health Program" Nutrients 7, no. 9: 7271-7284. https://doi.org/10.3390/nu7095338
APA StylePham, T.-M., Ekwaru, J. P., Setayeshgar, S., & Veugelers, P. J. (2015). The Effect of Changing Serum 25-Hydroxyvitamin D Concentrations on Metabolic Syndrome: A Longitudinal Analysis of Participants of a Preventive Health Program. Nutrients, 7(9), 7271-7284. https://doi.org/10.3390/nu7095338




