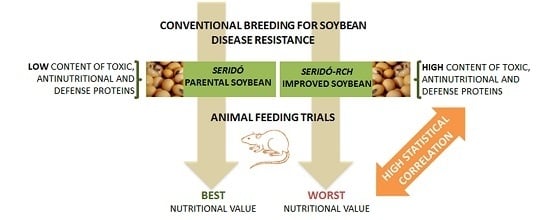
Increased Levels of Antinutritional and/or Defense Proteins Reduced the Protein Quality of a Disease-Resistant Soybean Cultivar
Abstract
:1. Introduction
2. Experimental Section
2.1. Biological and Chemical Reagents
2.2. Determination of Classical Antinutritional Proteins
2.3. Determination of Classical Plant Defense Proteins
2.4. Amino Acid Composition
2.5. Diets
| Ingredient | NPC | EW | Seridó | Seridó-RCH |
|---|---|---|---|---|
| maize starch | 555.6 | 422.4 | 409.4 | 399.6 |
| cassava starch | 111.1 | 111.1 | 100.0 | 100.0 |
| glucose | 166.7 | 166.7 | 150.0 | 150.0 |
| maize oil | 55.6 | 55.6 | 50.0 | 50.0 |
| vitamin mix a | 55.6 | 55.6 | 50.0 | 50.0 |
| mineral mix b | 55.6 | 55.6 | 50.0 | 50.0 |
| EW | - | 133.1 | - | - |
| Seridó | - | 187.6 | - | |
| Seridó-RCH | - | - | 197.4 | |
| l-methionine c | - | 2.3 | 2.3 | |
| l-tryptophan c | - | 0.7 | 0.7 | |
| Energy d (kcal/g) | 3.4 | 3.4 | 3.6 | 3.6 |
2.6. Feeding Trials
2.7. Chemical Analyses
2.8. Statistical Analyses
3. Results
3.1. Antinutritional and/or Defense Proteins
3.2. Amino Acid Composition
| Activity | Cultivar | |
|---|---|---|
| Seridó | Seridó-RCH | |
| trypsin inhibitor a | 37.50 ± 1.10 a | 59.71 ± 1.40 b |
| Lectin b | 0.10 ± 0.01 a | 0.45 ± 0.01 b |
| Urease c | 132,000 ± 11.32 a | 630,000 ± 17.44 b |
| Peroxidase d | 0.29 ± 0.04 a | 3.92 ± 0.01 b |
| Chitinase e | 17.76 ± 0.90 a | 23.50 ± 2.00 b |
| β-1,3-glucanase f | ND g | ND |
| Amino acid | Soybeans | Rat requirement | Requirement for children (3–10 years) | |
|---|---|---|---|---|
| Seridó | Seridó-RCH | |||
| Essential | ||||
| Thr | 37.0 | 38.5 | 40 | 25 |
| Val | 46.8 | 47.2 | 55 | 40 |
| Ile | 37.7 | 39.8 | 50 | 31 |
| Leu | 76.4 | 76.1 | 80 | 61 |
| Lys | 65.8 | 68.4 | 60 | 48 |
| Phe + Tyr | 107.2 | 107.0 | 90 | 41 |
| Met + Cys | 30.3 | 30.3 | 45 | 24 |
| Trp | 7.5 | 8.8 | 15 | 6.6 |
| Non-essential | ||||
| Asx | 116.7 | 118.8 | ||
| Glx | 188.4 | 186.4 | ||
| Ser | 42.5 | 41.5 | ||
| Gly | 38.7 | 39.3 | ||
| Ala | 41.2 | 41.9 | ||
| His | 29.0 | 30.3 | 25 | 16 |
| Arg | 81.5 | 74.7 | 50 | |
| Pro | 53.1 | 51.0 | ||
3.3. Nutritional Parameters
| Diets | ||||
|---|---|---|---|---|
| NPC | EW | Seridó | Seridó-RCH | |
| initial body weight (g) | 71.7 ± 2.8 a | 71.8 ± 2.6 a | 71.3 ± 2.2 a | 71.7 ± 2.4 a |
| final body weight (g) | 56.6 ± 2.9 c | 113.4 ± 8.5 a | 85.9 ± 7.0 b | 81.4 ± 6.4 b |
| body weight gain (g) | −15.2 ± 1.5 d | 42.6 ± 1.7 a | 14.4 ± 0.8 b | 9.8 ± 1.2 c |
| daily food intake (g) | 2.1 ± 0.6 b | 3.7 ± 0.6 a | 3.5 ± 0.7 a | 3.7 ± 0.7 a |
| net protein utilization (%) | - | 90.7 ± 1.2 a | 48.2 ± 1.0 b | 28.8 ± 1.0 c |
| protein digestibility (%) | - | 95.4 ± 1.0 a | 63.8 ± 1.0 b | 52.7 ± 1.6 c |
| biological value (%) | - | 95.1 ± 2.3 a | 75.6 ± 0.4 b | 54.7± 3.5 c |
| body nitrogen (g/kg) | 8.0 ± 0.8 c | 10.3 ± 1.0 a | 8.5 ± 0.4 b | 6.8 ± 0.4 d |
| Organ | Diets | |||
|---|---|---|---|---|
| NPC | EW | Seridó | Seridó-RCH | |
| stomach | 0.67 ± 0.03 b | 0.61 ± 0.04 ac | 0.63 ± 0.02 ab | 0.58 ± 0.04 c |
| intestine | 2.42 ± 0.44 a | 2.25 ± 0.20 a | 2.83 ± 0.22 b | 3.24 ± 0.13 c |
| cecum + colon | 0.67 ± 0.04 b | 0.70 ± 0.04 ab | 0.74 ± 0.06 a | 0.74 ± 0.06 a |
| liver | 4.52 ± 0.35 b | 5.19 ± 0.44 a | 4.29 ± 0.25 b | 4.57 ± 0.32 b |
| pancreas | 0.28 ± 0.02 a | 0.27 ± 0.02 a | 0.40 ± 0.04 b | 0.51 ± 0.05 c |
| thymus | 0.14 ± 0.01 d | 0.28 ± 0.02 a | 0.23 ± 0.02 b | 0.19 ± 0.01 c |
| spleen | 0.14 ± 0.01 b | 0.25 ± 0.01 a | 0.16 ± 0.01 b | 0.13 ± 0.02 b |
| kidneys | 0.90 ± 0.05 b | 0.79 ± 0.06 a | 0.75 ± 0.04 a | 0.77 ± 0.04 a |
| heart | 0.37 ± 0.04 b | 0.29 ± 0.01 a | 0.29 ± 0.02 a | 0.31 ± 0.02 a |
| lungs | 0.49 ± 0.05 a | 0.47 ± 0.03 a | 0.46 ± 0.05 a | 0.45 ± 0.02 a |
3.4. Relationships between Unintended Effects and Nutritional Performance
| Independent variable | Dependent variables | |||
|---|---|---|---|---|
| Diet Intake | Weight Gain | NPU | Digestibility | |
| lectin | −0.60 (−6.45) * | −3.33 (−4.27) * | −2.55 (−36.76) * | −8.43 (−8.26) * |
| trypsin inhibitor | −0.61 (−6.45) * | −3.01 (−4.63) * | −0.62 (−18.25) * | −5.22 (−10.41) * |
| peroxidase | −0.59 (−6.45) * | 2.86 (1.69) | −0.04 (−0.48) | 0.14 (0.13) |
| chitinase | −0.60 (−6.45) * | −1.96 (−7.79) * | −4.81 (−47.88) * | 3.99 (2.7) * |
| R2 | 0.81 | 0.93 | 0.99 | 0.98 |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ramanatha Rao, V.; Hodgkin, T. Genetic diversity and conservation and utilization of plant genetic resources. Plant Cell Tissue Organ Cult. 2002, 68, 1–19. [Google Scholar] [CrossRef]
- Rischer, H.; Oksman-Caldentey, K.M. Unintended effects in genetically modified crops: Revealed by metabolomics? Trends Biotechnol. 2006, 24, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Raboy, V. The future of crop breeding for nutritional quality. SABRAO J. Breed. Genet. 2013, 45, 100–111. [Google Scholar]
- Vasconcelos, I.M.; Maia, A.A.B.; Siebra, E.A.; Oliveira, J.T.A.; Carvalho, A.F.F.U.; Melo, V.M.; Carlini, C.R.; Castelar, L.I.M. Nutritional study of two Brazilian soybean (Glycine max) cultivars differing in the contents of antinutritional and toxic proteins. J. Nutr. Biochem. 2001, 12, 55–62. [Google Scholar] [CrossRef]
- Gilani, G.S.; Cockell, K.A.; Sepehr, E. Effects of antinutritional factors on protein digestibility and amino acid availability in foods. J. AOAC Int. 1989, 88, 967–987. [Google Scholar]
- Grant, G. Antinutritional effects of soybean: A review. Prog. Food Nutr. Sci. (Oxford) 1989, 13, 317–348. [Google Scholar]
- Brune, M.F.S.S.; Pinto, M.O.; Peluzio, M.C.G.; Moreira, M.A.; Barros, E.G. Biochemical and nutritional evaluation of a soybean line lacking the Kunitz trypsin inhibitor and lectins. Food Sci. Technol. 2010, 30, 657–663. [Google Scholar]
- Friedman, M.; Brandon, D.L.; Bates, A.H.; Hymowitz, T. Comparison of a commercial soybean cultivar and an isoline lacking the Kunitz trypsin inhibitor: Composition, nutritional value, and effects of heating. J. Agric. Food Chem. 1991, 39, 327–335. [Google Scholar] [CrossRef]
- Liener, I.E. Implications of antinutritional components in soybean foods. Crit. Rev. Food Sci. Nutr. 1994, 34, 31–67. [Google Scholar] [CrossRef] [PubMed]
- Haq, S.K.; Atif, S.M.; Khan, R.H. Protein proteinase inhibitor genes in combat against insects, pests, and pathogens: Natural and engineered phytoprotection. Arch. Biochem. Biophys. 2004, 431, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Vandenborre, G.; Smagghe, G.; Van Damme, E.J.M. Plant lectins as defense proteins against phytophagous insects. Phytochemistry 2011, 72, 1538–1550. [Google Scholar] [CrossRef] [PubMed]
- Campelo, G.J.A.; Kiihl, R.A.S.; Almeida, L.A. Características Agronômicas e Morfológicas das Cultivares de Soja Desenvolvidas Para as Regiões de Baixas Latitudes. Available online: http://www.cpatsa.embrapa.br/catalogo/livrorg/sojacultivares.pdf (accessed on 4 August 2013).
- Tome, D. Criteria and markers for protein quality assessment—A review. Br. J. Nutr. 2012, 108, S222–S229. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, I.M.; Siebra, E.A.; Maia, A.A.B.; Moreira, R.A.; Neto, A.F.; Campelo, G.J.A.; Oliveira, J.T.A. Composition, toxic and antinutritional factors of newly developed cultivars of Brazilian soybean (Glycine max). J. Sci. Food Agric. 1997, 75, 419–426. [Google Scholar] [CrossRef]
- Kakade, M.L.; Simons, N.R.; Liener, I.E. An evaluation of natural vs. synthetic substrates for measuring the antitryptic activity of soybean samples. Cereal Chem. 1969, 46, 518–526. [Google Scholar]
- Kaplan, A. The determination of urea, ammonia, and urease. Methods Biochem. Anal. 1969, 17, 311–324. [Google Scholar] [PubMed]
- Urbanek, H.; Kuzniak-Gebarowska, E.; Herka, K. Elicitation of defense responses in bean leaves by Botrytis cinerea polygalacturonase. Acta Physiol. Plant. 1991, 13, 43–50. [Google Scholar]
- Boller, T. Biochemical analysis of chitinases and β-1,3-glucanase. In Molecular Plant Pathology; Gurr, S.J., Mc Pherson, M.J., Bowles, D.J., Eds.; IRL Press: New York, NY, USA, 1993; pp. 23–29. [Google Scholar]
- Pintér-Szakács, M.; Molnár-Perl, H. Determination of tryptophan in unhydrolyzed food and feedstuffs by the acid ninhydrin method. J. Agric. Food Chem. 1990, 38, 720–726. [Google Scholar] [CrossRef]
- Milhome, M.A.L.; Lima, C.G.; de Lima, L.K.; Lima, F.A.F.; Sousa, D.O.B.; Nascimento, R.F. Occurrence of aflatoxins in cashew nuts produced in northeastern Brazil. Food Control 2014, 42, 34–37. [Google Scholar] [CrossRef]
- Coates, M.E.; Odonogue, P.N.; Payne, P.R. Nutritional and microbiological recommendation. In Laboratory Animal Handbook 2—Dietary Standards for Laboratory Rats and Mice; Coates, M.E., Ed.; Laboratory Animals Limited: London, UK, 1969; pp. 13–15. [Google Scholar]
- Miller, D.S.; Bender, A.E. The determination of the net utilization of protein by a shortened method. Br. J. Nutr. 1955, 9, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Triebold, H.O. Quantitative Analysis with Applications to Agricultural and Food Products; Van Nostrand Co.: New York, NY, USA, 1946. [Google Scholar]
- Baethgen, W.E.; Alley, M.M. A manual colorimetric procedure for measuring ammonium nitrogen in soil and plant Kjeldahl digests. Commun. Soil Sci. Plant Anal. 1989, 20, 961–969. [Google Scholar] [CrossRef]
- Morrison, D.F. Multivariate Statistical Methods; McGraw Hill Book Press: London, UK, 1978. [Google Scholar]
- Mundlak, Y. On the concept of non-significant functions and its implications for regression analysis. J. Econom. 1981, 16, 139–149. [Google Scholar] [CrossRef]
- WHO. Protein and Amino Acid Requirements in Human Nutrition—Report of Joint WHO/FAO/UNU Expert Consultation; World Health Organization Technical Report Series; WHO Press: Geneva, Switzerland, 2007; pp. 1–265. [Google Scholar]
- Iqbal, A.; Khalil, I.A.; Ateeq, N.; Khan, M.S. Nutritional quality of important food legumes. Food Chem. 2006, 97, 331–335. [Google Scholar] [CrossRef]
- Habib, H.; Fazili, K.M. Plant protease inhibitors: A defense strategy in plants. Biotechnol. Mol. Biol. Rev. 2007, 2, 68–85. [Google Scholar]
- Carrillo, L.; Herrero, I.; Cambra, I.; Sánchez-Monge, R.; Diaz, I.; Martinez, M. Differential in vitro and in vivo effect of barley cysteine and serine protease inhibitors on phytopathogenic microorganisms. Plant Physiol. Biochem. 2011, 49, 1191–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.Y.; Park, S.C.; Hwang, I.; Cheong, H.; Nah, J.W.; Hahm, K.S.; Park, Y. Protease inhibitors from plants with antimicrobial activity. Int. J. Mol. Sci. 2009, 10, 2860–2872. [Google Scholar] [CrossRef] [PubMed]
- De Hoff, P.L.; Brill, L.M.; Hirsch, A.M. Plant lectins: The ties that bind in root symbiosis and plant defense. Mol. Genet. Genomics 2009, 282, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Charungchitrak, S.; Petsom, A.; Sangvanich, P.; Karnchanatat, A. Antifungal and antibacterial activities of lectin from the seeds of Archidendron jiringa Nielsen. Food Chem. 2011, 126, 1025–1032. [Google Scholar] [CrossRef]
- Real-Guerra, R.; Stanisçuaski, F.; Carlini, C.R. Soybean urease: Over a hundred years of knowledge. In Comprehensive Survey of International Soybean Research—Genetics, Physiology, Agronomy and Nitrogen Relationships, 1st ed.; Board, J.E., Ed.; InTech: Dubrovnik, Croatia, 2013; pp. 317–339. [Google Scholar]
- Becker-Ritt, A.B.; Martinelli, A.H.; Mitidieri, S.; Feder, V.; Wassermann, G.E.; Santi, L.; Vainstein, M.H.; Oliveira, J.T.A.; Fiuza, L.M.; Pasquali, G.; et al. Antifungal activity of plant and bacterial ureases. Toxicon 2007, 50, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Wiebke-Strohm, B.; Pasquali, G.; Margis-Pinheiro, M.; Bencke, M.; Bücker-Neto, L.; Becker-Ritt, A.B.; Martinelli, A.H.S.; Rechenmacher, C.; Polacco, J.C.; Stolf, R.; et al. Ubiquitous urease affects soybean susceptibility to fungi. Plant Mol. Biol. 2012, 79, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Stam, J.M.; Kroes, A.; Li, Y.; Goes, R.; van Loon, J.J.A.; Poelman, E.H.; Dicke, M. Plant interactions with multiple insect herbivores: From community to genes. Annu. Rev. Plant Biol. 2014, 65, 689–713. [Google Scholar] [CrossRef] [PubMed]
- Cipollini, D.; Wang, Q.; Whitehill, J.G.A.; Powell, J.R.; Bonello, P.; Herms, D.A. Distinguishing defensive characteristics in the phloem of ash species resistant and susceptible to emerald ash borer. J. Chem. Ecol. 2011, 37, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shao, B.; Rao, P.; Deng, Z.; Xie, M. Limlin, a novel leguminous peroxidase with antifungal activity from Phaseolus limensis. J. Food Biochem. 2011, 35, 1206–1222. [Google Scholar] [CrossRef]
- Vega, K.; Kalkum, M. Chitin, chitinase responses, and invasive fungal infections. Int. J. Microbiol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ye, X.; Chen, J.; Rao, P. A novel chitinase isolated from Vicia faba and its antifungal activity. Food Res. Int. 2012, 45, 116–122. [Google Scholar] [CrossRef]
- Schultze, M.; Staehelin, C.; Brunner, F.; Genetet, I.; Legrand, M.; Fritig, B.; Kondorosi, E.; Kondorosi, A. Plant chitinase/lysozyme isoforms show distinct substrate specificity and cleavage site preference towards lipochitooligosaccharide Nod signals. Plant J. 1998, 16, 571–580. [Google Scholar] [CrossRef]
- Collinge, D.B.; Jorgensen, H.J.L.; Lund, O.S.; Lyngkjaer, M.F. Engineering pathogen resistance in crop plants: Current trends and future prospects. Annu. Rev. Phytopathol. 2010, 48, 269–291. [Google Scholar] [CrossRef] [PubMed]
- Paparini, A.; Romano-Spica, V. Public health issues related with the consumption of food obtained from genetically modified organisms. Biotechnol. Annu. Rev. 2004, 10, 85–122. [Google Scholar] [PubMed]
- Maity, J.P.; Chakraborty, S.; Kar, S.; Panja, S.; Jean, J.S.; Samal, A.C.; Chakraborty, A.; Santra, S.C. Effects of gamma irradiation on edible seed protein, amino acids and genomic DNA during sterilization. Food Chem. 2009, 114, 1237–1244. [Google Scholar] [CrossRef]
- El-Niely, H.F.G. Effect of radiation processing on antinutrients, in vitro protein digestibility and protein efficiency ratio bioassay of legume seeds. Radiat. Phys. Chem. 2007, 76, 1050–1057. [Google Scholar] [CrossRef]
- Kumta, U.S.; Tappel, A.L. Radiation damage to proteins. Nature 1961, 191, 1304–1305. [Google Scholar] [CrossRef] [PubMed]
- Afify, A.E.M.R.; Rashed, M.M.; Mahmoud, E.A.; El-Beltagi, H.S. Effect of gamma radiation on protein profile, protein fraction and solubility’s of three oil seeds: Soybean, peanut and sesame. Not. Bot. Horti Agrobot. Cluj-Napoca 2011, 39, 90–98. [Google Scholar]
- Muzquiz, M.; Varela, A.; Burbano, C.; Cuadrado, C.; Guillamón, E. Bioactive compounds in legumes: Pronutritive and antinutritive actions. Implications for nutrition and health. Phytochem. Rev. 2012, 11, 227–244. [Google Scholar] [CrossRef]
- Kumar, S.; Verma, A.K.; Das, M.; Jain, S.K.; Dwivedi, P.D. Clinical complications of kidney bean (Phaseolus vulgaris L.) consumption. Nutrition 2013, 29, 821–827. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, D.O.B.; Carvalho, A.F.U.; Oliveira, J.T.A.; Farias, D.F.; Castelar, I.; Oliveira, H.P.; Vasconcelos, I.M. Increased Levels of Antinutritional and/or Defense Proteins Reduced the Protein Quality of a Disease-Resistant Soybean Cultivar. Nutrients 2015, 7, 6038-6054. https://doi.org/10.3390/nu7075269
Sousa DOB, Carvalho AFU, Oliveira JTA, Farias DF, Castelar I, Oliveira HP, Vasconcelos IM. Increased Levels of Antinutritional and/or Defense Proteins Reduced the Protein Quality of a Disease-Resistant Soybean Cultivar. Nutrients. 2015; 7(7):6038-6054. https://doi.org/10.3390/nu7075269
Chicago/Turabian StyleSousa, Daniele O. B., Ana. F. U. Carvalho, José Tadeu A. Oliveira, Davi F. Farias, Ivan Castelar, Henrique P. Oliveira, and Ilka M. Vasconcelos. 2015. "Increased Levels of Antinutritional and/or Defense Proteins Reduced the Protein Quality of a Disease-Resistant Soybean Cultivar" Nutrients 7, no. 7: 6038-6054. https://doi.org/10.3390/nu7075269
APA StyleSousa, D. O. B., Carvalho, A. F. U., Oliveira, J. T. A., Farias, D. F., Castelar, I., Oliveira, H. P., & Vasconcelos, I. M. (2015). Increased Levels of Antinutritional and/or Defense Proteins Reduced the Protein Quality of a Disease-Resistant Soybean Cultivar. Nutrients, 7(7), 6038-6054. https://doi.org/10.3390/nu7075269