The Potential Benefits of Red Beetroot Supplementation in Health and Disease
Abstract
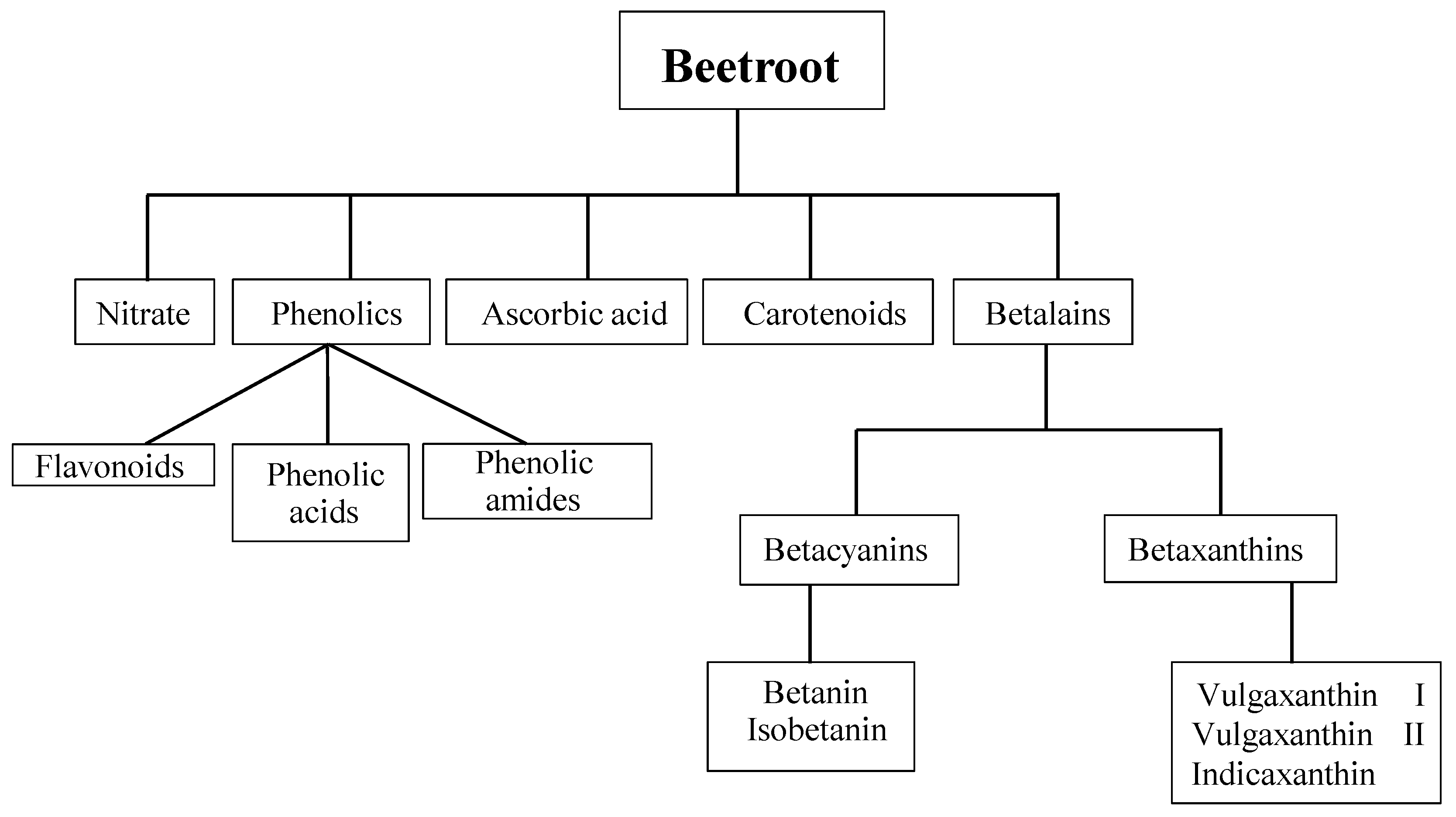
:1. Introduction
2. Bioavailability
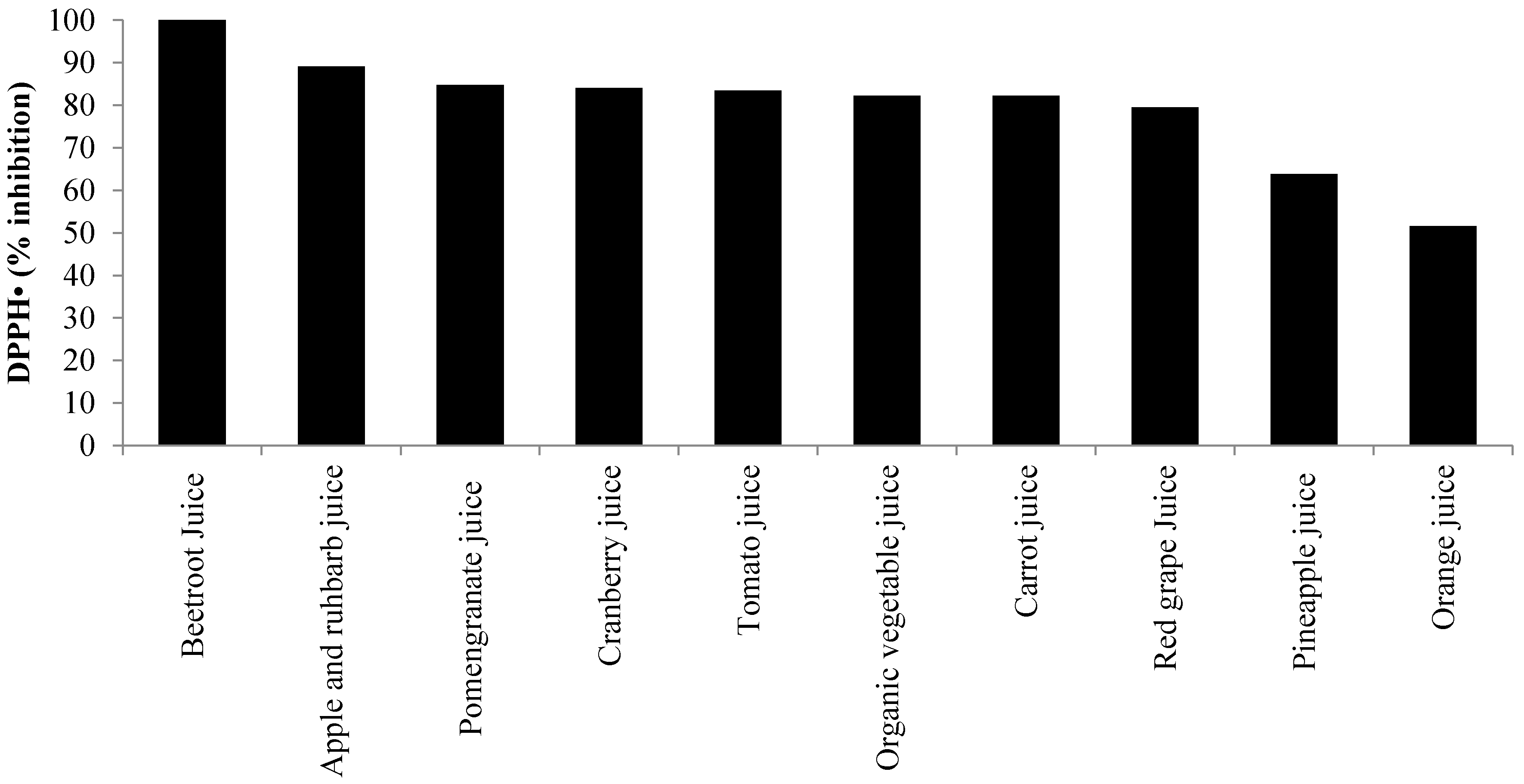
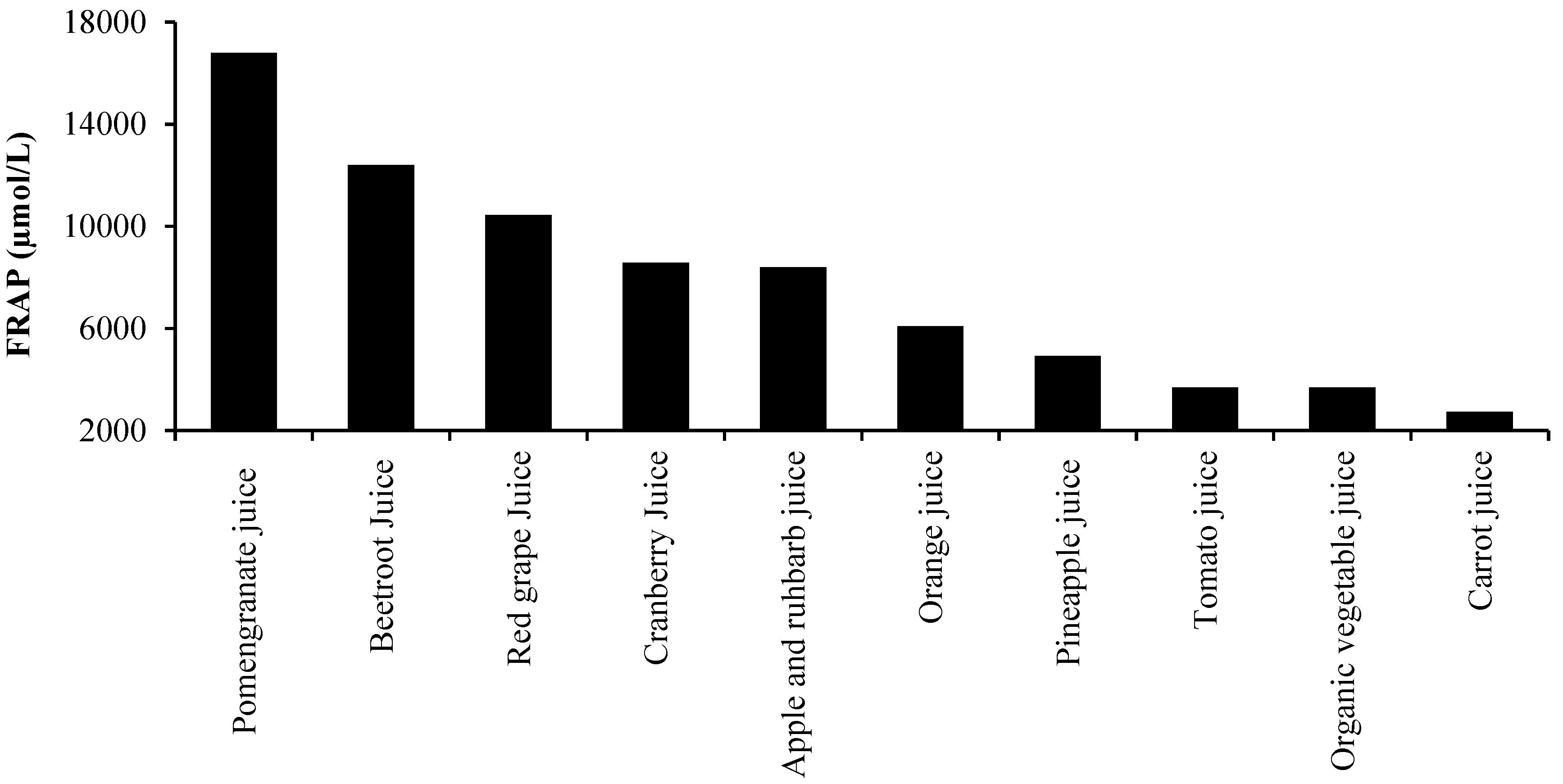
3. Oxidative Stress
| Authors | Cohort under Investigation | Dosage | Antioxidant Capacity of Treatment | Duration | Toxic inducing Protocol | Inflammation | Oxidative Stress | Enzymatic Antioxidant Activity |
|---|---|---|---|---|---|---|---|---|
| [54] | 48 male wistar rats | Beetroot juice delivered by gavage (8 mL∙kg∙bm∙day−1; ≈1.92 mL∙day−1) | 23.5 μmol Trolox equivalents∙mL−1 | 28 days | Intraperitoneal injection of CCl4 (2 mL∙kg∙bm−1) or NDEA (150 mL∙kg∙bm−1) | N/A | TBARS ↓ PC ↓ DNA damage ↓ | SOD ↑ GPX ↑ CAT ↑ GR ↑ |
| [63] | 80 male ICR mice | Betalains from beetroot delivered orally (0, 5, 20 and 80 mg∙kg∙bm∙day−1; ≈0–1.44 mg∙day−1) | N/A | 30 days | Exposed to cobalt-60-γ-gamma radiation (6.0 Gy, 1.5 Gy min−1) | N/A | MDA ↓ | SOD ↑ CAT ↑ GSH ↑ |
| [64] | 24 male wistar rats | Beetroot juice delivered by gavage (8 mL∙kg∙bm∙day−1) | N/A | 28 days | Intraperitoneal injection of NDEA (150 mL∙kg∙bm−1) | LDH ↓ AST ↓ ALT ↓ | DNA damage ↓ | GST ↑ |
| [27] | 10 osteoarthritic patients | Capsules made from beetroot extract delivered orally (70–200 mg∙day−1) | N/A | 10 days | N/A | TNF-α ↓ IL-6 ↓ RANTES ↓ GRO-α ↓ | AOPP ↓ | N/A |
| [23] | 48 albino wistar rats | Beetroot pomace extract delivered intraperitoneally (1–3 mL∙kg∙bm∙day−1; ≈0.2–0.6 ml∙day−1) | N/A | 7 days | Intraperitoneal injection of CCl4 (2 mL∙kg∙bm−1) | N/A | MDA ↓ | GSH ↑ GSHPx ↑ GR ↑ CAT ↑ |
| [65] | 24 albino wistar rats | Extracts of fresh beetroot delivered orally (250 and 500 mg∙kg∙bm∙day−1; ≈45–90 mg∙day−1) | 90.1% radical inhibition in the DPPH• assay (500 μg∙mL−1) | 28 days | Intraperitoneal injection of gentamicin (8 mg∙kg∙bm−1 for 8 days) | IL-6 ↓ TNF-α ↓ MPO ↓ NF-κB ↓ | MDA ↓ | CAT ↑ NP-SH ↑ |
| [58] | 24 female Sprague-dawley rats | Beetroot juice delivered by gavage (8 mL∙kg∙bm∙day−1; ≈1.92 mL∙day−1) | N/A | 28 days | Intraperitoneal injection of DMBA (10 mg∙kg∙bm−1 for 2 days) | LDH ↓ ALT↓ | N/A | GST ↑ NQO1 ↑ |
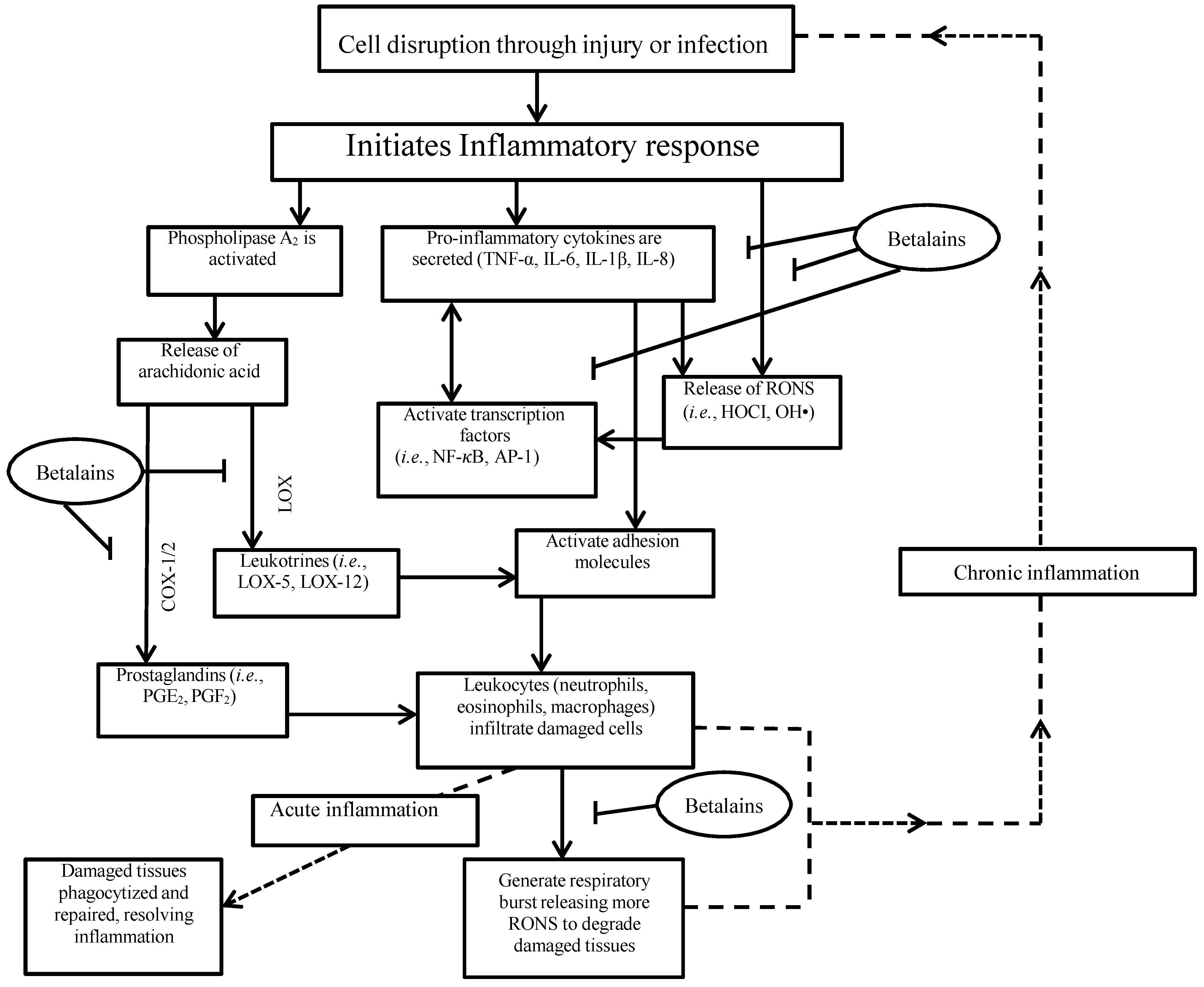
4. Inflammation
5. Endothelial Function
6. Cognitive Function
7. Conclusions
8. Future Directions
Author Contributions
Conflicts of Interest
References
- Ninfali, P.; Angelino, D. Nutritional and functional potential of Beta vulgaris cicla and rubra. Fitoterapia 2013, 89, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, V.G.; Weber, J.; Kneschke, E.M.; Denev, P.N.; Bley, T.; Pavlov, A.I. Antioxidant activity and phenolic content of betalain extracts from intact plants and hairy root cultures of the red beetroot Beta vulgaris cv. Detroit dark red. Plant Foods Hum. Nutr. 2010, 65, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Przyjemska, M.; Olejnik, A.; Dobrowolska-Zachwieja, A.; Grajek, W. In vitro effects of beetroot juice and chips on oxidative metabolism and apoptosis in neutrophils from obese individuals. Phytophera Res. 2009, 23, 49–55. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. 2008, 7, 156–167. [Google Scholar]
- Gilchrist, M.; Winyard, P.G.; Fulford, J.; Anning, C.; Shore, A.C.; Benjamin, N. Dietary nitrate supplementation improves reaction time in type 2 diabetes: Development and application of a novel nitrate-depleted beetroot juice placebo. Nitric Oxide 2014, 40, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Presley, T.D.; Morgan, A.R.; Bechtold, E.; Clodfelter, W.; Dove, R.W.; Jennings, J.M.; Kraft, R.A.; King, R.A.; Laurienti, P.J.; Rejeskib, J.; et al. Acute effect of a high nitrate diet on brain perfusion in older adults. Nitric Oxide 2011, 24, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Vanhatalo, A.; Bailey, S.J.; Blackwell, J.R.; di Menna, F.J.; Pavey, T.G.; Wilkerson, D.P.; Benjamin, N.; Winyard, P.G.; Jones, A.M. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am. J. Physiol. -Reg. I 2010, 299, 1121–1131. [Google Scholar]
- Wootton-Beard, P.C.; Brandt, K.; Fell, D.; Warner, S.; Ryan, L. Effects of a beetroot juice with high neobetanin content on the early-phase insulin response in healthy volunteers. J. Nutr. Sci. 2011, 3, 1–9. [Google Scholar]
- Bailey, S.J.; Winyard, P.; Vanhatalo, A.; Blackwell, J.R.; Dimenna, F.J.; Wilkerson, D.P.; Tarr, J.; Benjamin, N.; Jones, A.M. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J. Appl. Physiol. 2009, 107, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.J.; Patel, N.; Loukogeorgakis, S.; Okorie, M.; Aboud, Z.; Misra, S.; Rashid, R.; Miall, P.; Deanfield, J.; Benjamin, N.; et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension 2008, 51, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Jajja, A.; Sutyarjoko, A.; Lara, J.; Rennie, K.; Brandt, K.; Qadir, O.; Siervo, M. Beetroot supplementation lowers daily systolic blood pressure in older, overweight subjects. Nutr. Res. 2014, 34, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, D.A.; Goulding, M.G.; Nguyen, A.; Malaver, T.; Walker, C.F.; George, T.W.; Lovegrove, J.A. Acute ingestion of beetroot bread increases endothelium-independent vasodilation and lowers diastolic blood pressure in healthy men: A randomized controlled trial. J. Nutr. 2013, 143, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, D.A.; Kaffa, N.; George, T.W.; Methven, L.; Lovegrove, J.A. Blood pressure-lowering effects of beetroot juice and novel beetroot-enriched bread products in normotensive male subjects. Brit. J. Nutr. 2012, 108, 2066–2074. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, D.A.; George, T.W.; Lovegrove, J.A. The effects of dietary nitrate on blood pressure and endothelial function: A review of human intervention studies. Nutr. Res. Rev. 2013, 26, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Kapil, V.; Weitzberg, E.; Lundberg, J.O.; Ahluwalia, A. Clinical evidence demonstrating the utility of inorganic nitrate in cardiovascular health. Nitric Oxide 2014, 38, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Lidder, S.; Webb, A.J. Vascular effects of dietary nitrate (as found in green leafy vegetables and beetroot) via the nitrate-nitrite-nitric oxide pathway. Br. J. Clin. Pharmacol. 2013, 75, 677–696. [Google Scholar] [PubMed]
- Ormsbee, M.J.; Lox, J.; Arciero, P.J. Beetroot juice and exercise performance. J. Int. Soc. Sports Nutr. 2013, 5, 27–35. [Google Scholar]
- Machha, A.; Schechter, A.N. Dietary nitrite and nitrate: A review of potential mechanisms of cardiovascular benefits. Eur. J. Nutr. 2011, 50, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Carlström, M.; Larsen, F.J.; Weitzberg, E. Roles of dietary inorganic nitrate in cardiovascular health and disease. Cardiovas Res. 2011, 89, 525–532. [Google Scholar] [CrossRef]
- Kujala, T.S.; Vienola, M.S.; Klika, K.D.; Loponen, J.M.; Pihlaja, K. Betalain and phenolic compositions of four beetroot (Beta vulgaris) cultivars. Eur. Food. Res. Technol. 2002, 214, 505–510. [Google Scholar] [CrossRef]
- Wootton-Beard, P.C.; Ryan, L. A beetroot juice shot is a significant and convenient source of bioaccessible antioxidants. J. Funct. Foods 2011, 3, 329–334. [Google Scholar] [CrossRef]
- Lee, C.H.; Wettasinghe, M.; Bolling, B.W.; Ji, L.L.; Parkin, K.L. Betalains, phase II enzyme-inducing components from red beetroot (Beta vulgaris L.) extracts. Nutr. Cancer 2005, 53, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Vulić, J.J.; Ćebović, T.N.; Čanadanović-Brunet, J.M.; Ćetković, G.S.; Čanadanović, V.M.; Djilas, S.M.; Tumbas Šaponjac, V.T. In vivo and in vitro antioxidant effects of beetroot pomace extracts. J. Funct. Foods 2014, 6, 168–175. [Google Scholar] [CrossRef]
- Pavlov, A.; Georgiev, V.; Ilieva, M. Betalain biosynthesis by red beet (Beta vulgaris L.) hairy root culture. Process. Biochem. 2005, 40, 1531–1533. [Google Scholar] [CrossRef]
- Tesoriere, L.; Allegra, M.; Butera, D.; Livrea, M.A. Absorption, excretion, and distribution of dietary antioxidant betalains in LDLs: Potential health effects of betalains in humans. Am. J. Clin. Nutr. 2004, 80, 941–945. [Google Scholar] [PubMed]
- Vidal, P.J.; López-Nicolás, J.M.; Gandía-Herrero, F.; García-Carmona, F. Inactivation of lipoxygenase and cyclooxygenase by natural betalains and semi-synthetic analogues. Food. Chem. 2014, 154, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Pietrzkowski, Z.; Nemzer, B.; Spórna, A.; Stalica, P.; Tresher, W.; Keller, R.; Jiminez, R.; Michalowski, T.; Wybraniec, S. Influence of betalin-rich extracts on reduction of discomfort associated with osteoarthritis. New. Med. 2010, 1, 12–17. [Google Scholar]
- Das, S.; Williams, D.S.; Das, A.; Kukreja, R.C. Beet root juice promotes apoptosis in oncogenic MDA-MB-231 cells while protecting cardiomyocytes under doxorubicin treatment. J. Exp. Second. Sci. 2013, 2, 1–6. [Google Scholar]
- Kapadia, G.J.; Azuine, M.A.; Rao, G.S.; Arai, T.; Iida, A.; Tokuda, H. Cytotoxic effect of the red beetroot (Beta vulgaris L.) extract compared to doxorubicin (Adriamycin) in the human prostate (PC-3) and breast (MCF-7) cancer cell lines. Anti -Cancer Agent Med. Chem. 2011, 11, 280–284. [Google Scholar] [CrossRef]
- Kapadia, G.J.; Azuine, M.A.; Sridhar, R.; Okuda, Y.; Tsuruta, A.; Ichiishi, E.; Mukainakec, T.; Takasakid, M.; Konoshimad, T.; Nishinoc, H.; et al. Chemoprevention of DMBA-induced UV-B promoted, NOR-1-induced TPA promoted skin carcinogenesis, and DEN-induced phenobarbital promoted liver tumors in mice by extract of beetroot. Pharmacol. Res. 2003, 47, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, G.J.; Rao, G.S.; Ramachandran, C.; Iida, A.; Suzuki, N.; Tokuda, H. Synergistic cytotoxicity of red beetroot (Beta vulgaris L.) extract with doxorubicin in human pancreatic, breast and prostate cancer cell lines. J. Complement. Med. 2013, 10, 113–122. [Google Scholar]
- Toutain, P.L.; Bousquet-Mélou, A. Bioavailability and its assessment. J. Vet. Pharmacol. Ther. 2004, 27, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [PubMed]
- Ting, Y.; Jiang, Y.; Ho, C.T.; Huang, Q. Common delivery systems for enhancing in vivo bioavailability and biological efficacy of nutraceuticals. J. Funct. Foods 2014, 7, 112–128. [Google Scholar] [CrossRef]
- Van Velzen, A.G.; Sips, A.J. A.M.; Schothorst, R.C.; Lambers, A.C.; Meulenbelt, J. The oral bioavailability of nitrate from nitrate-rich vegetables in humans. Toxicol. Lett. 2008, 181, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Frank, T.; Stintzing, F.C.; Carle, R.; Bitsch, I.; Quaas, D.; Strass, G.; Netzel, M. Urinary pharmacokinetics of betalains following consumption of red beet juice in healthy humans. Pharmacol. Res. 2005, 52, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Kanner, J.; Harel, S.; Granit, R. Betalains A New Class of Dietary Cationized Antioxidants. J. Agric. Food. Chem. 2001, 49, 5178–5185. [Google Scholar] [CrossRef] [PubMed]
- Tesoriere, L.; Gentile, C.; Angileri, F.; Attanzio, A.; Tutone, M.; Allegra, M.; Livrea, M.A. Trans-epithelial transport of the betalain pigments indicaxanthin and betanin across Caco-2 cell monolayers and influence of food matrix. Eur. J. Nutr. 2013, 52, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230–242. [Google Scholar]
- Netzel, M.; Stintzing, F.C.; Quaas, D.; Strass, G.; Carle, R.; Bitsch, R.; Frank, T. Renal excretion of antioxidative constituents from red beet in humans. Food. Res. Int. 2005, 38, 1051–1058. [Google Scholar] [CrossRef]
- Kannan, K.; Jain, S.K. Oxidative stress and apoptosis. Pathophysiology 2000, 7, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Kohen, R.; Nyska, A. Oxidation of biological systems: Oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. 2002, 30, 620–650. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-κB, inflammation, and Metabolic Disease. Cell Metab. 2001, 13, 11–22. [Google Scholar] [CrossRef]
- Powers, S.K.; Jackson, M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [PubMed]
- Madamanchi, N.R.; Vendrov, A.; Runge, M.S. Oxidative stress and vascular disease. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Schinella, G.R.; Tournier, H.A.; Prieto, J.M.; Mordujovich de Buschiazzo, P.; Ríos, J.L. Antioxidant activity of anti-inflammatory plant extracts. Life Sci. 2002, 70, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacol. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517–520. [Google Scholar]
- Reddy, M.K.; Alexander-Lindo, R.L.; Nair, M.G. Relative inhibition of lipid peroxidation, cyclooxygenase enzymes, and human tumor cell proliferation by natural food colors. J. Agric. Food Chem. 2005, 53, 9268–9273. [Google Scholar] [CrossRef] [PubMed]
- Tesoriere, L.; Fazzari, M.; Angileri, F.; Gentile, C.; Livrea, M.A. In vitro digestion of betalainic foods. Stability and bioaccessibility of betaxanthins and betacyanins and antioxidative potential of food digesta. J. Agric. Food. Chem. 2008, 56, 10487–10492. [Google Scholar] [CrossRef] [PubMed]
- Wink, D.A.; Miranda, K.M.; Espey, M.G.; Pluta, R.M.; Hewett, S.J.; Colton, C.; Vitek, M.; Feelisch, M.; Grisham, M.B. Mechanisms of the antioxidant effects of nitric oxide. Antioxid. Redox Signal. 2001, 3, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Wink, D.A.; Hines, H.B.; Cheng, R.Y. S.; Switzer, C.H.; Flores-Santana, W.; Vitek, M.P.; Ridnour, L.A.; Colton, C.A. Nitric oxide and redox mechanisms in the immune response. J. Leukoc. Biol. 2011, 89, 873–891. [Google Scholar] [CrossRef] [PubMed]
- Kujawska, M.; Ignatowicz, E.; Murias, M.; Ewertowska, M.; Mikołajczyk, K.; Jodynis-Liebert, J. Protective effect of red beetroot against carbon tetrachloride- and N-nitrosodiethylamine-induced oxidative stress in rats. J. Agric. Food Chem. 2009, 57, 2570–2575. [Google Scholar] [CrossRef] [PubMed]
- Winkler, C.; Wirleitner, B.; Schroecksnadel, K. In vitro effects of beet root juice on stimulated and unstimulated peripheral blood mononuclear Cells. Am. J. Biochem. Biotechnol. 2005, 1, 180. [Google Scholar] [CrossRef]
- Wootton-Beard, P.C.; Moran, A.; Ryan, L. Stability of the total antioxidant capacity and total polyphenol content of 23 commercially available vegetable juices before and after in vitro digestion measured by FRAP, DPPH, ABTS and Folin-Ciocalteu methods. Food. Res. Int. 2011, 44, 217–224. [Google Scholar] [CrossRef]
- Ryan, L.; Prescott, S.L. Stability of the antioxidant capacity of twenty-five commercially available fruit juices subjected to an in vitro digestion. Int. J. Food Sci. Technol. 2010, 45, 1191–1197. [Google Scholar] [CrossRef]
- Szaefer, H.; Krajka-Kuźniak, V.; Ignatowicz, E.; Adamska, T.; Baer-Dubowska, W. Evaluation of the effect of beetroot juice on DMBA-induced damage in liver and mammary gland of female Sprague-Dawley rats. Phytother. Res. 2014, 28, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Esatbeyoglu, T.; Wagner, A.E.; Motafakkerazad, R.; Nakajima, Y.; Matsugo, S.; Rimbach, G. Free radical scavenging and antioxidant activity of betanin: Electron spin resonance spectroscopy studies and studies in cultured cells. Food. Chem. Toxicol. 2014, 73, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; McKercher, S.R.; Lipton, S.A. Reprint of: Nrf2/ARE-mediated antioxidant actions of pro-electrophilic drugs. Free Radic. Biol. Med. 2014, 66, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [PubMed]
- Krajka-Kuźniak, V.; Paluszczak, J.; Szaefer, H.; Baer-Dubowska, W. Betanin, a beetroot component, induces nuclear factor erythroid-2-related factor 2-mediated expression of detoxifying/antioxidant enzymes in human liver cell lines. Br. J. Nutr. 2013, 110, 2138–2149. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wang, Y.; Zhang, Z. Radioprotective activity of betalains from red beets in mice exposed to gamma irradiation. Eur. J. Pharmacol. 2009, 615, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Krajka-Kuźniak, V.; Szaefer, H.; Ignatowicz, E.; Adamska, T.; Baer-Dubowska, W. Beetroot juice protects against N-nitrosodiethylamine-induced liver injury in rats. Food. Chem. Toxicol. 2012, 50, 2027–2033. [Google Scholar] [CrossRef] [PubMed]
- El Gamal, A.A.; AlSaid, M.S.; Raish, M.; al-Sohaibani, M.; al-Massarani, S.M.; Ahmad, A.; Hefnawy, M.; al-Yahya, M.; Basoudan, O.A.; Rafatullah, S. Beetroot (Beta vulgaris L.) extract ameliorates gentamicin-induced nephrotoxicity associated oxidative stress, inflammation, and apoptosis in rodent model. Mediat. Inflamm. 2014, 2014, 983–952. [Google Scholar] [CrossRef]
- Halliwell, B. Free radicals, antioxidants, and human disease: Curiosity, cause, or consequence? Lancet 1994, 344, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Bell, P.G.; Walshe, I.H.; Davison, G.W.; Stevenson, E.; Howatson, G. Montmorency cherries reduce the oxidative stress and inflammatory responses to repeated days high-intensity stochastic cycling. Nutrients 2014, 6, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Howatson, G.; McHugh, M.P.; Hill, J.A.; Brouner, J.; Jewell, A.P.; van Someren, K.A.; Shave, R.; Howatson, S.A. Influence of tart cherry juice on indices of recovery following marathon running. Scand. J. Med. Sci. Sports 2010, 20, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.; Azevedo, I. Chronic inflammation in obesity and the metabolic syndrome. Mediat. Inflamm. 2010. [Google Scholar] [CrossRef]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Baek, S.J. Molecular targets of dietary polyphenols with anti-inflammatory properties. Yonsei. Med. J. 2005, 46, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Calixto, J.B.; Campos, M.M.; Otuki, M.F.; Santos, A.R. Anti-inflammatory compounds of plant origin. Part II. Modulation of pro-inflammatory cytokines, chemokines and adhesion molecules. Planta Med. 2004, 70, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.K.; Surh, Y.J. Emerging avenues linking inflammation and cancer. Free Radic. Biol. Med. 2012, 52, 2013–2037. [Google Scholar] [CrossRef] [PubMed]
- García-Lafuente, A.; Guillamón, E.; Villares, A.; Rostagno, M.A.; Martínez, J.A. Flavonoids as anti-inflammatory agents: Implications in cancer and cardiovascular disease. Inflamm. Res. 2009, 58, 537–552. [Google Scholar] [CrossRef] [PubMed]
- O’Byrne, K.J.; Dalgleish, A.G. Chronic immune activation and inflammation as the cause of malignancy. Br. J. Cancer 2001, 85, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Page, J.; Henry, D. Consumption of NSAIDs and the development of congestive heart failure in elderly patients: An under recognized public health problem. Arch. Intern. Med. 2000, 160, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Wang, Y.; Bai, B.; Yang, X.; Han, J. Betanin attenuates oxidative stress and inflammatory reaction in kidney of paraquat-treated rat. Food Chem Toxicol. 2015, 78, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Lechner, J.F.; Wang, L.S.; Rocha, C.M.; Larue, B.; Henry, C.; McIntyre, C.M.; Riedl, K.M.; Schwartz, S.J.; Stoner, G.D. Drinking water with red beetroot food color antagonizes esophageal carcinogenesis in N-nitrosomethylbenzylamine-treated rats. J. Med. Food 2010, 13, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Gladwin, M.T.; Schechter, A.N.; Kim-Shapiro, D.B.; Patel, R.P.; Hogg, N.; Shiva, S.; Lundberg, J.O. The emerging biology of the nitrite anion. Nat Chem Biol. 2005, 1, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Kim-Shapiro, D.B.; Gladwin, M.T. Mechanisms of nitrite bioactivation. Nitric Oxide 2014, 38, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Davignon, J.; Ganz, P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004, 109, 27–32. [Google Scholar] [CrossRef]
- Celermajer, D.S. Endothelial dysfunction: Does it matter? Is it reversible? J. Am. Coll. Cardiol. 1997, 30, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Joris, P.J.; Mensink, R.P. Beetroot juice improves in overweight and slightly obese men postprandial endothelial function after consumption of a mixed meal. Atherosclerosis 2013, 231, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Kenjale, A.A.; Ham, K.L.; Stabler, T.; Robbins, J.L.; Johnson, J.L.; Vanbruggen, M.; Privette, G.; Yim, E.; Kraus, W.E.; Allen, J.D. Dietary nitrate supplementation enhances exercise performance in peripheral arterial disease. J. Appl. Physiol. 2011, 110, 1582–1591. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, M.; Winyard, P.G.; Aizawa, K.; Anning, C.; Shore, A.; Benjamin, N. Effect of dietary nitrate on blood pressure, endothelial function, and insulin sensitivity in type 2 diabetes. Free Radic. Biol. Med. 2013, 60, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Frombaum, M.; le Clanche, S.; Bonnefont-Rousselot, D.; Borderie, D. Antioxidant effects of resveratrol and other stilbene derivatives on oxidative stress and NO bioavailability: Potential benefits to cardiovascular diseases. Biochimie 2012, 94, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Harrison, D.G. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ. Res. 2000, 87, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Bond, V., Jr.; Curry, B.H.; Adams, R.G.; Asadi, M.S.; Millis, R.M.; Haddad, G.E. Effects of dietary nitrates on systemic and cerebrovascular hemodynamics. Cardiol. Res. Pract. 2013, 435–629. [Google Scholar]
- Spilt, A.; Weverling-Rijnsburger, A.W.E.; Middelkoop, H.A.M.; van Der Flier, W.M.; Gussekloo, J.; de Craen, A.J.M.; Westendorp, R.G.J. Late-onset dementia: Structural brain damage and total cerebral blood flow. Radiology 2005, 236, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, C.P.; Downey, L.A.; Croft, K.D.; Scholey, A.; Stough, C.; Yang, X.; Considine, M.J.; Ward, N.C.; Puddey, I.B.; Swinny, E.; et al. The acute effect of flavonoid-rich apples and nitrate-rich spinach on cognitive performance and mood in healthy men and women. Food Funct. 2014, 5, 849–858. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, J.C.; Stefano, G.B. Evidence that Alzheimer’s disease is a microvascular disorder: The role of constitutive nitric oxide. Brain Res. Rev. 2000, 34, 119–136. [Google Scholar] [CrossRef]
- Poels, M.M.F.; Ikram, M.A.; Vernooij, M.W.; Krestin, G.P.; Hofman, A.; Niessen, W.J.; van der Lugt, A.; Breteler, M.M.B. Total cerebral blood flow in relation to cognitive function: The Rotterdam Scan Study. J. Cereb. Blood Flow Metab. 2008, 28, 1652–1655. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.; Fulford, J.; Vanhatalo, A.; Blackwell, J.R.; French, O.; Bailey, S.J.; Gichrist, M.; Winyard, P.G.; Jones, A.M. Effects of short-term dietary nitrate supplementation on blood pressure, O2 uptake kinetics, and muscle and cognitive function in older adults. Am. J. Physiol. -Reg. I 2013, 304, 73–83. [Google Scholar]
- Thompson, K.G.; Turner, L.; Prichard, J.; Dodd, F.; Kennedy, D.O.; Haskell, C.; Blackwell, J.R.; Jones, A.M. Influence of dietary nitrate supplementation on physiological and cognitive responses to incremental cycle exercise. Respir. Physiol. Neurobiol. 2014, 193, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Lansley, K.E.; Winyard, P.G.; Bailey, S.J.; Vanhatalo, A.; Wilkerson, D.P.; Blackwell, J.R.; Gilchrist, M.; Benjamin, N.; Jones, A.M. Acute dietary nitrate supplementation improves cycling time trial performance. Med. Sci. Sports Exerc. 2011, 43, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Cermak, N.M.; Gibala, M.J.; van Loon, L.J.C. Nitrate supplementation’s improvement of 10-km time-trial performance in trained cyclists. Int. J. Sports Nutr. Exerc. 2012, 22, 64–71. [Google Scholar]
- Jones, A.M.; Bailey, S.J.; Vanhatalo, A.; Fulford, J.; Gilchrist, M.; Benjamin, N.; Winyard, P.G. Reply to Lundberg, Larsen, and Weitzberg. J. Appl. Physiol. 2011, 111, 619. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Larsen, F.J.; Weitzberg, E. Supplementation with nitrate and nitrite salts in exercise: A word of caution. J. Appl. Physiol. 2011, 111, 616–617. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M. Dietary nitrate supplementation and exercise performance. Sports Med. 2014, 44, 35–45. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clifford, T.; Howatson, G.; West, D.J.; Stevenson, E.J. The Potential Benefits of Red Beetroot Supplementation in Health and Disease. Nutrients 2015, 7, 2801-2822. https://doi.org/10.3390/nu7042801
Clifford T, Howatson G, West DJ, Stevenson EJ. The Potential Benefits of Red Beetroot Supplementation in Health and Disease. Nutrients. 2015; 7(4):2801-2822. https://doi.org/10.3390/nu7042801
Chicago/Turabian StyleClifford, Tom, Glyn Howatson, Daniel J. West, and Emma J. Stevenson. 2015. "The Potential Benefits of Red Beetroot Supplementation in Health and Disease" Nutrients 7, no. 4: 2801-2822. https://doi.org/10.3390/nu7042801
APA StyleClifford, T., Howatson, G., West, D. J., & Stevenson, E. J. (2015). The Potential Benefits of Red Beetroot Supplementation in Health and Disease. Nutrients, 7(4), 2801-2822. https://doi.org/10.3390/nu7042801