Fish Consumption, Long-Chain Omega-3 Polyunsaturated Fatty Acid Intake and Risk of Metabolic Syndrome: A Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Data Sources and Searches
2.2. Study Selection
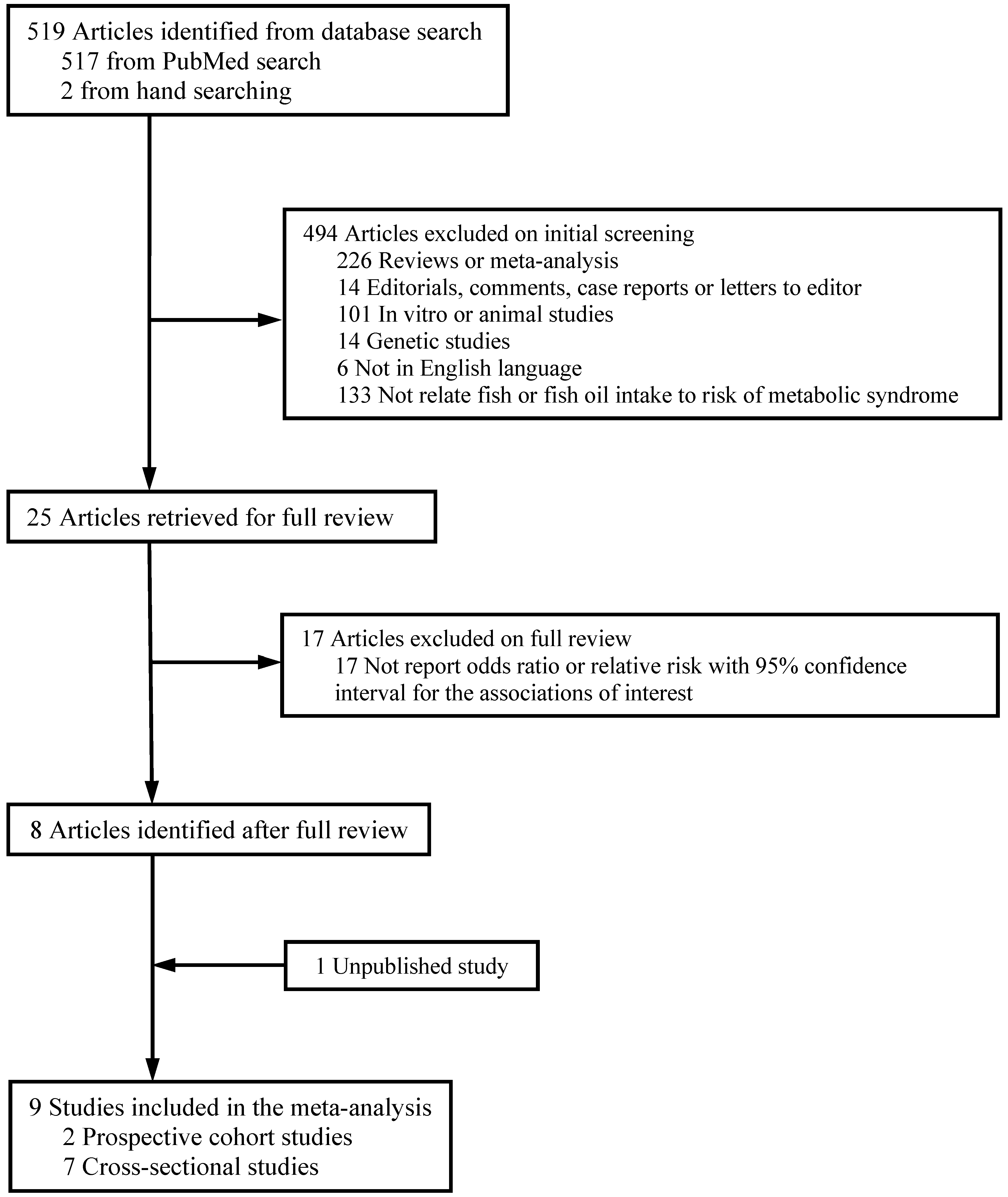
2.3. Data Extraction
2.4. Statistical Analysis
3. Results
3.1. Study Characteristics
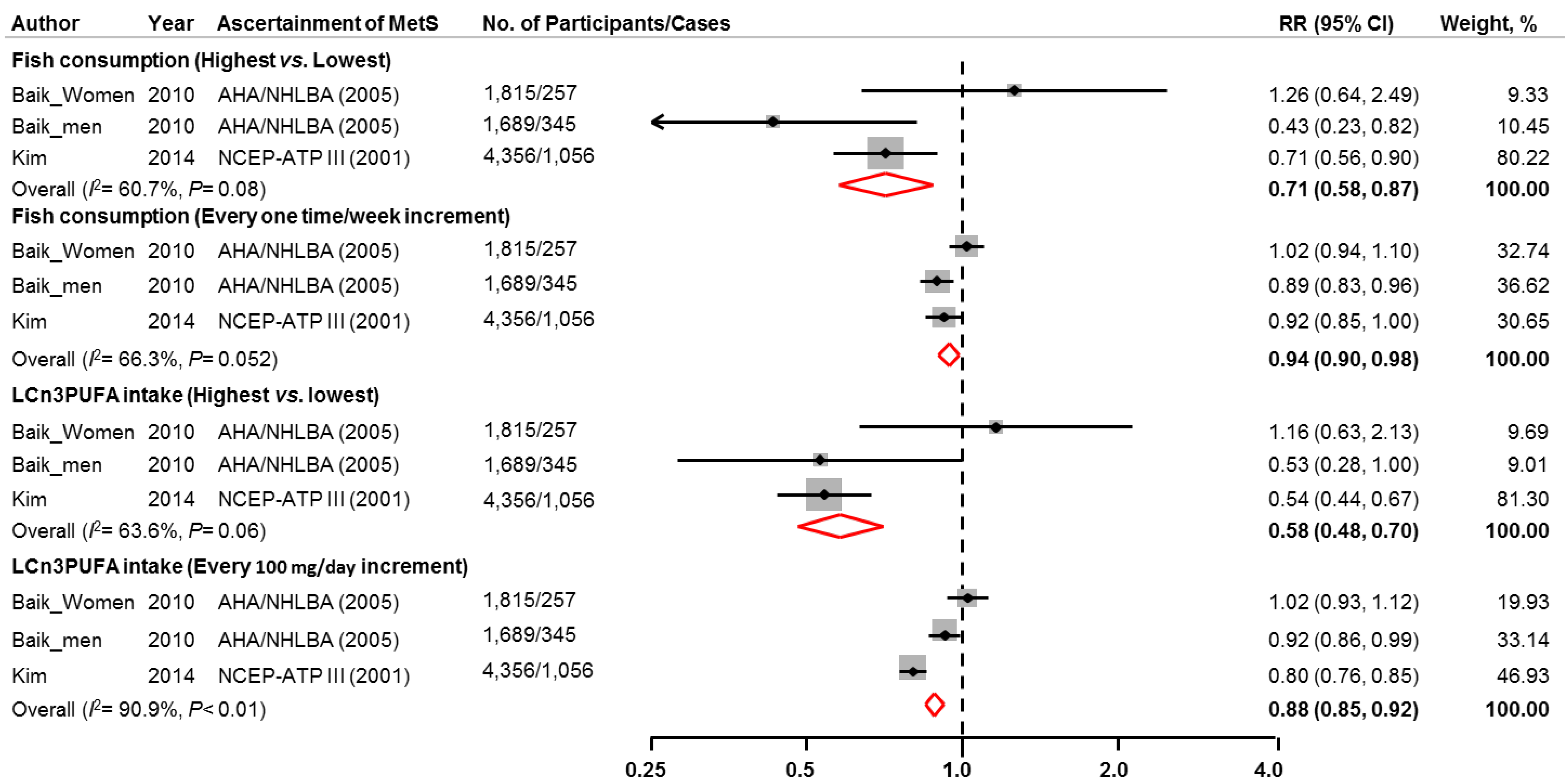
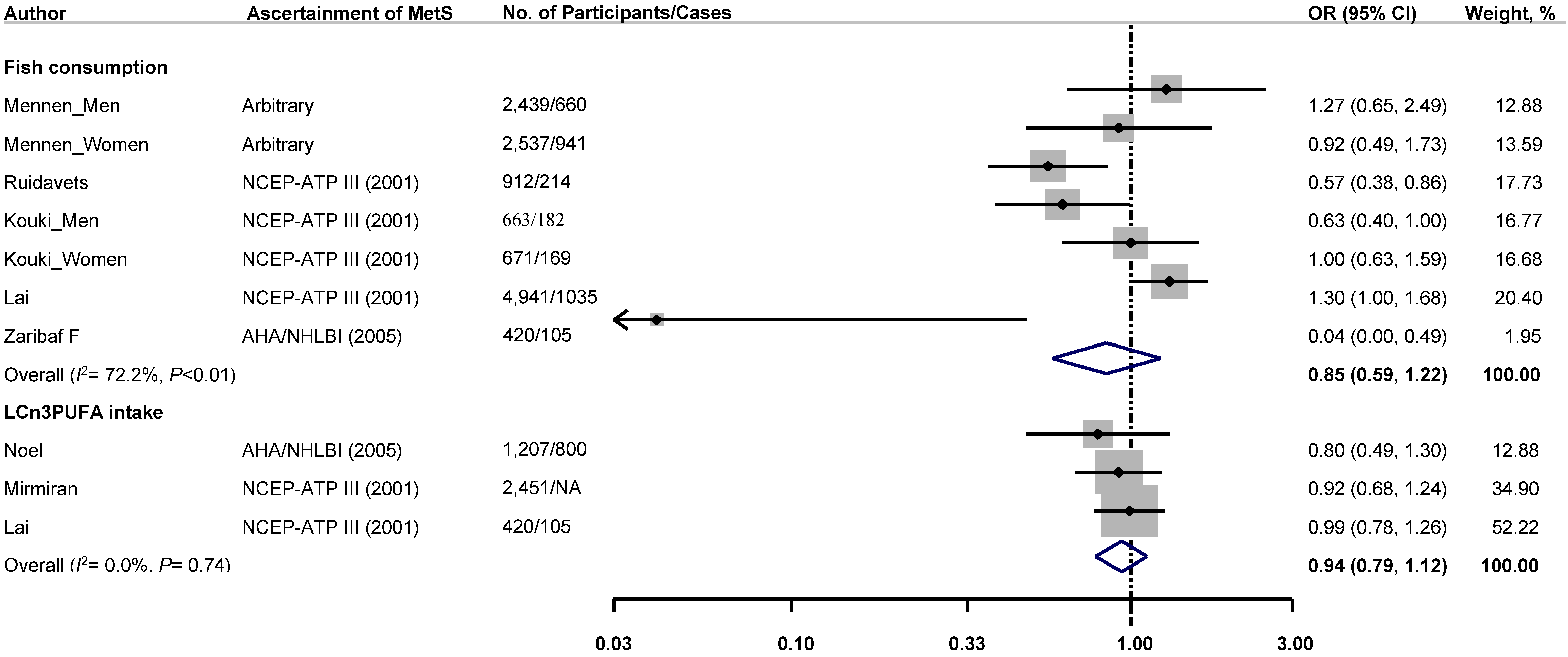
3.2. Association between Intake of Fish or LCω3PUFAs and Risk of Metabolic Syndrome
3.3. Publication Bias
| Source | Participants (n) | Age (years) | Men (%) | Duration of Follow-Up (Years) | Exposure Assessment | Exposure Categories | Metabolic Syndrome Ascertainment | No. of Cases | Adjusted Variables |
|---|---|---|---|---|---|---|---|---|---|
| Cross-Sectional Studies | |||||||||
| Mennen et al. [11], 2000, DESIR study, France | 2439 | 30–64 | 100 | N/A | Self-administered questionnaire | Fish intake | Arbitrary criteria | 660 | Age, waist-hip ratio and energy intake. |
| (portions /week): | |||||||||
| <2; | |||||||||
| 2–4; | |||||||||
| >4; | |||||||||
| Mennen et al. [11] 2000, DESIR study, France | 2537 | 30–64 | 0 | N/A | Self-administered questionnaire | Fish intake | Arbitrary criteria | 941 | Age, waist-hip ratio and energy intake. |
| <2; | |||||||||
| 2–4; | |||||||||
| >4; | |||||||||
| Ruidavets et al. [14], 2007, MONICA study, France | 912 | 45–64 | 100 | N/A | 3-day food record | Fish intake (g/day): Tertiles | NCEP-ATP III | 214 | Age, center, physical activity, level of education, smoking habits, alcohol intake, drugs for hypertension and dyslipidaemia, energy intake (without alcohol), dieting, and diet quality index. |
| Noel et al. [13], 2010, BPRH Study, USA | 1207 | 45–75 | ~30 (exact proportion: NA) | N/A | Self-administered questionnaire | n-3 PUFA: Quintiles of fat intake as a percentage of total energy | AHA/NHLBI | ~800 (exact number: NA) | Age, gender, smoking and alcohol use, physical activity, education, fish oil supplement use, acculturation, total energy, total fat, dietary fiber, lipid-lowering medication use and BMI. |
| Kouki et al. [24], 2011, DR’s EXTRA study, Finland | 663 | 57–78 | 100 | N/A | 4-day food record | Fish intake (g/day): | NCEP-ATP III | 182 | Age, smoking, alcohol consumption, education and VO2max. |
| <18.5; | |||||||||
| 18.5–59.5; | |||||||||
| >59.5; | |||||||||
| Kouki et al. [24], 2011, DR’s EXTRA study, Finland | 671 | 57–78 | 0 | N/A | 4-day food record | Fish intake (g/day): | NCEP-ATP III | 169 | Age, smoking, alcohol consumption, education and VO2max. |
| <18.0; | |||||||||
| 18.0–51.0; | |||||||||
| >51.0; | |||||||||
| Mirmiran et al. [12], 2012, TLGS, Iran | 2451 | 19–84 | 46 | N/A | Interviewer-administered questionnaire | Fish oil (EPA + DHA, mg/day): | NCEP-ATP III * | NA | Age, gender, smoking status, physical activity, total energy intake, percentage of energy from carbohydrate, protein, saturated fatty acid, monounsaturated fatty acid, oleic acid, and total fiber. |
| ≤29; | |||||||||
| 30–66; | |||||||||
| 67–135; | |||||||||
| ≥136. | |||||||||
| Lai et al. [10], 2013, NHLBI Family Heart Study, USA | 4941 | 52.1 (mean) | 46 | N/A | Self-administered questionnaire | Fish intake (times/week): | NCEP-ATP III | 1035 | Age, gender, race, alcohol intake, smoking, exercise, TV watching, energy intake, multivitamin use, fruits and vegetables intake, and risk group using generalized estimating equations. |
| 0; | |||||||||
| 1; | |||||||||
| 2; | |||||||||
| ≥3. | |||||||||
| Dietary n-3 PUFA (quintiles, mean (g/day)): | |||||||||
| Q1: 0.04; | |||||||||
| Q2: 0.11; | |||||||||
| Q3: 0.18; | |||||||||
| Q4: 0.28; | |||||||||
| Q5: 0.64. | |||||||||
| Zaribaf et al. [25], 2014, Iran | 420 | 35.2 (mean) | 0 | N/A | Self-administered questionnaire | Energy-adjusted fish intake (g/day): Tertiles | AHA/NHLBI | 105 | Age, energy intake, physical activity, socioeconomic status, medication use, marital and menopausal status, dietary intakes of red meat, whole and refined grains, fruits, vegetables, legume and nuts, dairy products, fiber and oils, BMI. |
| Prospective Studies | |||||||||
| Baik et al. [26], 2010, Korean Genome Epidemiology Study, Korea | 1689 | 40–69 | 100 | 4 | Self-administered questionnaire | Fish intake (times/week): | AHA/NHLBI * | 345 | Age, BMI, income, occupation, marital status, education level, smoking status, alcohol intake, physical activity, daily intake of energy, fat, dietary fiber, consumption of red meat, dairy products, sweetened carbonated beverage, use of multivitamin supplements, and baseline report of a physician diagnosis of diabetes or hypertension. |
| <1; | |||||||||
| 1–4; | |||||||||
| 5–6; | |||||||||
| Daily. | |||||||||
| n-3 PUFA (percentile, median (mg)): | |||||||||
| <10th: 37; | |||||||||
| 10th–50th: 138; | |||||||||
| 50th–90th: 375; | |||||||||
| >90th: 786 mg. | |||||||||
| Baik et al. [26] , 2010, Korean Genome Epidemiology Study, Korea | 1815 | 40–69 | 0 | 4 | Self-administered questionnaire | Fish intake (times/week): | AHA/NHLBI * | 257 | Age, BMI, income, occupation, marital status, education level, smoking status, alcohol intake, physical activity, daily intakes of energy, fat, dietary fiber, red meat, dairy products, and sweetened carbonated beverage, use of multivitamin supplements, baseline report of a physician diagnosis of diabetes or hypertension, menopausal status, and postmenopausal hormone use. |
| <1; | |||||||||
| 1–4; | |||||||||
| 5–6; | |||||||||
| Daily. | |||||||||
| n-3 PUFA (percentile, median (mg)): | |||||||||
| <10th: 29; | |||||||||
| 10th–50th: 125; | |||||||||
| 50th–90th: 360; | |||||||||
| >90th: 563. | |||||||||
| Kim et al. (Under journal review) CARDIA study, USA | 4356 | 18–30 | 47 | 25 | Interviewer-administered questionnaire | Fish intake: | NCEP-ATP III | 1069 | Age, gender, ethnicity, study center, education, smoking status, family history of diabetes, physical activity, alcohol consumption, and baseline BMI. Fried fish was also adjusted when non-fried fish was the exposure. |
| <1/month; | |||||||||
| 1–3/month; | |||||||||
| 1/week; | |||||||||
| 2–4/week; | |||||||||
| ≥5/week. | |||||||||
| Fish oil (Quintiles, median (g/day)): | |||||||||
| Q1: 0.03; | |||||||||
| Q2: 0.07; | |||||||||
| Q3: 0.11; | |||||||||
| Q4: 0.18; | |||||||||
| Q5: 0.33. | |||||||||
3.4. Sensitivity Analysis
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ford, E.S.; Giles, W.H.; Dietz, W.H. Prevalence of the metabolic syndrome among us adults: Findings from the third national health and nutrition examination survey. JAMA J. Am. Med. Assoc. 2002, 287, 356–359. [Google Scholar] [CrossRef]
- Mozumdar, A.; Liguori, G. Persistent increase of prevalence of metabolic syndrome among U.S. adults: NHANES III to NHANES 1999–2006. Diabetes Care 2011, 34, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Esmaillzadeh, A.; Kimiagar, M.; Mehrabi, Y.; Azadbakht, L.; Hu, F.B.; Willett, W.C. Fruit and vegetable intakes, C-reactive protein, and the metabolic syndrome. Am. J. Clin. Nutr. 2006, 84, 1489–1497. [Google Scholar] [PubMed]
- Van Meijl, L.E.; Vrolix, R.; Mensink, R.P. Dairy product consumption and the metabolic syndrome. Nutr. Res. Rev. 2008, 21, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Sahyoun, N.R.; Jacques, P.F.; Zhang, X.L.; Juan, W.; McKeown, N.M. Whole-grain intake is inversely associated with the metabolic syndrome and mortality in older adults. Am. J. Clin. Nutr. 2006, 83, 124–131. [Google Scholar] [PubMed]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002, 106, 2747–2757. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, Y.A.; Portois, L.; Malaisse, W.J. n-3 Fatty acids and the metabolic syndrome. Am. J. Clin. Nutr. 2006, 83, 1499s–1504s. [Google Scholar] [PubMed]
- He, K. Fish, long-chain omega-3 polyunsaturated fatty acids and prevention of cardiovascular disease—Eat fish or take fish oil supplement? Prog. Cardiovasc. Dis. 2009, 52, 95–114. [Google Scholar] [CrossRef] [PubMed]
- Lorente-Cebrian, S.; Costa, A.G.; Navas-Carretero, S.; Zabala, M.; Martinez, J.A.; Moreno-Aliaga, M.J. Role of omega-3 fatty acids in obesity, metabolic syndrome, and cardiovascular diseases: A review of the evidence. J. Physiol. Biochem. 2013, 69, 633–651. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.H.; Petrone, A.B.; Pankow, J.S.; Arnett, D.K.; North, K.E.; Ellison, R.C.; Hunt, S.C.; Djousse, L. Association of dietary omega-3 fatty acids with prevalence of metabolic syndrome: The national heart, lung, and blood institute family heart study. Clin. Nutr. (Edinb. Scotl.) 2013, 32, 966–969. [Google Scholar] [CrossRef]
- Mennen, L.I.; Lafay, L.; Feskens, E.J.M.; Novak, M.; Lépinay, P.; Balkau, B. Possible protective effect of bread and dairy products on the risk of the metabolic syndrome. Nutr. Res. 2000, 20, 335–347. [Google Scholar] [CrossRef]
- Mirmiran, P.; Hosseinpour-Niazi, S.; Naderi, Z.; Bahadoran, Z.; Sadeghi, M.; Azizi, F. Association between interaction and ratio of omega-3 and omega-6 polyunsaturated fatty acid and the metabolic syndrome in adults. Nutrition 2012, 28, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Noel, S.E.; Newby, P.K.; Ordovas, J.M.; Tucker, K.L. Adherence to an (n-3) fatty acid/fish intake pattern is inversely associated with metabolic syndrome among Puerto Rican adults in the greater Boston area. J. Nutr. 2010, 140, 1846–1854. [Google Scholar] [CrossRef] [PubMed]
- Ruidavets, J.B.; Bongard, V.; Dallongeville, J.; Arveiler, D.; Ducimetiere, P.; Perret, B.; Simon, C.; Amouyel, P.; Ferrieres, J. High consumptions of grain, fish, dairy products and combinations of these are associated with a low prevalence of metabolic syndrome. J. Epidemiol. Community Health 2007, 61, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of observational studies in epidemiology (moose) group. JAMA J. Am. Med. Assoc. 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Normand, S.T. Tutorial in biostatistics. Meta-analysis: Formulating, evaluating, combining, and reporting. Stat. Med. 2000, 19, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.; Ioannidis, J.P.; Schmid, C.H. Summing up evidence: One answer is not always enough. Lancet 1998, 351, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Cochran, W.G. The combination of estimates from different experiments. Biometrics 1954, 10, 101–129. [Google Scholar] [CrossRef]
- Hardy, R.J.; Thompson, S.G. Detecting and describing heterogeneity in meta-analysis. Stat. Med. 1998, 17, 841–856. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Duval, S.; Tweedie, R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J. Am. Stat. Assoc. 2000, 95, 89–98. [Google Scholar]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA J. Am. Med. Assoc. 2001, 285, 2486–2497. [Google Scholar]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American heart association/national heart, lung, and blood institute scientific statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [PubMed]
- Kouki, R.; Schwab, U.; Hassinen, M.; Komulainen, P.; Heikkila, H.; Lakka, T.A.; Rauramaa, R. Food consumption, nutrient intake and the risk of having metabolic syndrome: The Dr’s extra study. Eur. J. Clin. Nutr. 2011, 65, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Zaribaf, F.; Falahi, E.; Barak, F.; Heidari, M.; Keshteli, A.H.; Yazdannik, A.; Esmaillzadeh, A. Fish consumption is inversely associated with the metabolic syndrome. Eur. J. Clin. Nutr. 2014, 68, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Baik, I.; Abbott, R.D.; Curb, J.D.; Shin, C. Intake of fish and n-3 fatty acids and future risk of metabolic syndrome. J. Amer. Diet. Assoc. 2010, 110, 1018–1026. [Google Scholar] [CrossRef]
- Brady, L.M.; Williams, C.M.; Lovegrove, J.A. Dietary PUFA and the metabolic syndrome in Indian Asians living in the UK. Proc. Nutr. Soc. 2004, 63, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.S.; Bryant, S.P.; Mishra, G.D.; Krebs, J.D.; Browning, L.M.; Miller, G.J.; Jebb, S.A. Oily fish reduces plasma triacylglycerols: A primary prevention study in overweight men and women. Nutrition 2006, 22, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Xun, P.; Hou, N.; Daviglus, M.; Liu, K.; Morris, J.S.; Shikany, J.M.; Sidney, S.; Jacobs, D.R.; He, K. Fish oil, selenium and mercury in relation to incidence of hypertension: A 20-year follow-up study. J. Intern. Med. 2011, 270, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Xun, P.; He, K. Fish consumption and incidence of diabetes: Meta-analysis of data from 438,000 individuals in 12 independent prospective cohorts with an average 11-year follow-up. Diabetes Care 2012, 35, 930–938. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-S.; Xun, P.; He, K. Fish Consumption, Long-Chain Omega-3 Polyunsaturated Fatty Acid Intake and Risk of Metabolic Syndrome: A Meta-Analysis. Nutrients 2015, 7, 2085-2100. https://doi.org/10.3390/nu7042085
Kim Y-S, Xun P, He K. Fish Consumption, Long-Chain Omega-3 Polyunsaturated Fatty Acid Intake and Risk of Metabolic Syndrome: A Meta-Analysis. Nutrients. 2015; 7(4):2085-2100. https://doi.org/10.3390/nu7042085
Chicago/Turabian StyleKim, Yong-Seok, Pengcheng Xun, and Ka He. 2015. "Fish Consumption, Long-Chain Omega-3 Polyunsaturated Fatty Acid Intake and Risk of Metabolic Syndrome: A Meta-Analysis" Nutrients 7, no. 4: 2085-2100. https://doi.org/10.3390/nu7042085
APA StyleKim, Y.-S., Xun, P., & He, K. (2015). Fish Consumption, Long-Chain Omega-3 Polyunsaturated Fatty Acid Intake and Risk of Metabolic Syndrome: A Meta-Analysis. Nutrients, 7(4), 2085-2100. https://doi.org/10.3390/nu7042085





