Effectiveness of Dietary Allergen Exclusion Therapy on Eosinophilic Colitis in Chinese Infants and Young Children ≤ 3 Years of Age
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Design and Data Collection
2.2. Definition of Eosinophilic Colitis
2.3. Serum Allergen-Specific Immunoglobulin E (sIgE) Test
2.4. Treatment Regimen for Eosinophilic Colitis
2.5. Statistical Analysis
3. Results
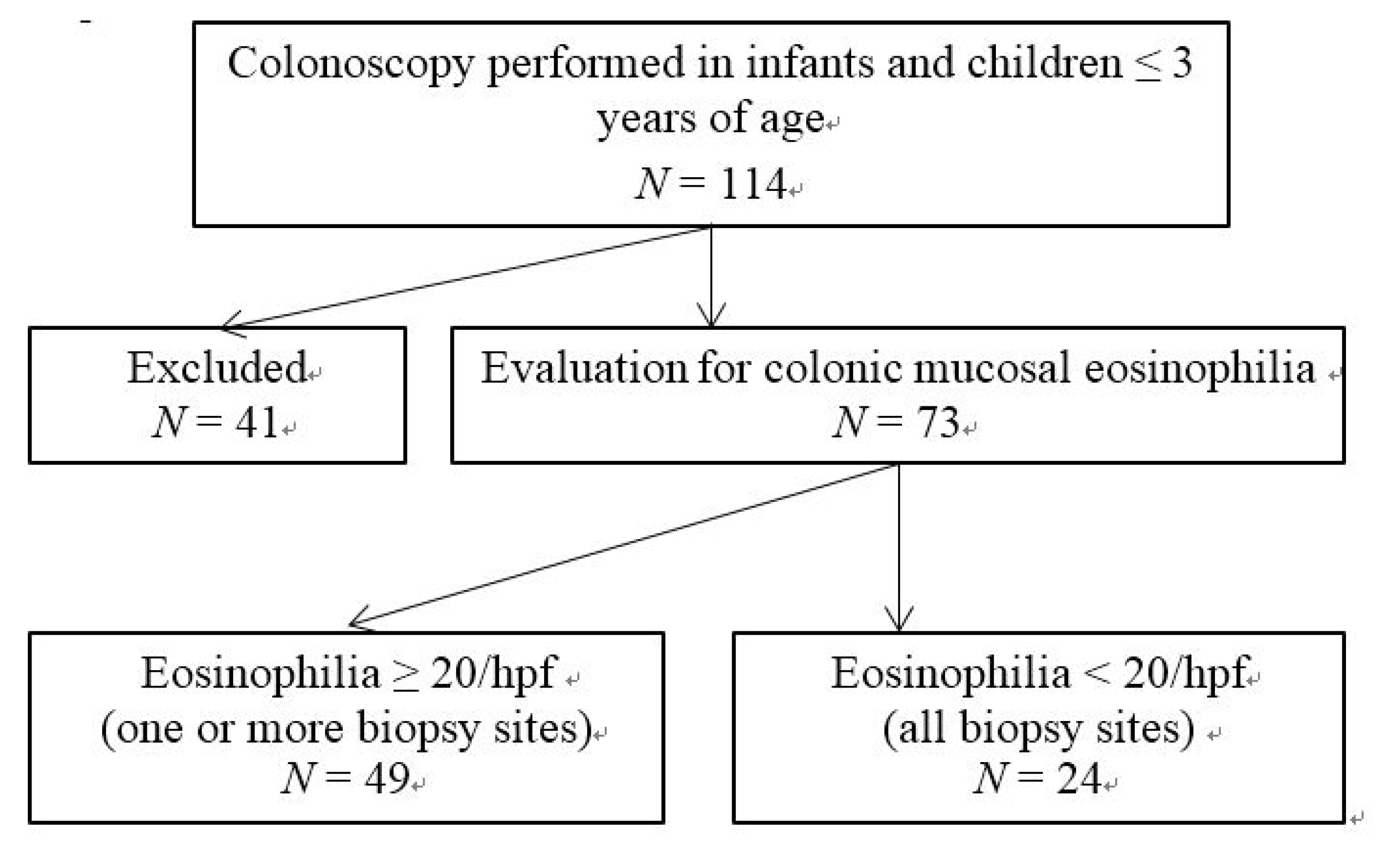
3.1. Patient Selection, Presenting Symptoms and Patient Characteristics
| Symptoms | Eosinophilic Colitis (n = 49) | Non-Eosinophilic Colitis (n = 24) |
|---|---|---|
| Diarrhea | 23 (46.9%) | 12 (50%) |
| Blood in stool | 20 (40.8%) | 5 (20.8%) |
| Abdominal pain/irritability | 4 (8.2%) | 4 (16.7%) |
| Poor weight gain | 1 (2.0%) | 2 (8.3% |
| Paleness | 1 (2.0%) | 1 (8.3%) |
| Basic characteristics | Eosinophilic Colitis (n = 49) | Non-Eosinophilic Colitis (n = 24) |
|---|---|---|
| Age (mean ± SD) | 20.9 ± 8.8 | 17.6 ± 8.6 |
| <12 months | 12 (24.5.0%) | 4 (16.7%) |
| 12 months–36 months | 37 (75.5.0%) | 20 (83.3%) |
| Youngest age | 3 months | 4 months |
| Sex, Male/Female | 33/16 | 16/8 |
| Feeding patterns: | ||
| Breast feeding | 24 (49.0%) | 13 (54.2%) |
| Formula feeding | 8(16.3%) | 4 (16.7%) |
| Mixed feeding | 17 (34.7%) | 7 (29.1% ) |
| Time to add Solid food: | ||
| ≤4 months | 7 (14.3%) | 2 (8.3%) |
| 4–6 months | 38 (77.6%) | 20 (83.3%) |
| ≥6 months | 4 (8.2%) | 2 (8.3% ) |
3.2. Clinical Features and Laboratory Tests in Children with Eosinophilic Colitis
| Symptoms and Laboratory Tests | Eosinophilic Colitis (n = 49) | Non-Eosinophilic Colitis (n = 24) |
|---|---|---|
| Abdominal pain | 11 (22.4%) | 6 (25.0%) |
| Nausea/vomiting | 13 (26.5%) | 7 (29.1%) |
| Blood in stool | 32 (65.3%) | 10 (41.7%) |
| Poor appetite | 10 (20.4% ) | 4(16.7%) |
| Bloating/distention | 6 (12.2% ) | 3 (12.5%) |
| Irritability with stooling | 7 (14.3%) | 3 (12.5%) |
| Bristol Stool Form Scale 1–2 | 8 (16.3%) | 2 (8.3%) |
| Bristol Stool Form Scale 6–7 | 19 (38.8%) | 9 (37.5%) |
| Stool OB (+) | 22 (44.9%) | 8 (33.3%) |
| Anemia (HB < 110 g L−1) | 15(30.6%) | 8 (33.3%) |
| Blood eosinophil > 5% | 21 (42.9%) | 6(25.0%) |
| Total serum IgE elevation | 22 (44.9%) | 7 (29.2%) |
| Hypoalbuminemia (<30 g L−1) | 4 (8.2%) | 1 (4.2%) |
| sIgE (+) | 29 (59.2%) * | 7(29.1%) |
| milk | 22 (44.9%) | 6 (12.5%) |
| egg | 14 (28.5%) | 3 (12.5%) |
| Milk + egg | 11(22.4%) | 2 (8.3%) |
3.3. Treatment Outcomes for Infants and Children with Eosinophilic Colitis

4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Furuta, G.T.; Forbes, D.; Boey, C.; Dupont, C.; Putnam, P.; Roy, S.; Sabrá, A.; Salvatierra, A.; Yamashiro, Y.; Husby, S.; et al. Eosinophilic Gastrointestinal Diseases Working Group. Eosinophilic gastrointestinal diseases (EGIDs). J. Pediatr. Gastroenterol. Nutr. 2008, 47, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Aceves, S.; Hirano, I.; Furuta, G.T.; Collins, M.H. Eosinophilic gastrointestinal diseases—Clinically diverse and histopathologically confounding. Semin. Immunopathol. 2012, 34, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Jawairia, M.; Shahzad, G.; Mustacchia, P. Eosinophilic gastrointestinal diseases: Review and update. ISRN Gastroenterol. 2012, 2012, 463689. [Google Scholar] [PubMed]
- DeBrosse, C.W.; Rothenberg, M.E. Allergy and eosinophilic-associated gastrointestinal disorders (EGID). Curr. Opin. Immunol. 2008, 20, 703–708. [Google Scholar] [CrossRef]
- Lucendo, A.J. Eosinophilic diseases of the gastrointestinal tract. Scand. J. Gastroenterol. 2010, 45, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.L.; Atkins, D.; Fleischer, D.; Pan, Z.; Ruybal, J.; Furuta, G.T. Factors contributing to adherence to dietary treatment of eosinophilic gastrointestinal diseases. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 430–432. [Google Scholar] [CrossRef] [PubMed]
- Ishimura, N.; Furuta, K.; Sato, S.; Ishihara, S.; Kinoshita, Y. Limited role of allergy testing in patients with eosinophilic gastrointestinal disorders. J. Gastroenterol. Hepatol. 2013, 28, 1306–1313. [Google Scholar] [CrossRef] [PubMed]
- Gonsalves, N. Food allergies and eosinophilic gastrointestinal illness. Gastroenterol. Clin. N. Am. 2007, 36, 75–91. [Google Scholar] [CrossRef]
- Fleischer, D.M.; Atkins, D. Evaluation of patient with suspected eosinophilic gastrointestinal disease. Immunol. Allergy Clin. N. Am. 2009, 29, 53–63. [Google Scholar] [CrossRef]
- Bischoff, S.C. Food allergy and eosinophilic gastroenteritis and colitis. Curr. Opin. Allergy Clin. Immunol. 2010, 10, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.A.; Assa’ad, A.; Burks, A.W.; Jones, S.M.; Sampson, H.A.; Wood, R.A.; Plaut, M.; Cooper, S.F.; Fenton, M.J.; Arshad, S.H.; et al. Guidelines for the Diagnosis and Management of Food Allergy in the United States: Summary of the NIAID-Sponsored Expert Panel Report. J. Allergy Clin. Immunol. 2010, 126, 1105–1118. [Google Scholar] [CrossRef] [PubMed]
- Arias, A.; González-Cervera, J.; Tenias, J.M.; Lucendo, A.J. Efficacy of dietary interventions for inducing histologic remission in patients with eosinophilic esophagitis: A systematic review and meta-analysis. Gastroenterology 2014, 146, 1639–1648. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, J.B.; Schwartz, S.; Amsden, K.; Kagalwalla, A.F. Elimination diets in the management of eosinophilic esophagitis. J. Asthma Allergy 2014, 7, 85–94. [Google Scholar] [PubMed]
- Behjati, S.; Zilbauer, M.; Heuschkel, R.; Phillips, A.; Salvestrini, C.; Torrente, F.; Bates, A.W. Defining eosinophilic colitis in children: Insights from a retrospective case series. J. Pediatr. Gastroenterol. Nutr. 2009, 49, 208–215. [Google Scholar] [CrossRef] [PubMed]
- OKpara, N.; Aswad, B.; Baffy, G. Eosinophilic colitis. World J. Gastroenterol. 2009, 15, 2975–2979. [Google Scholar] [CrossRef] [PubMed]
- Lozinsky, A.C.; Morais, M.B. Eosinophilic colitis in infants. J. Pediatr. 2014, 90, 16–21. [Google Scholar] [CrossRef]
- Lowichik, A.; Weinberg, A.G. A quantitative evaluation of mucosal eosinophils in the pediatric gastrointestinal tract. Mod. Pathol. 1996, 9, 110–114. [Google Scholar] [PubMed]
- DeBrosse, C.W.; Case, J.W.; Putnam, P.E.; Collins, M.H.; Rothenberg, M.E. Quantity and distribution of eosinophils in the gastrointestinal tract of children. Pediatr. Dev. Pathol. 2006, 9, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.G. Normal quantity and distribution of mast cells and eosinophils in the pediatric colon. Pediatr. Dev. Pathol. 2011, 14, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Gaertner, W.B.; Macdonald, J.E.; Kwaan, M.R.; Shepela, C.; Madoff, R.; Jessurun, J.; Melton, G.B. Eosinophilic colitis: University of Minnesota experience and literature review. Gastroenterol. Res. Pract. 2011, 2011, 857508. [Google Scholar] [CrossRef] [PubMed]
- Alfadda, A.A.; Storr, M.A.; Shaffer, E.A. Eosinophilic colitis: An update on pathophysiology and treatment. Br. Med. Bull. 2011, 100, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, S.; Niggemann, B.; Arato, A.; Dias, J.A.; Heuschkel, R.; Husby, S.; Mearin, M.L.; Papadopoulou, A.; Ruemmele, F.M.; Staiano, A.; et al. Diagnostic approach and management of cow’s-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Friesen, C.A.; Kearns, G.L.; Andre, L.; Neustrom, M.; Roberts, C.C.; Abdel-Rahman, S.M. Clinical efficacy and pharmacokinetics of montelukast in dyspeptic children with duodenal eosinophilia. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Friesen, C.A.; Sandridge, L.; Andre, L.; Roberts, C.C.; Abdel-Rahman, S.M. Mucosal eosinophilia and response to H1/H2 antagonist and cromolyn therapy in pediatric dyspepsia. Clin. Pediatr. 2006, 45, 143–147. [Google Scholar] [CrossRef]
- Melamed, I.; Feanny, S.J.; Sherman, P.M.; Roifman, C.M. Benefit of ketotifen in patients with eosinophilic gastroenteritis. Am. J. Med. 1991, 90, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.J.; Chu, C.H.; Lin, S.C.; Shih, S.C.; Wang, T.E. Eosinophilic gastroenteritis: Clinical experience with 15 patients. World J. Gastroenterol. 2003, 9, 2813–2816. [Google Scholar] [PubMed]
- García-Ara, C.; Boyano-Martínez, T.; Díaz-Pena, J.M.; Martín-Muñoz, F.; Reche-Frutos, M.; Martín-Esteban, M. Specific IgE levels in the diagnosis of immediate hypersensitivity to cows’ milk protein in the infant. J. Allergy Clin. Immunol. 2001, 107, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Erwin, E.A.; James, H.R.; Gutekunst, H.M.; Russo, J.M.; Kelleher, K.J.; Platts-Mills, T.A. Serum IgE measurement and detection of food allergy in pediatric patients with eosinophilic esophagitis. Ann. Allergy Asthma Immunol. 2010, 104, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Sánchez, J.; Gómez Torrijos, E.; López Viedma, B.; de la Santa Belda, E.; Martín Dávila, F.; García Rodríguez, C.; Feo Brito, F.; Olmedo Camacho, J.; Reales Figueroa, P.; Molina-Infante, J. Efficacy of IgE-targeted vs. empiric six-food elimination diets for adult eosinophilic oesophagitis. Allergy 2014, 69, 936–942. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Geng, L.; Chen, P.; Wang, F.; Xu, Z.; Liang, C.; Li, H.; Fang, T.; Friesen, C.A.; Gong, S.; et al. Effectiveness of Dietary Allergen Exclusion Therapy on Eosinophilic Colitis in Chinese Infants and Young Children ≤ 3 Years of Age. Nutrients 2015, 7, 1817-1827. https://doi.org/10.3390/nu7031817
Yang M, Geng L, Chen P, Wang F, Xu Z, Liang C, Li H, Fang T, Friesen CA, Gong S, et al. Effectiveness of Dietary Allergen Exclusion Therapy on Eosinophilic Colitis in Chinese Infants and Young Children ≤ 3 Years of Age. Nutrients. 2015; 7(3):1817-1827. https://doi.org/10.3390/nu7031817
Chicago/Turabian StyleYang, Min, Lanlan Geng, Peiyu Chen, Fenghua Wang, Zhaohui Xu, Cuiping Liang, Huiwen Li, Tiefu Fang, Craig A. Friesen, Sitang Gong, and et al. 2015. "Effectiveness of Dietary Allergen Exclusion Therapy on Eosinophilic Colitis in Chinese Infants and Young Children ≤ 3 Years of Age" Nutrients 7, no. 3: 1817-1827. https://doi.org/10.3390/nu7031817
APA StyleYang, M., Geng, L., Chen, P., Wang, F., Xu, Z., Liang, C., Li, H., Fang, T., Friesen, C. A., Gong, S., & Li, D. (2015). Effectiveness of Dietary Allergen Exclusion Therapy on Eosinophilic Colitis in Chinese Infants and Young Children ≤ 3 Years of Age. Nutrients, 7(3), 1817-1827. https://doi.org/10.3390/nu7031817





