Zinc in Early Life: A Key Element in the Fetus and Preterm Neonate
Abstract
:1. Introduction
- Explore the role of zinc in early life including embryogenesis, fetal and neonatal life.
- Analyze the criteria used for the diagnosis of zinc deficiency in preterm neonates.
- Investigate the consequences of zinc deficiency in preterm neonates.
- Evaluate the adequacy of current recommendations on zinc for preterm neonates.
- Suggest new possible research perspectives on the use of zinc in early life.
2. Evidence Selection Method
| Medical Subject Headings and Terms | Zinc AND Embryogenesis | Zinc AND Fetus | Zinc AND Preterm Neonate or Zinc AND Preterm Newborn |
|---|---|---|---|
| Eligible articles | 196 | 100 | 155 |
| Excluded articles (reasons) | 188 (unrelated articles) | 86 (unrelated articles) | 96 (unrelated articles) |
| Selected articles, n | 8 | 14 | 59 |
| -Human | 4 | 11 | 55 |
| -Animal | 4 | 3 | 4 |
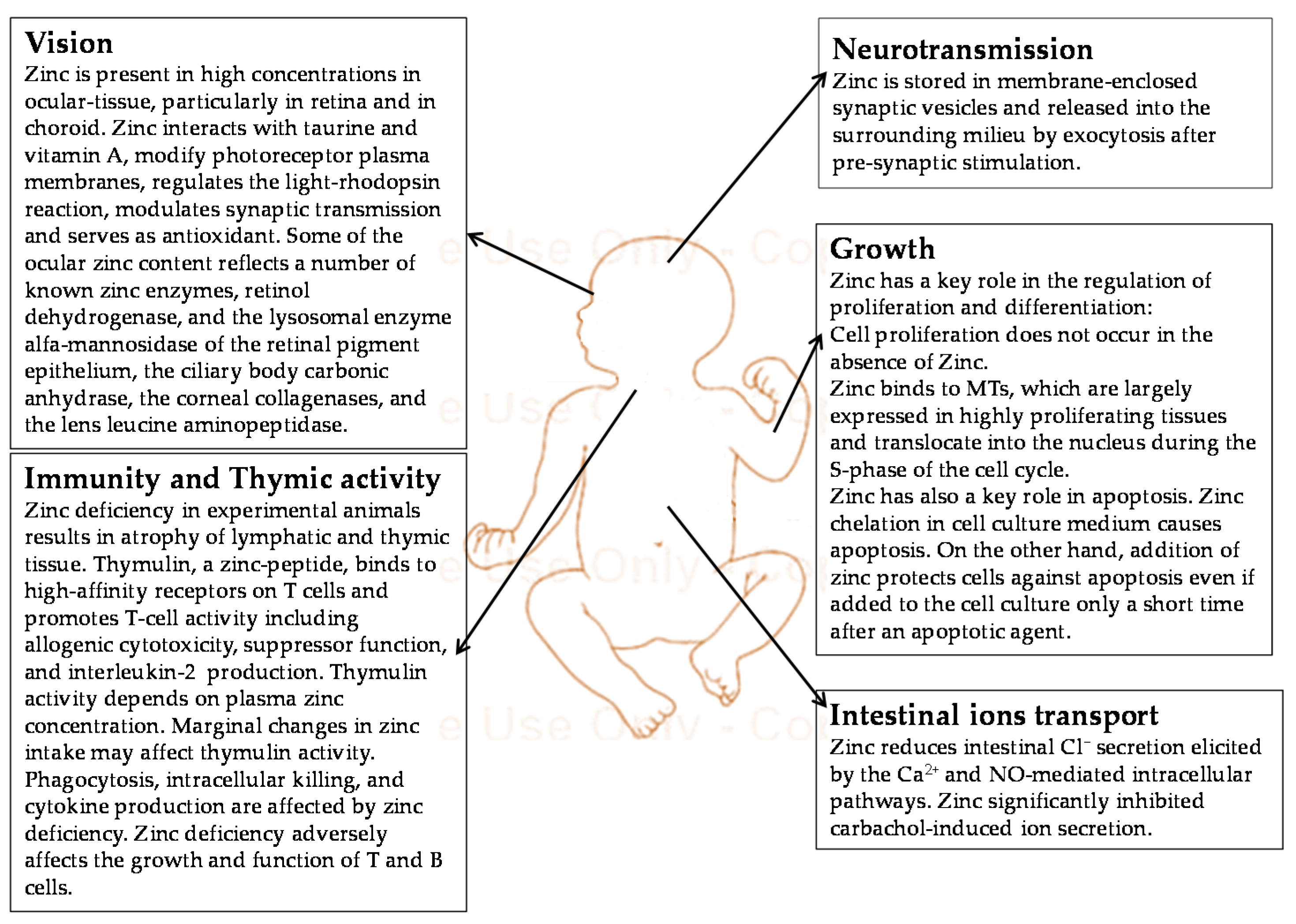
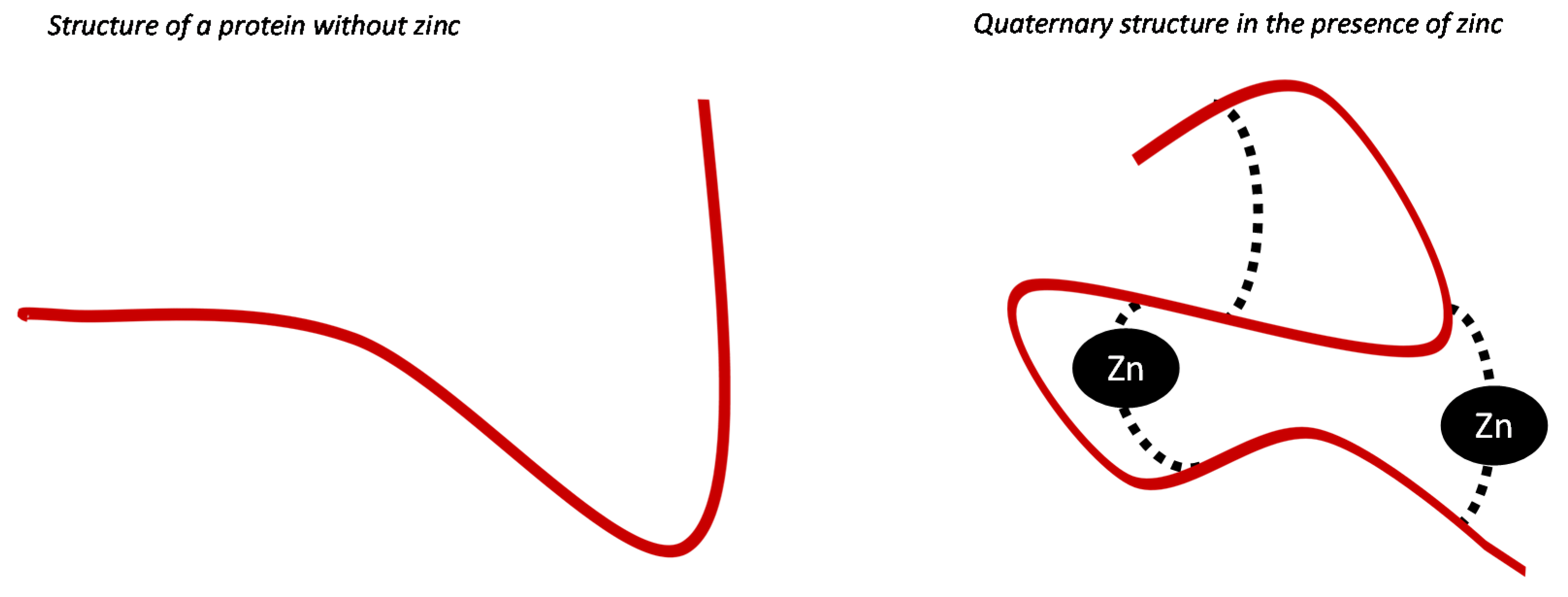
3. Function of Zinc during Fetal and Neonatal Life
3.1. The Role of Zinc in Embryogenesis
| A. Evidence from Experimental Animal Model. | |||||
|---|---|---|---|---|---|
| Study | Model | Study Design | Main Results | ||
| Hurley et al., 1969 [25] | Pregnant rats | Severe zinc deficiency induced by the use of a diet containing isolated soybean protein (treated with a chelating agent). Controls fed with zinc-supplemented diet | 98% of full-term fetuses with congenital malformations of the tail (72%), finger (64%), lungs (54%), palate (42%), brain (47%), eye (42%), feet (38%), urogenital tract (21%) | ||
| Hicory et al., 1979 [26] | Pregnant rats | Eighteen rats fed with zinc deficient diet and 18 fed with zinc supplemented diet during pregnancy | Malformations of the trunk and limbs in fetuses of zinc deficient mothers | ||
| Rogers et al., 1985 [27] | Long-Evans hooded pregnant rats and fetuses | Determination of teratogenicity of maternal Zn deficiency in the Long-Evans hooded rat, examining the effects of Zn deficiency on Zn, Fe, and Cu concentrations in maternal and fetal tissues. Evaluation of the effects of Zn deficiency on the risk of abdominal and skeletal malformations | All fetuses presented malformations when zinc was supplemented at low doses | ||
| Falchuk et al., 2001 [28] | Frog embryos | Deprivation of zinc in embryos to evaluate the effects on metallo-proteins activity and on organ formation and development | Agenesis of dorsal organs (including brain, eyes and spinal cord) in embryos developed in the absence of zinc. | ||
| B. Evidence from Clinical Studies in Human. | |||||
| Study | Population | Study Design | Results | ||
| Velie et al., 1999 [29] | Mothers of infants with neural tube defect (NTD) compared with mothers of healthy neonates (controls) | Retrospective study on pre-conceptional use of vitamin, mineral, and food supplements, by filling a specific questionnaire | Risk of NTDs decreased with the increase in maternal pre-conceptional zinc intake | ||
| Cengiz et al., 2004 [30] | Mothers of infants with neural tube defect diagnosed in the second trimester of gestation compared with mothers of healthy neonates (controls) | Case-control study to investigate the relationship between maternal micronutrient serum level (including zinc) and NTD occurrence in neonates | No strict correlation between zinc concentrations and NTD | ||
| Zeyreks et al., 2009 [31] | Mothers of infants with neural tube defect (NTD) compared with mothers of healthy neonates (controls) | Case-control study to investigate the relation between cord blood and maternal micronutrient serum levels of (including zinc) and NTD occurrence in neonates | The mean maternal serum zinc level in mothers of neonates with NTD was significantly lower than those of controls | ||
| Dey et al., 2010 [32] | Mothers of infants with neural tube defects (NTD) compared with mothers of healthy neonates (controls). | Hospital-based case-control study conducted with the objective of finding the relationship between serum zinc levels in newborns and their mothers and NTDs in a Bangladeshi population | NTD were more likely in subjects born from mothers with lower serum zinc level | ||
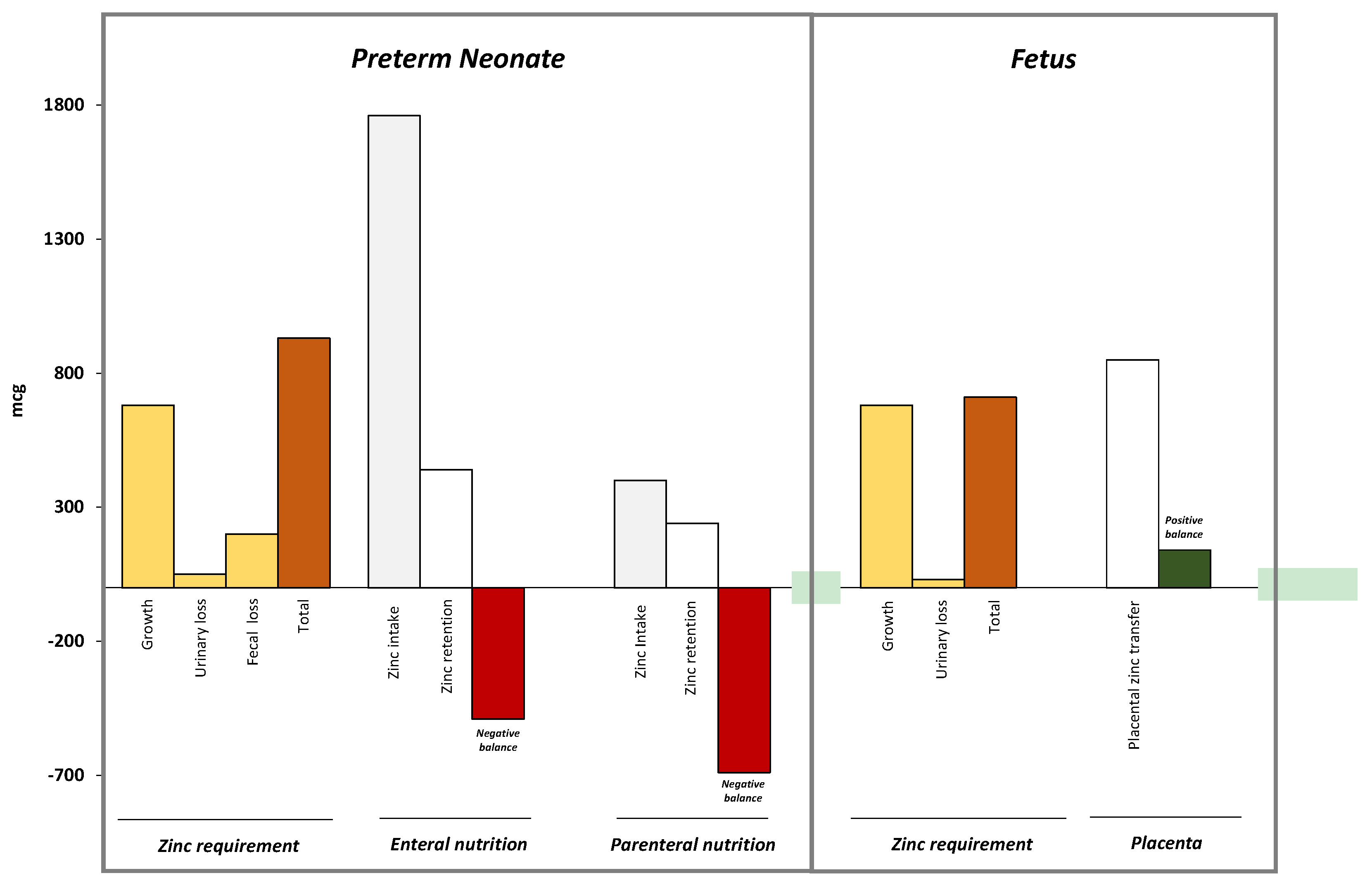
3.2. Metabolism of Zinc in Fetal Life
| Risk of Zinc Deficiency | Diet Characteristics |
|---|---|
| Low | Adequate protein content mainly from non-vegetable sources (i.e., meat or fish), low cereals intake (phytate intake <500 mg/day) |
| Moderate | Mixed diet containing animal or fish protein, vegetarian or vegan diet not based on cereal or flours (phytate intake of 500–1500 mg/day) |
| High | Low animal protein intake, high unrefined, unfermented and ungerminated * cereals intake (phytate intake >1500 mg/day) |
4. Neonatal Zinc Metabolism
| Nutrient | Mechanism |
|---|---|
| Proteins [64,65,66,67,68] | Protein is a major source of zinc, thus increased protein intake results in increased zinc intake High amounts of protein in enteral nutrition improve zinc absorption Casein in cow milk reduces zinc absorption |
| Lipids [69] | Fecal zinc increases in subjects with steatorrhea Medium-chain triglycerides improve zinc absorption |
| Copper [70] | Slight increase in copper intake does not interfere with zinc absorption if zinc intake is satisfactory. The effects of increased copper intake in subjects with low intake of zinc still remain to be defined. |
| Iron [71] | Iron administered at high doses (i.e., iron-zinc ratio of 25:1 molar) reduce zinc absorption. Duration of iron supplementation does not affect zinc status |
| Vitamin A [72] | Severe vitamin A deficiency may reduce absorption and lymphatic transport of zinc by altering synthesis of zinc-dependent protein |
| Folic acid [73] | Supplementation with folate may impair zinc absorption by insoluble chelate formation |
5. Diagnosis of Zinc Deficiency in Preterm Neonate
| Biologic Samples Used for Zinc Concentrations Measurement | Characteristics | Limitations on the Use in Preterm Neonate |
|---|---|---|
| Serum or plasma [76] | It is the only biochemical indicator recommended by WHO to assess zinc status. Levels vary according to zinc intakes It may be used to predict response to zinc supplementation It is readily available as an early marker of severe deficit | Adventitious zinc can easily be added to samples by environmental exposure and inappropriate sample handling |
| Zinc is released from hemolysed red blood cells into the serum. | ||
| Low specificity (serum zinc concentrations decrease with a number of conditions such as infection, trauma, stress, steroid use, metabolic redistribution of zinc from the plasma to the tissues, concurrent nutrient deficiency). | ||
| Starvation can induce the release of zinc in the circulation. | ||
| The time of the day when blood samples are drawn has a significant effect on serum zinc concentrations (serum zinc is higher in morning samples than in afternoon or evening samples) | ||
| Intracellular concentrations (erythrocytes, platelets, leucocytes) [76,77] | Provides information on zinc status over a longer time period (independent of serum turn-over). | Absence of standardization and reference values in neonates |
| Large volumes of blood required for the assay | ||
| Sophisticated technology useful to isolate cells | ||
| Metalloenzymes [78] | Rapid response to zinc supplementation | No data on diagnostic accuracy in preterm neonates. |
| Hair [79] | Provides information on zinc status over a longer time period Easy to collect | Variability with age, sex, season, hair growth rate, and hair color |
| No standardized methods for collection, washing, and analysis of hair samples in neonates |
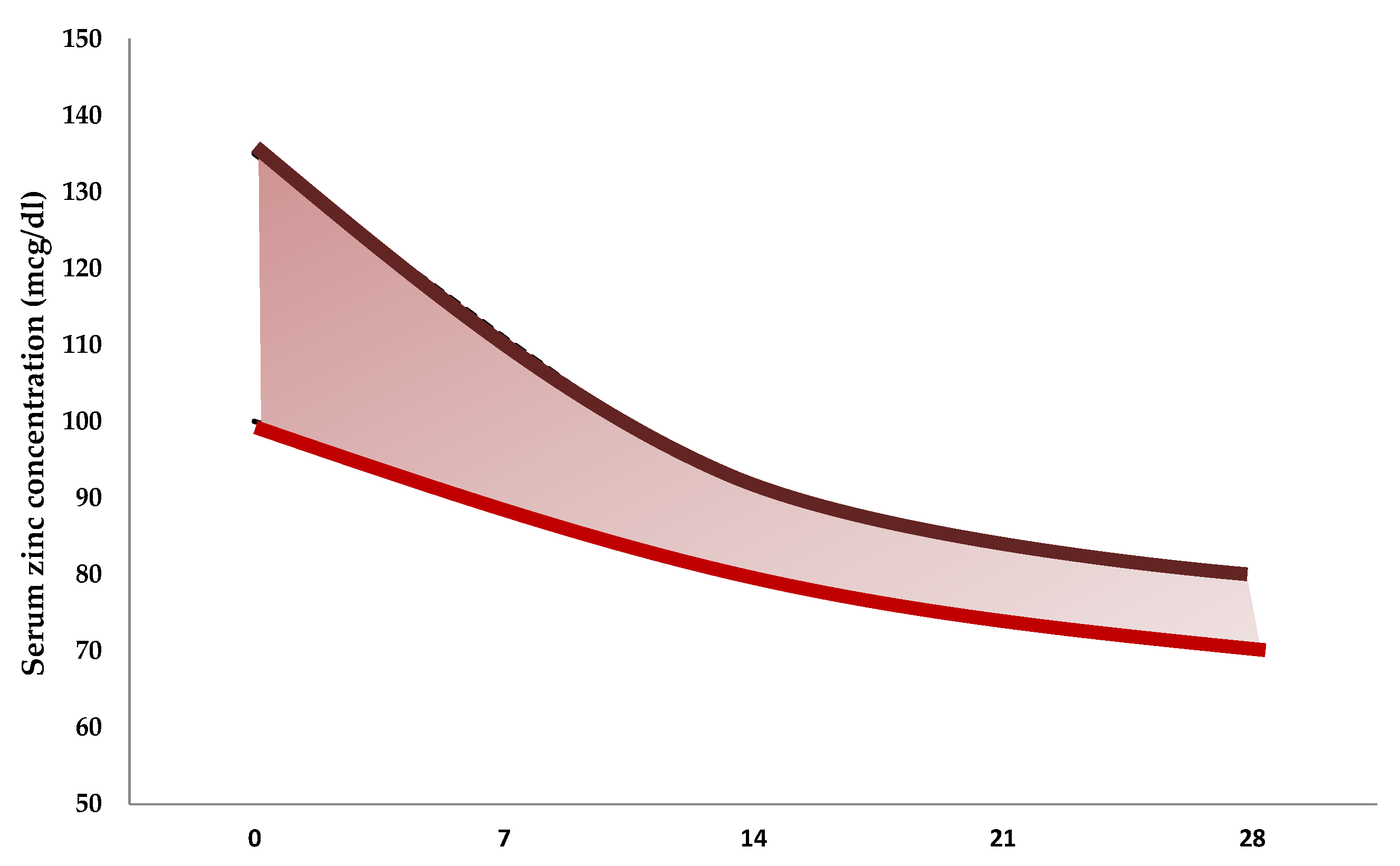
| Reference | Number of Neonates | Gestational Age at Birth, Weeks | Birth Weight, g | Mean Values ± Standard Deviation (µg/dL) |
|---|---|---|---|---|
| Jeswani et al., 1991 [81] | 25 | <37 | 1790 ± 380 | 94 ± 18 |
| 25 | >37 | 2800 ± 200 | 129 ± 14 | |
| 10 | >37 | 1880 ± 150 | 112 ± 9 | |
| Wasowicz et al., 1993 [82] | 51 | >37 | 81 ± 24 | |
| 51 | <37 | 93 ± 12 | ||
| 23 | 1500–2499 | 85 ± 13 | ||
| 41 | 2500–4750 | 81 ± 27 | ||
| 13 | 24–36 | 92 ± 12 | ||
| 15 | 37–38 | 87 ± 33 | ||
| 36 | 39–41 | 78 ± 19 | ||
| Iqbal et al., 2001 [83] | 3 | 28–33 | 90 ± 47 | |
| 29 | 34–36 | 88 ± 30 | ||
| 22 | 37–39 | 83 ± 39 | ||
| 11 | 40–41 | 79 ± 24 | ||
| 11 | 1000–1500 | 103 ± 37 | ||
| 16 | 1600–2000 | 81 ± 25 | ||
| 10 | 2100–2500 | 79 ± 29 | ||
| 18 | 2600–3000 | 83 ± 43 | ||
| 10 | 3100–4000 | 81 ± 14 | ||
| Perveen et al., 2002 [84] | 11 | 24–28 | 116 ± 45 | |
| 11 | 29–33 | 94 ± 19 | ||
| 9 | 34–37 | 89 ± 15 | ||
| 11 | 38–42 | 87 ± 9 | ||
| Galinier et al., 2005 [85] | 53 | 26–31 | 160 ± 27 | |
| 76 | 31–33 | 137 ± 30 | ||
| 66 | 33–34 | 125 ± 23 | ||
| 53 | 34–37 | 128 ± 18 | ||
| 262 | >37 | 3234 ± 358 | 123 ± 20 | |
| Tsuzuki et al., 2013 [86] | 14 | 36 ± 2 | 2388 ± 465 | 89 ± 14 |
| 30 | 39 ± 1 | 3043 ± 321 | 86 ± 16 |
6. Zinc Deficiency and Neonatal Complications
6.1. Dermatitis
6.2. Growth Retardation
6.3. Necrotizing Enterocolitis
6.4. Neurologic Damage
6.5. Bronchopulmonary Dysplasia
6.6. Infections
6.7. Retinopathy of Prematurity
7. Supplementation of Zinc
| Institute/Scientific Societies/Academic Groups | Publication Year | Population of Neonates | Dose (mg/Kg/Day) |
|---|---|---|---|
| Enteral route | |||
| The American Academy of Pediatrics Committee on nutrition [123] | 1985 | All | 0.6 |
| Committee on Nutrition of the Preterm Infant, European Society of Paediatric Gastroenterology and Nutrition [124] | 1987 | All | 0.7–1.4 |
| Zlotkin et al. [125] | 1996 | 0–14 days of life | 0.5–0.8 |
| >14 day of life | 1 | ||
| Klein et al. [126] | 2002 | Birth weight <1 Kg | 2 |
| Birth weight 1–2 Kg | 1.7 | ||
| Birth weight >2 Kg | 1.3 | ||
| Hambidge et al. [127] | 2006 | Birth weight <1 Kg | 2.4 |
| Birth weight 1–2 Kg | 2 | ||
| Birth weight 2–3.5 Kg | 1.6 | ||
| ESPGHAN Committee on Nutrition [128] | 2010 | Birth weight <1.8 Kg | 1.1–2.0 |
| Griffin et al. [129] | 2013 | Human milk feed | 2.3–2.4 |
| Formula feed | 1.8–2.4 | ||
| Parenteral route | |||
| American Society of Clinical Nutrition [130] | 1988 | All | 0.4 |
| Zlotkin et al. [125] | 1996 | Transitional period | 0.15 |
| Stable period | 0.4 | ||
8. Excessive Exposure to Zinc
9. Conclusions
- Study of the role of zinc in infertility and multiple abortions
- Identification of diet and environmental determinants of zinc absorption in otherwise well-nourished pregnant women
- Evaluation of zinc metabolism in neonates with alcoholic syndrome
- Definition of determinants of zinc absorption in preterm neonates
- Calculation of diagnostic power of zinc concentration assessment in cells and hairs, and of metalloenzyme in preterm neonates
- Definition of modalities for zinc supplementation in preterm neonates (doses, administration route, duration of therapy)
- Evaluation of the usefulness of individualized zinc supplementation in human milk feeding for preterm neonates
- Study of the relation between zinc and vitamin A in preterm neonates
- Study of the relation between zinc deficiency and occurrence of morbidities (i.e., NEC, brain damage, BPD, infectious diseases, ROP) in preterm neonates.
- Determination of zinc efficacy in reducing severe complications of prematurity and the study of the related mechanisms
Author Contributions
Conflicts of Interest
References
- King, J.C. Zinc: An Essential but Elusive Nutrient. Am. J. Clin. Nutr. 2011, 94, 679S–684S. [Google Scholar] [CrossRef] [PubMed]
- Vallee, B.L.; Auld, D.S. Zinc Coordination, Function, and Structure of Zinc Enzymes and Other Proteins. Biochemistry 1990, 19, 5647–5659. [Google Scholar] [CrossRef]
- Hambidge, K.M.; Krebs, N.F. Zinc in the Fetus and Neonate. In Fetal Neonatal Physiol; Elsevier Saunders: Philadelphia, PA, USA, 2004; pp. 342–347. [Google Scholar]
- Vallee, B.L.; Auld, D.S. Active Zinc Binding Sites of Zinc Metalloenzymes. Matrix Suppl. 1992, 1, 5–19. [Google Scholar] [PubMed]
- Palmiter, R.D. The Elusive Function of Metallothioneins. Proc. Natl. Acad. Sci. USA 1998, 21, 8428–8430. [Google Scholar] [CrossRef]
- Hijova, E. Metallothioneins and zinc: Their functions and interactions. Bratisl. Lek. Listy 2004, 105, 230–234. [Google Scholar] [PubMed]
- Hanas, J.S.; Hazuda, D.J.; Bogenhagen, D.F.; Wu, F.Y.; Wu, C.W. Xenopus Transcription factor a requires zinc for binding to the 5 S RNA gene. J. Biol. Chem. 1983, 10, 14120–14125. [Google Scholar]
- Miller, J.; McLachlan, A.D.; Klug, A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985, 4, 1609–1614. [Google Scholar] [PubMed]
- Shang, Z.; Liao, Y.D.; Wu, F.Y.; Wu, C.W. Zinc Release from Xenopus transcription factor IIIA induced by chemical modifications. Biochemistry 1989, 12, 9790–9795. [Google Scholar] [CrossRef]
- Evans, R.M. The steroid and thyroid hormone receptor superfamily. Science 1988, 13, 889–895. [Google Scholar] [CrossRef]
- Fukada, T.; Yamasaki, S.; Nishida, K.; Murakami, M.; Hirano, T. Zinc homeostasis and signaling in health and diseases: Zinc signaling. J. Biol. Inorg. Chem. 2011, 16, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Zinc in human health: Effect of zinc on immune cells. Mol. Med. 2008, 14, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S.; Meftah, S.; Abdallah, J.; Kaplan, J.; Brewer, G.J.; Bach, J.F.; Dardenne, M. Serum thymulin in human zinc deficiency. J. Clin. Investig. 1988, 82, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Summersgill, H.; England, H.; Lopez-Castejon, G.; Lawrence, C.B.; Luheshi, N.M.; Pahle, J.; Mendes, P.; Brough, D. Zinc depletion regulates the processing and secretion of IL-1β. Cell Death Dis. 2014, 30, e1040. [Google Scholar] [CrossRef] [PubMed]
- Grahn, B.H.; Paterson, P.G.; Gottschall-Pass, K.T.; Zhang, Z. Zinc and the eye. J. Am. Coll. Nutr. 2001, 20, 106–118. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, R.S. The Role of Zinc in Growth and Cell Proliferation. J. Nutr. 2000, 130, 1500S–1508S. [Google Scholar] [PubMed]
- Bobat, R.; Coovadia, H.; Stephen, C.; Naidoo, K.L.; McKerrow, N.; Black, R.E.; Moss, W.J. Safety and Efficacy of Zinc Supplementation for Children with HIV-1 Infection in South Africa: A randomised double-blind placebo-controlled trial. Lancet 2005, 366, 1862–1867. [Google Scholar] [CrossRef]
- Wastney, M.E.; Angelus, P.A.; Barnes, R.M.; Subramanian, K.N. Zinc absorption, distribution, excretion, and retention by healthy preterm infants. Pediatr. Res. 1999, 45, 191. [Google Scholar] [CrossRef] [PubMed]
- Zlotkin, S.H.; Cherian, M.G. Hepatic Metallothionein as a Source of Zinc and Cysteine during the First Year of Life. Pediatr. Res. 1988, 24, 326–329. [Google Scholar] [CrossRef] [PubMed]
- Falchuk, K.H. The Molecular Basis for the Role of Zinc in Developmental Biology. Mol. Cell Biochem. 1998, 188, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Record, I.R. Zinc Deficiency and the Developing Embryo. Neurotoxicology 1987, 8, 369–378. [Google Scholar] [PubMed]
- Simmer, K.; Lort-Phillips, L.; James, C.; Thompson, R.P. A Double-blind Trial of Zinc Supplementation in Pregnancy. Eur. J. Clin. Nutr. 1991, 45, 139–144. [Google Scholar] [PubMed]
- Hambidge, K.M.; Hackshaw, A. Neural Tube Defects and Serum Zinc. Br. J. Obstet. Gynaecol. 1993, 100, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Keen, C.L.; Hurley, L.S.; Lönnerdal, B. Effects of Zinc Deficiency on Prenatal and Postnatal Development. Fed. Proc. 1987, 42, 1735–1739. [Google Scholar]
- Hurley, L.S. Zinc Deficiency in the Developing Rats. Am. J. Clin. Nutr. 1969, 22, 1332–1339. [Google Scholar] [PubMed]
- Hickory, W.; Nanda, R.; Catalanotto, F.A. Fetal Skeletal Malformations Associated with Moderate Zinc Deficiency during Pregnancy. J. Nutr. 1979, 109, 883–891. [Google Scholar] [PubMed]
- Rogers, J.M.; Keen, C.L.; Hurley, L.S. Zinc Deficiency in Pregnant Long-Evans Hooded Rats: Teratogenicity and Tissue Trace Elements. Teratology 1985, 31, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Falchuk, K.H.; Montorzi, M. Zinc Physiology and Biochemistry in Oocytes and Embryos. Biometals 2001, 14, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Velie, E.M.; Block, G.; Shaw, G.M.; Samuels, S.J.; Schaffer, D.M.; Kulldorff, M. Maternal Supplemental and Dietary Zinc and the Occurrence of Neural Tube Defects in California. Am. J. Epidemiol. 1999, 150, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, B.; Söylemez, F.; Oztürk, E.; Cavdar, A.O. Serum Zinc, Selenium, Copper, and Lead Levels in Women with Second-Trimester Induced Abortion Resulting from Neural Tube Defects: A Preliminary Study. Biol. Trace Elem. Res. 2004, 97, 225–235. [Google Scholar] [CrossRef]
- Zeyrek, D.; Soran, M.; Cakmak, A.; Kocyigit, A.; Iscan, A. Serum Copper and Zinc Levels in Mothers and Cord Blood of Their Newborn Infants with Neural Tube Defects: A Case-control Study. Indian Pediatr. 2009, 46, 675–680. [Google Scholar] [PubMed]
- Dey, A.C.; Shahidullah, M.; Mannan, M.A.; Noor, M.K.; Saha, L.; Rahman, S.A. Maternal and Neonatal Serum Zinc Level and its Relationship with Neural Tube Defects. J. Health Popul. Nutr. 2010, 28, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Masters, D.G.; Keen, C.L.; Lönnerdal, B.; Hurley, L.S. Zinc deficiency teratogenicity: The protective role of maternal tissue catabolism. J. Nutr. 1983, 113, 905–912. [Google Scholar] [PubMed]
- Miller, S.I.; del Villano, B.C.; Flynn, A.; Krumhansl, M. Interaction of alcohol and zinc in fetal dysmorphogenesis. Pharmacol. Biochem. Behav. 1983, 18, 311–315. [Google Scholar] [CrossRef]
- Zidenberg-Cherr, S.; Rosenbaum, J.; Keen, C.L. Influence of ethanol consumption on maternal-fetal transfer of zinc in pregnant rats on day 14 of pregnancy. J. Nutr. 1988, 118, 865–870. [Google Scholar] [PubMed]
- Da Cunha Ferreira, R.M.; Marquiegui, I.M.; Elizaga, I.V. Teratogenicity of zinc deficiency in the rat: Study of the fetal skeleton. Teratology 1989, 39, 181–194. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Keen, C.L.; Uriu-Adams, J.Y.; Skalny, A.; Grabeklis, A.; Grabeklis, S.; Green, K.; Yevtushok, L.; Wertelecki, W.W.; Chambers, C.D. The Plausibility of Maternal Nutritional Status Being a Contributing Factor to the Risk for Fetal Alcohol Spectrum Disorders the Potential Influence of Zinc Status as an Example. Biofactors 2010, 36, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Keppen, L.D.; Pysher, T.; Rennert, O.M. Zinc Deficiency Acts as a Co-teratogen with Alcohol in Fetal Alcohol Syndrome. Pediatr. Res. 1985, 19, 944–947. [Google Scholar] [CrossRef] [PubMed]
- McClain, C.J.; Kasarskis, E.J., Jr.; Allen, J.J. Functional Consequences of Zinc Deficiency. Prog. Food Nutr. Sci. 1985, 9, 185–226. [Google Scholar] [PubMed]
- Wastney, M.E.; Angelus, P.; Barnes, R.M.; Subramanian, K.N. Zinc Kinetics in Preterm Infants: A Compartmental Model Based on Stable Isotope Data. Am. J. Physiol. 1996, 271, 1452–1459. [Google Scholar]
- Donangelo, C.M.; King, J.C. Maternal Zinc Intakes and Homeostatic Adjustments during Pregnancy and Lactation. Nutrients 2012, 4, 782–798. [Google Scholar] [CrossRef] [PubMed]
- Bax, C.M.; Bloxam, D.L. Two Major Pathways of Zinc (II) Acquisition by Human Placental Syncytiotrophoblast. J. Cell. Physiol. 1995, 164, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Ota, E.; Mori, R.; Middleton, P.; Tobe-Gai, R.; Mahomed, K.; Miyazaki, C.; Bhutta, Z.A. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst. Rev. 2015, 2, 10. [Google Scholar]
- Goldenberg, R.L.; Tamura, T.; Neggers, Y.; Copper, R.L.; Johnston, K.E.; DuBard, M.B.; Hauth, J.C. The Effect of Zinc Supplementation on Pregnancy Outcome. JAMA 1995, 9, 463–468. [Google Scholar] [CrossRef]
- King, J.C. Determinants of Maternal Zinc Status during Pregnancy. Am. J. Clin. Nutr. 2000, 71, 1334S–1343S. [Google Scholar] [PubMed]
- Sandstead, H.H.; Freeland-Graves, J.H. Dietary Phytate, Zinc and Hidden Zinc Deficiency. J. Trace Elem. Med. Biol. 2014, 28, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Hambidge, K.M.; Miller, L.V.; Westcott, J.E.; Krebs, N.F. Dietary Reference Intakes for Zinc may Require Adjustment for Phytate Intake Based upon Model Predictions. J. Nutr. 2008, 138, 2363–2366. [Google Scholar] [CrossRef] [PubMed]
- Sur, D.; Gupta, D.N.; Mondal, S.K.; Ghosh, S.; Manna, B.; Rajendran, K.; Bhattacharya, S.K. Impact of Zinc Supplementation on Dhiarroeal Morbidity and Growth Pattern of Low Birth Weight Infants in Kolkata India: A randomized, double-blind, placebo-controlled, community-based study. Pediatrics 2003, 112, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Wulf, K.; Wilhelm, A.; Spielmann, M.; Wirth, S.; Jenke, A.C. Frequency of Symptomatic Zinc Deficiency in very Low Birth Weight Infants. Klin. Padiatr. 2013, 225, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Hemalatha, P.; Bhaskaram, P.; Kumar, P.A.; Khan, M.M.; Islam, M.A. Zinc Status of Breastfed and Formula-fed Infants of Different Gestational Ages. J. Trop. Pediatr. 1997, 43, 52–54. [Google Scholar] [CrossRef] [PubMed]
- Giles, E.; Doyle, L.W. Zinc in extremely Low-Birthweight or Very Preterm Infants. Neoreviews 2007, 8, e165–e172. [Google Scholar] [CrossRef]
- Picciano, M.F.; Guthrie, H.A. Copper, Iron, and Zinc Contents of Mature Human Milk. Am. J. Clin. Nutr. 1976, 29, 242–254. [Google Scholar] [PubMed]
- Walravens, P.A.; Chakar, A.; Mokni, R.; Denise, J.; Lemonnier, D. Zinc supplements in breastfed infants. Lancet 1992, 9, 683–685. [Google Scholar] [CrossRef]
- Castillo-Durán, C.; Rodríguez, A.; Venegas, G.; Alvarez, P.; Icaza, G. Zinc supplementation and growth of infants born small for gestational age. J. Pediatr. 1995, 127, 206–211. [Google Scholar] [CrossRef]
- Ehrenkranz, R.A.; Gettner, P.A.; Nelli, C.M.; Sherwonit, E.A.; Williams, J.E.; Ting, B.T.; Janghorbani, M. Zinc and Copper Nutritional Studies in very Low Birth Weight Infants: Comparison of stable isotopic extrinsic tag and chemical balance. Pediatr. Res. 1989, 26, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Passariello, A.; Terrin, G.; Baldassarre, M.E.; de Curtis, M.; Paludetto, R.; Berni Canani, R. Diarrhea in neonatal intensive care unit. World J. Gastroenterol. 2010, 7, 2664–2668. [Google Scholar] [CrossRef]
- Dauncey, M.J.; Shaw, J.C.; Urman, J. The Absorption and Retention of Magnesium, Zinc and Copper by Low Birth Weight Infants Fed Pasteurized Human Breast Milk. Pediatr. Res. 1977, 11, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.C. Trace elements in the Fetus and Young Infant. I. Zinc. Am. J. Dis. Child. 1979, 133, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.S.; Dewolfe, M.S. Changes in Serum Zinc Concentrations of Some Canadian Full Term and Low Birthweight Infants from Birth to Six Months. Acta Paedtr. 1981, 70, 497–500. [Google Scholar] [CrossRef]
- Tyrala, E.E.; Manser, J.I.; Brodsky, N.L.; Tran, N. Serum Zinc Concentrations in Growing Premature Infants. Acta Paediatr. Scand. 1983, 72, 695–698. [Google Scholar] [CrossRef] [PubMed]
- McMaster, D.; Lappin, T.R.; Halliday, H.L.; Patterson, C.C. Serum Copper and Zinc Levels in the Preterm Infant. A longitudinal Study of the First Year of Life. Biol. Neonate. 1983, 44, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, S.L.; Lönnerdal, B. Zinc transporters in the rat mammary gland respond to marginal zinc and vitamin A intakes during lactation. J. Nutr. 2002, 132, 3280–3285. [Google Scholar] [PubMed]
- Kumar, L.; Michalczyk, A.; McKay, J.; Ford, D.; Kambe, T.; Hudek, L.; Varigios, G.; Taylor, P.E.; Ackland, M.L. Altered Expression of Two Zinc Transporters, SLC30A5 and SLC30A6underlies a Mammary Gland Disorder of Reduced Zinc Secretion into Milk. Genes Nutr. 2015, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Sandström, B.; Cederblad, A. Zinc Absorption from Composite Meals. II. Influence of the main protein source. Am. J. Clin. Nutr. 1980, 33, 1778–1783. [Google Scholar] [PubMed]
- Snedeker, S.M.; Greger, J.L. Metabolism of Zinc, Copper and Iron as affected by Dietary Protein, Cysteine and histidine. J. Nutr. 1983, 113, 644–652. [Google Scholar] [PubMed]
- Sandström, B.; Almgren, A.; Kivistö, B.; Cederblad, A. Effect of Protein Level and Protein Source on Zinc Absorption in Humans. J. Nutr. 1989, 119, 48–53. [Google Scholar] [PubMed]
- Hurrell, R.F.; Lynch, S.R.; Trinidad, T.P.; Dassenko, S.A.; Cook, J.D. Iron Absorption in Humans as Influenced by Bovine Milk Proteins. Am. J. Clin. Nutr. 1989, 49, 546–552. [Google Scholar] [PubMed]
- Sandström, B.; Sandberg, A.S. Inhibitory Effects of Isolated Inositol Phosphates on Zinc Absorption in Humans. J. Trace Elem. Electrolytes Health. Dis. 1992, 6, 99–103. [Google Scholar] [PubMed]
- Voyer, M.; Davakis, M.; Antener, I.; Valleur, D. Zinc Balances in Preterm Infants. Biol. Neonate 1982, 42, 87–92. [Google Scholar] [CrossRef] [PubMed]
- August, D.; Janghorbani, M.; Young, V.R. Determination of Zinc and Copper Absorption at Three Dietary Zn-Cu by Using Stable Isotope Methods in Young Adult and Elderly Subjects. Am. J. Clin. Nutr. 1989, 50, 1457–1463. [Google Scholar] [PubMed]
- Sandström, B.; Davidsson, L.; Cederblad, A.; Lönnerdal, B. Oral iron, Dietary Ligands and Zinc Absorption. J. Nutr. 1985, 115, 411–414. [Google Scholar] [PubMed]
- Christian, P.; Shahid, F.; Rizvi, A.; Klemm, R.D.; Bhutta, Z.A. Treatment Response to Standard of Care for Severe Anemia in Pregnant Women and Effect of Multivitamins and Enhanced Anthelminthics. Am. J. Clin. Nutr. 2009, 89, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Milne, D.B.; Canfield, W.K.; Mahalko, J.R.; Sandstead, H.H. Effect of Oral Folic Acid Supplements on Zinc, Copper, and Iron Absorption and Excretion. Am. J. Clin. Nutr. 1984, 39, 535–539. [Google Scholar] [PubMed]
- Smith, J.C., Jr.; Brown, E.D.; McDaniel, E.G.; Chan, W. Alterations in Vitamin A Metabolism during Zinc Deficiency and Food and Growth Restriction. J. Nutr. 1976, 106, 569–574. [Google Scholar] [PubMed]
- Christian, P.; West, K.P., Jr. Interactions between Zinc and Vitamin A: An update. Am. J. Clin. Nutr. 1998, 68, 435S–441S. [Google Scholar] [PubMed]
- Gibson, R.S.; Hess, S.Y.; Hotz, C.; Brown, K.H. Indicators of Zinc Status at the Population Level: A review of the evidence. Br. J. Nutr. 2008, 99, S14–23. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.P. Assessment of Zinc Status. Proc. Nutr. Soc. 1991, 50, 19–28. [Google Scholar] [CrossRef] [PubMed]
- King, J.C. Assessment of Zinc Status. J. Nutr. 1990, 120, 1474–1479. [Google Scholar] [PubMed]
- Hambidge, K.M. Hair Analyses: Worthless for vitamins, limited for minerals. Am. J. Clin. Nutr. 1982, 36, 943–949. [Google Scholar] [PubMed]
- Hambidge, K. Copper. In Neonatal Nutrition and Metabolism, 2nd ed.; Thureen, P., Hay, W., Jr., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 281–292. [Google Scholar]
- Jeswani, R.M.; Vani, S.N. A study of Serum Zinc Levels in Cord Blood of Neonates and their Mothers. Indian J. Pediatr. 1991, 58, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Wasowicz, W.; Wolkanin, P.; Bednarski, M.; Gromadzinska, J.; Sklodowska, M.; Grzybowska, K. Plasma trace element (Se, Zn, Cu) concentrations in maternal and umbilical cord blood in Poland. Relation with birth weight, gestational age, and parity. Biol. Trace Elem. Res. 1993, 38, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.S.; Shahidullah, M.; Islam, M.N.; Akhter, S.; Banu, S. Serum Zinc and Copper Levels in the Maternal Blood and Cord Blood of Neonates. Indian J. Pediatr. 2001, 68, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Perveen, S.; Altaf, W.; Vohra, N.; Bautista, M.L.; Harper, R.G.; Wapnir, R.A. Effect of Gestational Age on Cord Blood Plasma Copper, Zinc, Magnesium and Albumin. Early Hum. Dev. 2002, 69, 15–23. [Google Scholar] [CrossRef]
- Galinier, A.; Périquet, B.; Lambert, W.; Garcia, J.; Assouline, C.; Rolland, M.; Thouvenot, J.P. Reference Range for Micronutrients and Nutritional Marker Proteins in Cord Blood of Neonates Appropriated for Gestational Ages. Early Hum. Dev. 2005, 81, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, S.; Morimoto, N.; Hosokawa, S.; Matsushita, T. Associations of Maternal and Neonatal Serum Trace Element Concentrations with Neonatal Birth Weight. PLoS ONE 2013, 27, e75627. [Google Scholar] [CrossRef] [PubMed]
- Terrin, G.; Berni Canani, R.; Passariello, A.; Messina, F.; Conti, M.G.; Caoci, S.; Smaldore, A.; Bertino, E.; de Curtis, M. Zinc supplementation reduces morbidity and mortality in very-low-birth-weight preterm neonates: A hospital-based randomized, placebo-controlled trial in an industrialized country. Am. J. Clin. Nutr. 2013, 98, 1468–1474. [Google Scholar] [CrossRef] [PubMed]
- Arlette, J.P.; Johnston, M.M. Zinc Deficiency Dermatosis in Premature Infants Receiving Prolonged Parenteral Alimentation. J. Am. Acad. Dermatol. 1981, 5, 37–42. [Google Scholar] [CrossRef]
- Islam, M.N.; Chowdhury, A.K.; Siddika, M.; Hossain, M.A.; Hossain, M.K. Effect of Zinc on Growth of Preterm Babies. Mymensingh Med. J. 2009, 18, 125–130. [Google Scholar] [PubMed]
- Prasad, A.S. Zinc Deficiency in Human Subjects. Prog. Clin. Biol. Res. 1983, 129, 1–33. [Google Scholar] [PubMed]
- Zattra, E.; Belloni Fortina, A. Transient Symptomatic Zinc Deficiency Resembling Acrodermatitis Enteropathica in a Breast-fed Premature Infant: Case report and brief review of the literature. G. Ital. Dermatol. Venereol. 2013, 148, 699–702. [Google Scholar] [PubMed]
- Perafán-Riveros, C.; França, L.F.; Alves, A.C.; Sanches, J.A., Jr. Acrodermatitis Enteropathica: Case report and review of the literature. Pediatr. Dermatol. 2002, 19, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Chowdhury, M.A.; Siddika, M.; Qurishi, S.B.; Bhuiyan, M.K.; Hoque, M.M.; Akhter, S. Effect of Oral Zinc Supplementation on the Growth of Preterm Infants. Indian Pediatr. 2010, 47, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Ram Kumar, T.V.; Ramji, S. Effect of Zinc Supplementation on Growth in very Low Birth Weight Infants. J. Trop. Pediatr. 2012, 58, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Itabashi, K.; Saito, T.; Ogawa, Y.; Uetani, Y. Incidence and Predicting Factors of Hypozincemia in very-low-birth-weight Infants at Near-term Postmenstrual Age. Biol. Neonate 2003, 83, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Golden, B.E.; Golden, M.H. Effect of Zinc on Lean Tissue Synthesis during Recovery from Malnutrition. Eur. J. Clin. Nutr. 1992, 46, 697–706. [Google Scholar] [PubMed]
- Laureano, A.; Brás, S.; Carvalho, R.; Amaro, C.; Cardoso, J. Transient Symptomatic Zinc Deficiency in a Preterm Exclusively Breast-fed Infant. Dermatol. Online J. 2014, 20, 14. [Google Scholar]
- Terrin, G.; Scipione, A.; de Curtis, M. Update in Pathogenesis and Prospective in Treatment of Necrotizing Enterocolitis. Biomed. Res. 2014, 2014, 543765. [Google Scholar] [CrossRef] [PubMed]
- Terrin, G.; Passariello, A.; Canani, R.B.; Manguso, F.; Paludetto, R.; Cascioli, C. Minimal enteral feeding reduces the risk of sepsis in feed-intolerant very low birth weight newborns. Acta Pediatr. 2009, 98, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.I.; Thompson, D.; Kovar, I.Z.; Copeman, P.W.; Barltrop, D. Zinc Deficiency in a Preterm Neonate with Necrotizing Enterocolitis. J. R. Soc. Med. 1984, 77, 40–41. [Google Scholar] [PubMed]
- Suwendi, E.; Iwaya, H.; Lee, J.S.; Hara, H.; Ishizuka, S. Zinc Deficiency Induces Dysregulation of Cytokine Productions in an Experimental Colitis of Rats. Biomed. Res. 2012, 33, 329–336. [Google Scholar] [CrossRef] [PubMed]
- De Queiroz, C.A.; Fonseca, S.G.; Frota, P.B.; Figueiredo, I.L.; Aragão, K.S.; Magalhães CEde Carvalho, C.B.; Lima, A.Â.; Ribeiro, R.A.; Guerrant, R.L.; Moore, S.R.; et al. Zinc Treatment Ameliorates Diarrhea and Intestinal Inflammation in Undernourished Rats. BMC Gastroenterol. 2014, 5, 136. [Google Scholar] [CrossRef] [PubMed]
- Bolick, D.T.; Kolling, G.L.; Moore, J.H., 2nd; de Oliveira, L.A.; Tung, K.; Philipson, C.; Viladomiu, M.; Hontecillas, R.; Bassaganya-Riera, J.; Guerrant, R.L. Zinc Deficiency Alters Host Response and Pathogen Virulence in a Mouse Model of Enteroaggregative Escherichia coli-induced Diarrhea. Gut Microbes 2014, 5, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Vallee, B.L.; Falchuk, K.H. The Biochemical Basis of Zinc Pphysiology. Physiol. Rev. 1993, 73, 79–118. [Google Scholar] [PubMed]
- Berni Canani, R.; Secondo, A.; Passariello, A.; Buccigrossi, V.; Canzoniero, L.M.; Ruotolo, S.; Puzone, C.; Porcaro, F.; Pensa, M.; Braucci, A.; et al. Zinc Inhibits Calcium-mediated and Nitric Oxide-mediated ion Secretion in Human Enterocytes. Eur. J. Pharmacol. 2010, 25, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Kloubert, V.; Rink, L. Zinc as a Micronutrient and its Preventive Role of Oxidative Damage in cells. Food Funct. 2015, 6, 3195–3204. [Google Scholar] [CrossRef] [PubMed]
- Howell, G.A.; Welch, M.G.; Frederickson, C.J. Stimulation-induced Uptake and Release of Zinc in Hippocampal Slices. Nature 1984, 308, 736–738. [Google Scholar] [CrossRef] [PubMed]
- Frederickson, C.J.; Koh, J.Y.; Bush, A.I. The Neurobiology of Zinc in Health and Disease. Nat Rev. Neurosci. 2005, 6, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Adamo, A.M.; Oteiza, P.I. Zinc Deficiency and Neurodevelopment: The case of neurons. Biofactors 2010, 36, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Ho, E.; Ames, B.N. Low Intracellular Zinc Induces Oxidative DNA Damage, Disrupts p53, NFkappa B, and AP1 DNA binding, and affects DNA Repair in a Rat Glioma Cell Line. Proc. Natl. Acad. Sci. USA 2002, 99, 16770–16775. [Google Scholar] [CrossRef] [PubMed]
- Ramel, S.E.; Georgieff, M.K. Preterm Nutrition and the Brain. World Rev. Nutr. Diet. 2014, 110, 190–200. [Google Scholar] [PubMed]
- Zysman-Colman, Z.; Tremblay, G.M.; Bandeali, S.; Landry, J.S. Bronchopulmonary Dysplasia—Trends over three decades. Paediatr. Child. Health 2013, 18, 86–90. [Google Scholar] [PubMed]
- Merritt, T.A.; Deming, D.D.; Boynton, B.R. The “new” bronchopulmonary dysplasia challenges and commentary. Semin. Fetal Neonatal. Med. 2009, 14, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Biniwale, M.A.; Ehrenkranz, R.A. The Role of Nutrition in The Prevention and Management of Bronchopulmonary Dysplasia. Semin. Perinatol. 2006, 30, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Zinc is an Antioxidant and Anti-Inflammatory Agent: Its Role in Human Health. Front. Nutr. 2014, 1, 14. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Bromberg, P.A.; Samet, J.M. Zinc ions as effectors of environmental oxidative lung injury. Free Radic. Biol. Med. 2013, 65, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, P.; Benedetti, G.; Albarède, F.; Miossec, P. Zinc and its Role in Immunity and Inflammation. Autoimmun. Rev. 2015, 14, 277–285. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.M.; Manji, K.P.; Kisenge, R.; Aboud, S.; Spiegelman, D.; Fawzi, W.W.; Duggan, C.P. Daily Zinc but Not Multivitamin Supplementation Reduces Diarrhea and Upper Respiratory Infections in Tanzanian Infants: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Nutr. 2015, 145, 2153–2160. [Google Scholar] [CrossRef] [PubMed]
- Beligere, N.; Perumalswamy, V.; Tandon, M.; Mittal, A.; Floora, J.; Vijayakumar, B.; Miller, M.T. Retinopathy of Prematurity and Neurodevelopmental Disabilities in Premature Infants. Semin. Fetal Neonatal. Med. 2015, 20, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Fu, Z.J.; Lo, A.C. Hypoxia-induced Oxidative Stress in Ischemic Retinopathy. Oxid. Med. Cell. Longev. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ding, Y.; Chen, L. Effect of Trace Elements on Retinopathy of Prematurity. J. Huazhong. Univ. Sci. Technolog. Med. Sci. 2007, 27, 590–592. [Google Scholar] [CrossRef] [PubMed]
- Caulfield, L.E.; Zavaleta, N.; Shankar, A.H.; Merialdi, M. Potential Contribution of Maternal Zinc Supplementation during Pregnancy to Maternal and Child Survival. J. Clin. Nutr. 1998, 68, 499S–508S. [Google Scholar]
- Anonymous. American Academy of Pediatrics Committee on Nutrition: Nutritional Needs of Low-birth-weight infants. Pediatrics 1985, 75, 976–986. [Google Scholar]
- Committee on Nutrition of the Preterm Infant; European Society of Paediatric Gastroenterology and Nutrition. Nutrition and Feeding of Preterm Infants. Acta Paediatr. Scand. Suppl. 1987, 336, 1–14. [Google Scholar]
- Zlotkin, S.H.; Atkinson, S.; Lockitch, G. Trace Elements in Nutrition for Premature Infants. Clin. Perinatol. 1996, 22, 223–240. [Google Scholar]
- Klein, C.J. Nutrient requirements for preterm infant formulas. J. Nutr. 2002, 132, 1395S–1577S. [Google Scholar] [PubMed]
- Hambidge, K.M.; Krebs, N.F.; Westcott, J.E.; Miller, L.V. Changes in Zinc Absorption during Development. J. Pediatr. 2006, 149, S64–S68. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, C.; Buonocore, G.; Carnielli, V.P.; de Curtis, M.; Darmaun, D.; Decsi TDomellöf, M.; Embleton, N.D.; Fusch, C.; Genzel-Boroviczeny, O.; Goulet, O.; et al. Enteral nutrient supply for preterm infants: Commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Griffin, I.J.; Domellöf, M.; Bhatia, J.; Anderson, D.M.; Kler, N. Zinc and copper requirements in preterm infants: An examination of the current literature. Early Hum. Dev. 2013, 89, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Greene, H.L.; Hambidge, K.M.; Schanler, R.; Tsang, R.C. Guidelines for the use of vitamins, trace elements, calcium, magnesium, and phosphorus in infants and children receiving total parenteral nutrition: Report of the Subcommittee on Pediatric Parenteral Nutrient Requirements from the Committee on Clinical Practice Issues of the American Society for Clinical Nutrition. Am. J. Clin. Nutr. 1988, 48, 1324–1342. [Google Scholar] [PubMed]
- Schanler, R.J.; Shulman, R.J.; Prestridge, L.L. Parenteral Nutrient Needs of very Low Birth Weight Infants. J. Pediatr. 1994, 125, 961–968. [Google Scholar] [CrossRef]
- Higashi, A.; Ikeda, T.; Iribe, K.; Matsuda, I. Zinc Balance in Premature Infants Given the Minimal Dietary Zinc Requirement. J. Pediatr. 1988, 112, 262–266. [Google Scholar] [CrossRef]
- Altigani, M.; Murphy, J.F.; Gray, O.P. Plasma Zinc Concentration and Catch up Growth in Preterm Infants. Acta Paediatr. Scand. Suppl. 1989, 357, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Marriott, L.D.; Foote, K.D.; Kimber, A.C.; Delves, H.T.; Morgan, J.B. Zinc, Copper, Selenium and Manganese Blood Levels in Preterm Infants. Arch. Dis. Child. Fetal. Neonatal. 2007, 92, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Applegate, T.J. Effects of Dietary Copper Supplementation and Copper Source on Digesta pH, Calcium, Zinc, and Copper Complex Size in the Gastrointestinal Tract of the Broiler Chicken. Poult. Sci. 2007, 86, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.C., Jr. The Vitamin A—Zinc Connection: A review. Acad. Sci. 1980, 355, 62–74. [Google Scholar] [CrossRef]
- Ahmed, F.; Barua, S.; Mohiduzzaman, M.; Shaheen, N.; Bhuyan, M.A.; Margetts, B.M.; Jackson, A.A. Interactions between Growth and Nutrient Status in School-age children of Urban Bangladesh. Am. J. Clin. Nutr. 1993, 58, 334–338. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terrin, G.; Berni Canani, R.; Di Chiara, M.; Pietravalle, A.; Aleandri, V.; Conte, F.; De Curtis, M. Zinc in Early Life: A Key Element in the Fetus and Preterm Neonate. Nutrients 2015, 7, 10427-10446. https://doi.org/10.3390/nu7125542
Terrin G, Berni Canani R, Di Chiara M, Pietravalle A, Aleandri V, Conte F, De Curtis M. Zinc in Early Life: A Key Element in the Fetus and Preterm Neonate. Nutrients. 2015; 7(12):10427-10446. https://doi.org/10.3390/nu7125542
Chicago/Turabian StyleTerrin, Gianluca, Roberto Berni Canani, Maria Di Chiara, Andrea Pietravalle, Vincenzo Aleandri, Francesca Conte, and Mario De Curtis. 2015. "Zinc in Early Life: A Key Element in the Fetus and Preterm Neonate" Nutrients 7, no. 12: 10427-10446. https://doi.org/10.3390/nu7125542
APA StyleTerrin, G., Berni Canani, R., Di Chiara, M., Pietravalle, A., Aleandri, V., Conte, F., & De Curtis, M. (2015). Zinc in Early Life: A Key Element in the Fetus and Preterm Neonate. Nutrients, 7(12), 10427-10446. https://doi.org/10.3390/nu7125542