Magnesium and Space Flight
Abstract
:1. Introduction
2. Experimental Section
2.1. Studies and Participants
2.1.1. Biological Sample Collections
2.1.2. Space Flight
2.2. Bed Rest
2.3. Biochemical Analyses
2.4. Statistical Methods
3. Results
3.1. Serum and Urinary Magnesium
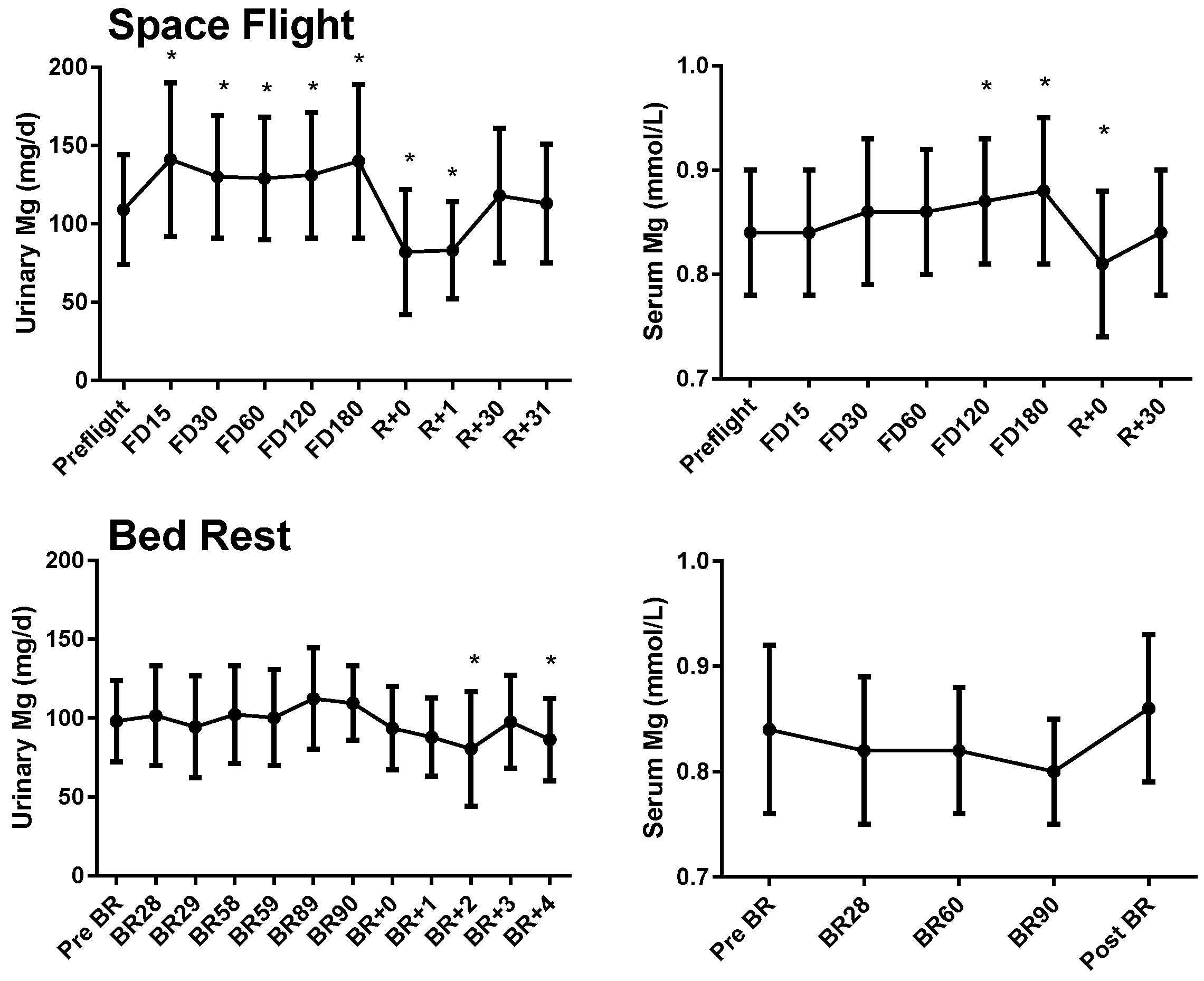
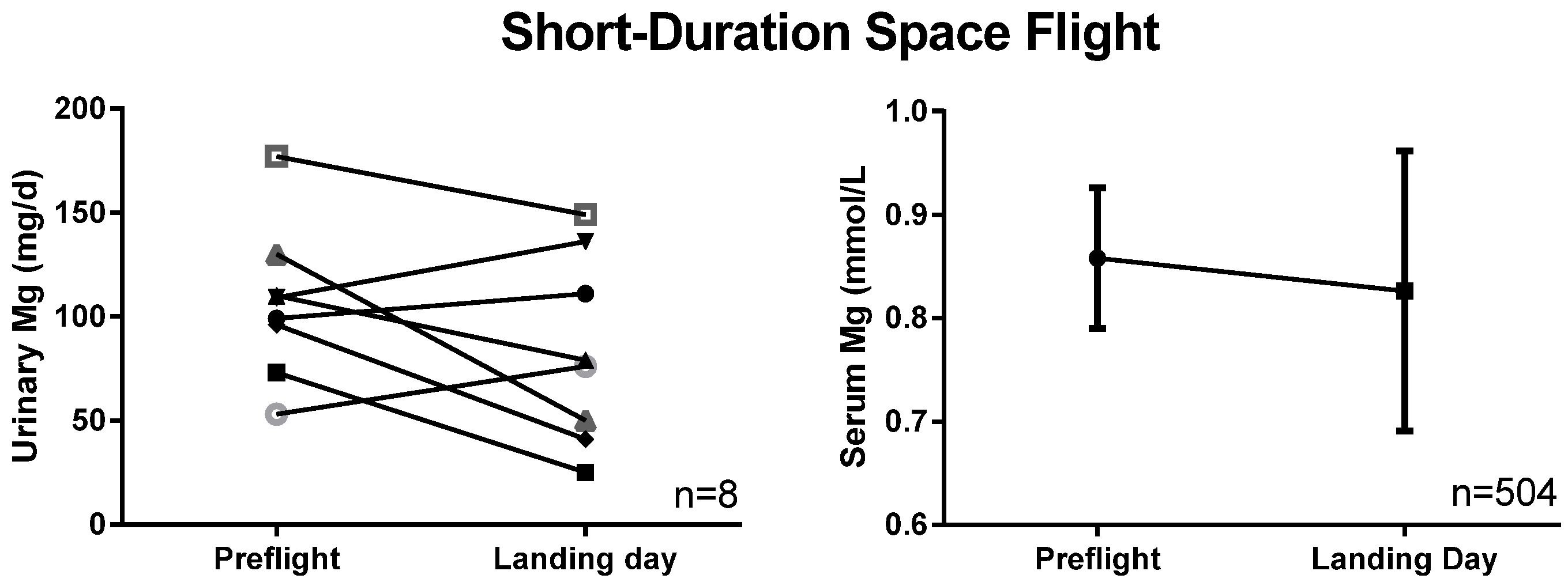
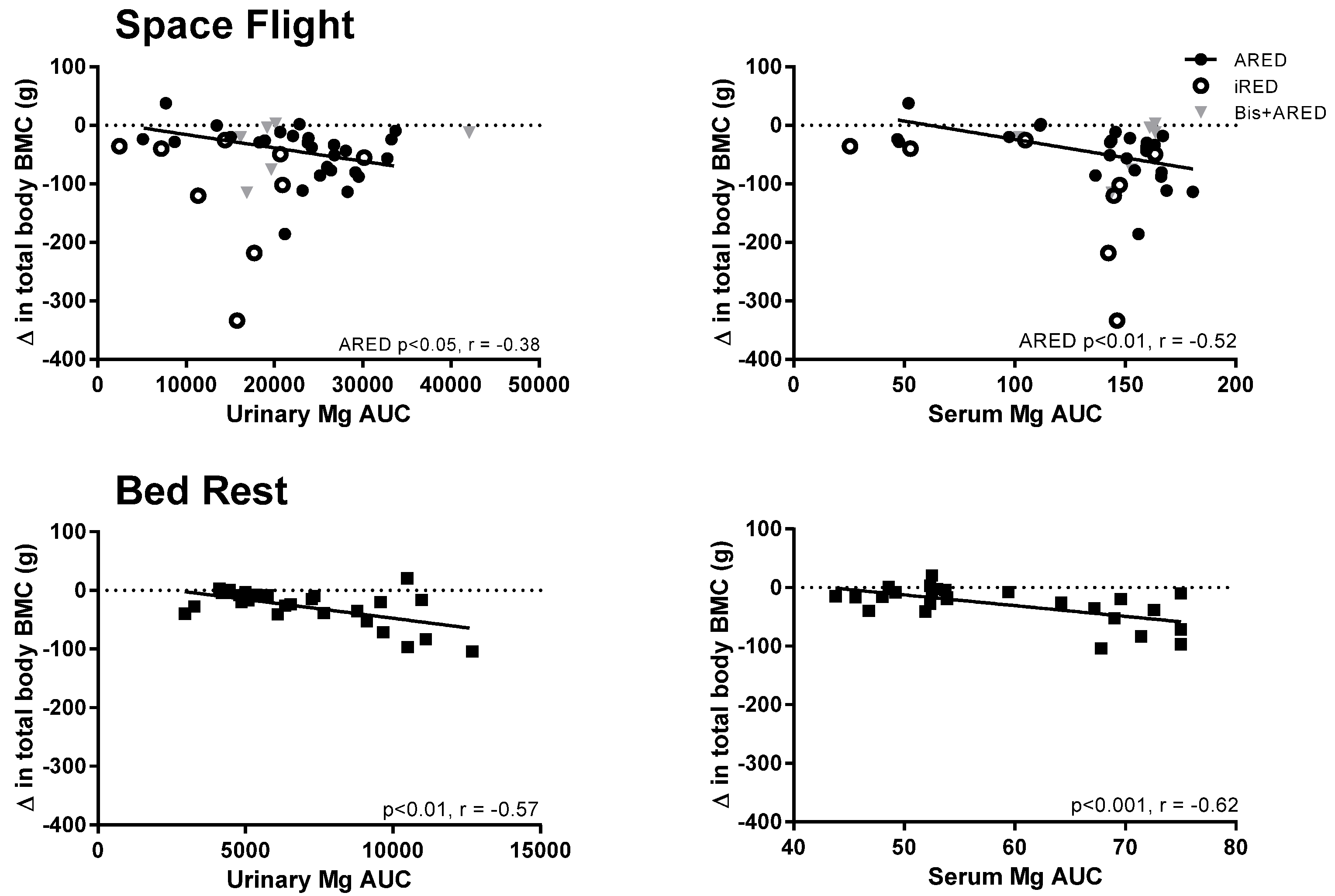
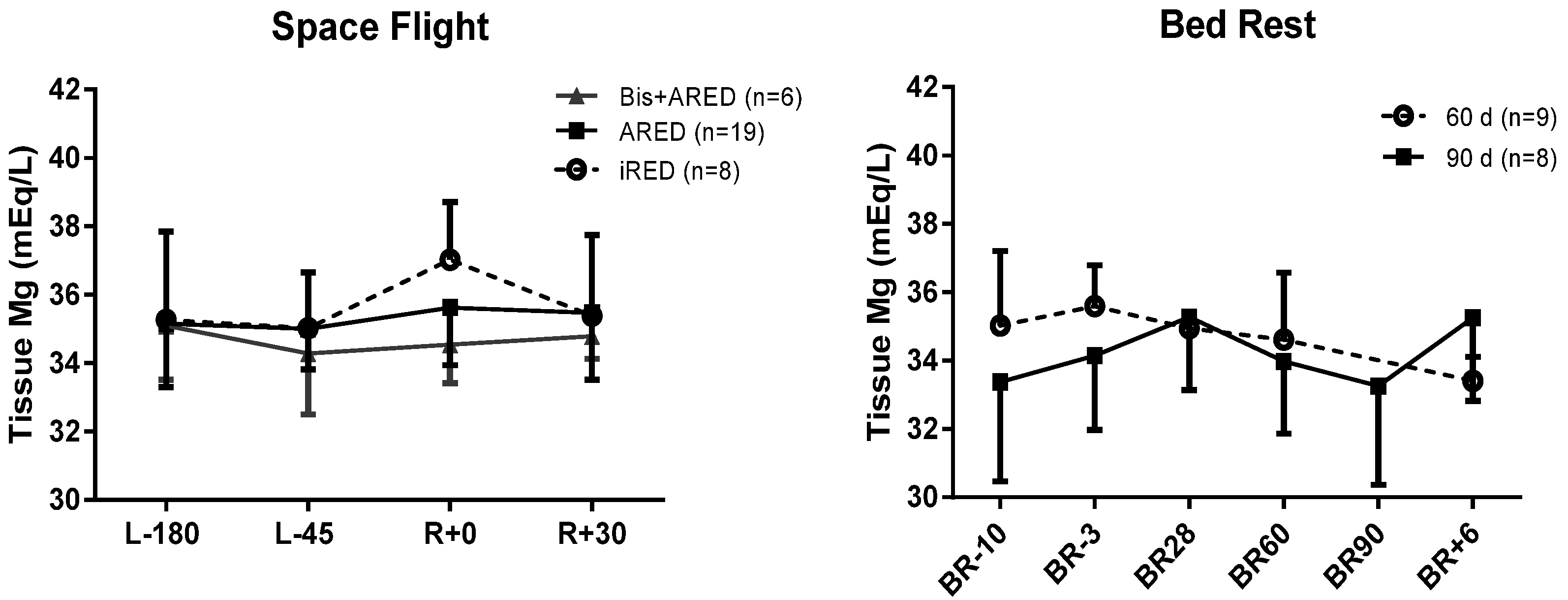
3.2. Tissue Magnesium
4. Discussion
Supplementary materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Volpe, S.L. Magnesium in disease prevention and overall health. Adv. Nutr. 2013, 4, 378S–383S. [Google Scholar] [CrossRef] [PubMed]
- De Baaij, J.H.; Hoenderop, J.G.; Bindels, R.J. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Zwart, S.R.; Heer, M. Human Adaptation to Spaceflight: The Role of Nutrition (NP-2014-10-018-JSC); National Aeronautics and Space Administration Lyndon B. Johnson Space Center: Houston, TX, USA, 2014.
- Ayuk, J.; Gittoes, N.J. Contemporary view of the clinical relevance of magnesium homeostasis. Ann. Clin. Biochem. 2014, 51, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Shechter, M. Magnesium and cardiovascular system. Magnes. Res. 2010, 23, 60–72. [Google Scholar] [PubMed]
- Haigney, M.C.; Silver, B.; Tanglao, E.; Silverman, H.S.; Hill, J.D.; Shapiro, E.; Gerstenblith, G.; Schulman, S.P. Noninvasive measurement of tissue magnesium and correlation with cardiac levels. Circulation 1995, 92, 2190–2197. [Google Scholar] [CrossRef] [PubMed]
- Hayhoe, R.P.; Lentjes, M.A.; Luben, R.N.; Khaw, K.T.; Welch, A.A. Dietary magnesium and potassium intakes and circulating magnesium are associated with heel bone ultrasound attenuation and osteoporotic fracture risk in the EPIC-Norfolk cohort study. Am. J. Clin. Nutr. 2015, 102, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Orchard, T.S.; Larson, J.C.; Alghothani, N.; Bout-Tabaku, S.; Cauley, J.A.; Chen, Z.; Lacroix, A.Z.; Wactawski-Wende, J.; Jackson, R.D. Magnesium intake, bone mineral density, and fractures: Results from the Women's Health Initiative Observational Study. Am. J. Clin. Nutr. 2014, 99, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Heer, M.; Zwart, S.R. Nutrition and bone health in space. In Nutrition and Bone Health, 2nd ed.; Holick, M., Nieves, J., Eds.; Springer: New York, NY, USA, 2015; pp. 687–705. [Google Scholar]
- Smith, S.M.; Zwart, S.R.; Kloeris, V.; Heer, M. Nutritional Biochemistry of Space Flight; Nova Science Publishers: New York, NY, USA, 2009. [Google Scholar]
- Sibonga, J.D. Spaceflight-induced bone loss: Is there an osteoporosis risk? Curr. Osteoporos. Rep. 2013, 11, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Sibonga, J.D.; Cavanagh, P.R.; Lang, T.F.; LeBlanc, A.D.; Schneider, V.S.; Shackelford, L.C.; Smith, S.M.; Vico, L. Adaptation of the skeletal system during long-duration spaceflight. Clin. Rev. Bone Miner. Metab. 2008, 5, 249–261. [Google Scholar] [CrossRef]
- Smith, S.M.; Heer, M.A.; Shackelford, L.C.; Sibonga, J.D.; Ploutz-Snyder, L.; Zwart, S.R. Benefits for bone from resistance exercise and nutrition in long-duration spaceflight: Evidence from biochemistry and densitometry. J. Bone Miner. Res. 2012, 27, 1896–1906. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Zwart, S.R.; Heer, M.; Hudson, E.K.; Shackelford, L.; Morgan, J.L. Men and women in space: Bone loss and kidney stone risk after long-duration spaceflight. J. Bone Miner. Res. 2014, 29, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Leach, C.S.; Alexander, W.C.; Johnson, P.C. Endocrine, electrolyte, and fluid volume changes associated with Apollo missions. In Biomedical Results from Apollo (NASA SP-368); Johnston, R.S., Dietlein, L.F., Berry, M.D., Eds.; National Aeronautics and Space Administration: Washington, DC, USA, 1975; pp. 163–184. [Google Scholar]
- Smith, S.M.; Zwart, S.R.; Block, G.; Rice, B.L.; Davis-Street, J.E. The nutritional status of astronauts is altered after long-term space flight aboard the International Space Station. J. Nutr. 2005, 135, 437–443. [Google Scholar] [PubMed]
- Leach, C.S.; Rambaut, P.C. Biochemical responses of the Skylab crewmen: An overview. In Biomedical Results from Skylab (NASA SP-377); Johnston, R.S., Dietlein, L.F., Eds.; National Aeronautics and Space Administration: Washington, DC, USA, 1977; pp. 204–216. [Google Scholar]
- Leach-Huntoon, C.S.; Schneider, H.; Cintron, N.M.; Landry, R. Combined blood investigations. In Results of the Life Sciences DSOs Conducted aboard the Space Shuttle 1981–1986; Bungo, M.W., Bagian, T.M., Bowman, M.A., Levitan, B.M., Eds.; Space Biomedical Research Institute, Johnson Space Center: Houston, TX, USA, 1987; pp. 7–11. [Google Scholar]
- Zwart, S.R.; Launius, R.D.; Coen, G.K.; Morgan, J.L.; Charles, J.B.; Smith, S.M. Body mass changes during long-duration spaceflight. Aviat. Space Environ. Med. 2014, 85, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Grigoriev, A.I.; Morukov, B.V.; Oganov, V.S.; Rakhmanov, A.S.; Buravkova, L.B. Effect of exercise and bisphosphonate on mineral balance and bone density during 360 day antiorthostatic hypokinesia. J. Bone Miner. Res. 1992, 7, S449–S455. [Google Scholar] [CrossRef] [PubMed]
- Zerwekh, J.E.; Odvina, C.V.; Wuermser, L.A.; Pak, C.Y. Reduction of renal stone risk by potassium-magnesium citrate during 5 weeks of bed rest. J. Urol. 2007, 177, 2179–2184. [Google Scholar] [CrossRef] [PubMed]
- Zwart, S.R.; Oliver, S.A.M.; Fesperman, J.V.; Kala, G.; Krauhs, J.; Ericson, K.; Smith, S.M. Nutritional status assessment before, during, and after long-duration head-down bed rest. Aviat. Space Environ. Med. 2009, 80, A15–A22. [Google Scholar] [CrossRef] [PubMed]
- Sakowski, C.; Starc, V.; Smith, S.M.; Schlegel, T.T. Sedentary long-duration head-down bed rest and ECG repolarization heterogeneity. Aviat. Space Environ. Med. 2011, 82, 416–423. [Google Scholar] [CrossRef]
- Morgan, J.L.; Heer, M.; Hargens, A.R.; Macias, B.R.; Hudson, E.K.; Shackelford, L.C.; Zwart, S.R.; Smith, S.M. Sex-specific responses of bone metabolism and renal stone risk during bed rest. Physiol. Rep. 2014. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.L.; Zwart, S.R.; Heer, M.; Ploutz-Snyder, R.; Ericson, K.; Smith, S.M. Bone metabolism and nutritional status during 30-day head-down-tilt bed rest. J. Appl. Physiol. 2012, 113, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Zwart, S.R.; Booth, S.L.; Peterson, J.W.; Wang, Z.; Smith, S.M. Vitamin K status in spaceflight and ground-based models of spaceflight. J. Bone Miner. Res. 2011, 26, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Zwart, S.R.; Morgan, J.L.; Smith, S.M. Iron status and its relations with oxidative damage and bone loss during long-duration space flight on the International Space Station. Am. J. Clin. Nutr. 2013, 98, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Heer, M.; Wang, Z.; Huntoon, C.L.; Zwart, S.R. Long-duration space flight and bed rest effects on testosterone and other steroids. J. Clin. Endocrinol. Metab. 2012, 97, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; McCoy, T.; Gazda, D.; Morgan, J.L.; Heer, M.; Zwart, S.R. Space flight calcium: Implications for astronaut health, spacecraft operations, and Earth. Nutrients 2012, 4, 2047–2068. [Google Scholar] [CrossRef] [PubMed]
- Zwart, S.R.; Kala, G.; Smith, S.M. Body iron stores and oxidative damage in humans increased during and after a 10- to 12-day undersea dive. J. Nutr. 2009, 139, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, A.; Matsumoto, T.; Jones, J.; Shapiro, J.; Lang, T.; Shackelford, L.; Smith, S.M.; Evans, H.; Spector, E.; Ploutz-Snyder, R.; et al. Bisphosphonates as a supplement to exercise to protect bone during long-duration spaceflight. Osteoporos. Int. 2013, 24, 2105–2114. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Wastney, M.E.; Morukov, B.V.; Larina, I.M.; Nyquist, L.E.; Abrams, S.A.; Taran, E.N.; Shih, C.Y.; Nillen, J.L.; Davis-Street, J.E.; et al. Calcium metabolism before, during, and after a 3-mo spaceflight: Kinetic and biochemical changes. Am. J. Physiol. 1999, 277, R1–R10. [Google Scholar] [PubMed]
- Smith, S.M.; Wastney, M.E.; O'Brien, K.O.; Morukov, B.V.; Larina, I.M.; Abrams, S.A.; Davis-Street, J.E.; Oganov, V.; Shackelford, L.C. Bone markers, calcium metabolism, and calcium kinetics during extended-duration space flight on the Mir space station. J. Bone Miner. Res. 2005, 20, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Spector, E.R.; Smith, S.M.; Sibonga, J.D. Skeletal effects of long-duration head-down bed rest. Aviat. Space Environ. Med. 2009, 80, A23–A28. [Google Scholar] [CrossRef] [PubMed]
- Meck, J.V.; Dreyer, S.A.; Warren, L.E. Long-duration head-down bed rest: Project overview, vital signs, and fluid balance. Aviat. Space Environ. Med. 2009, 80, A1–A8. [Google Scholar] [CrossRef] [PubMed]
- Silver, B.B. Development of cellular magnesium nano-analysis in treatment of clinical magnesium deficiency. J. Am. Coll. Nutr. 2004, 23, 732S–737S. [Google Scholar] [CrossRef] [PubMed]
- Pokan, R.; Hofmann, P.; von Duvillard, S.P.; Smekal, G.; Wonisch, M.; Lettner, K.; Schmid, P.; Shechter, M.; Silver, B.; Bachl, N. Oral magnesium therapy, exercise heart rate, exercise tolerance, and myocardial function in coronary artery disease patients. Br. J. Sports Med. 2006, 40, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Shechter, M.; Saad, T.; Shechter, A.; Koren-Morag, N.; Silver, B.B.; Matetzky, S. Comparison of magnesium status using X-ray dispersion analysis following magnesium oxide and magnesium citrate treatment of healthy subjects. Magnes. Res. 2012, 25, 28–39. [Google Scholar] [PubMed]
- Haigney, M.C.; Berger, R.; Schulman, S.; Gerstenblith, G.; Tunin, C.; Silver, B.; Silverman, H.S.; Tomaselli, G.; Calkins, H. Tissue magnesium levels and the arrhythmic substrate in humans. J. Cardiovasc. Electrophysiol. 1997, 8, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Shechter, M.; Sharir, M.; Labrador, M.J.; Forrester, J.; Silver, B.; Bairey Merz, C.N. Oral magnesium therapy improves endothelial function in patients with coronary artery disease. Circulation 2000, 102, 2353–2358. [Google Scholar] [CrossRef] [PubMed]
- Topf, J.M.; Murray, P.T. Hypomagnesemia and hypermagnesemia. Rev. Endocr. Metab. Disord. 2003, 4, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Leach Huntoon, C.S.; Grigoriev, A.I.; Natochin, Y.V. Fluid and Electrolyte Regulation in Spaceflight; Univelt, Inc.: San Diego, CA, USA, 1998; Volume 94, p. 219. [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; National Academy Press: Washington, DC, USA, 1997. [Google Scholar]
- Smith, S.M.; Abrams, S.A.; Davis-Street, J.E.; Heer, M.; O'Brien, K.O.; Wastney, M.E.; Zwart, S.R. Fifty years of human space travel: Implications for bone and calcium research. Annu. Rev. Nutr. 2014, 34, 377–400. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, A.D.; Spector, E.R.; Evans, H.J.; Sibonga, J.D. Skeletal responses to space flight and the bed rest analog: A review. J. Musculoskelet. Neuronal Interact. 2007, 7, 33–47. [Google Scholar] [PubMed]
- Inniss, A.M.; Rice, B.L.; Smith, S.M. Dietary support of long-duration head-down bed rest. Aviat. Space Environ. Med. 2009, 80 (Suppl. 5), A9–A14. [Google Scholar] [CrossRef] [PubMed]
- Trappe, S.; Costill, D.; Gallagher, P.; Creer, A.; Peters, J.R.; Evans, H.; Riley, D.A.; Fitts, R.H. Exercise in space: Human skeletal muscle after 6 months aboard the International Space Station. J. Appl. Physiol. 2009, 106, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Speich, M.; Pineau, A.; Ballereau, F. Minerals, trace elements and related biological variables in athletes and during physical activity. Clin. Chim. Acta 2001, 312, 1–11. [Google Scholar] [CrossRef]
- Leach, C.S.; Leonard, J.I.; Rambaut, P.C.; Johnson, P.C. Evaporative water loss in man in a gravity-free environment. J. Appl. Physiol. 1978, 45, 430–436. [Google Scholar] [PubMed]
- Fortney, S.M.; Mikhaylov, V.; Lee, S.M.; Kobzev, Y.; Gonzalez, R.R.; Greenleaf, J.E. Body temperature and thermoregulation during submaximal exercise after 115-day spaceflight. Aviat. Space Environ. Med. 1998, 69, 137–141. [Google Scholar] [PubMed]
- Lee, S.M.C.; Williams, W.J.; Schneider, S.M. Role of skin blood flow and sweating rate in exercise thermoregulation after bed rest. J. Appl. Physiol. 2002, 92, 2026–2034. [Google Scholar] [CrossRef] [PubMed]
- Kupetsky-Rincon, E.A.; Uitto, J. Magnesium: Novel applications in cardiovascular disease—A review of the literature. Ann. Nutr. Metab. 2012, 61, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Kass, L.; Weekes, J.; Carpenter, L. Effect of magnesium supplementation on blood pressure: A meta-analysis. Eur. J. Clin. Nutr. 2012, 66, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Crucian, B.E.; Zwart, S.R.; Mehta, S.; Uchakin, P.; Quiriarte, H.D.; Pierson, D.; Sams, C.F.; Smith, S.M. Plasma cytokine concentrations indicate that in vivo hormonal regulation of immunity is altered during long-duration spaceflight. J. Interferon Cytokine Res. 2014, 34, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Crucian, B.; Stowe, R.; Mehta, S.; Uchakin, P.; Quiriarte, H.; Pierson, D.; Sams, C. Immune system dysregulation occurs during short duration spaceflight on board the space shuttle. J. Clin. Immunol. 2013, 33, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Norsk, P.; Asmar, A.; Damgaard, M.; Christensen, N.J. Fluid shifts, vasodilatation and ambulatory blood pressure reduction during long duration spaceflight. J. Physiol. 2015, 593, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Leach, C.S.; Alfrey, C.P.; Suki, W.N.; Leonard, J.I.; Rambaut, P.C.; Inners, L.D.; Smith, S.M.; Lane, H.W.; Krauhs, J.M. Regulation of body fluid compartments during short-term spaceflight. J. Appl. Physiol. 1996, 81, 105–116. [Google Scholar] [PubMed]
- Fu, Q.; Levine, B.D.; Pawelczyk, J.A.; Ertl, A.C.; Diedrich, A.; Cox, J.F.; Zuckerman, J.H.; Ray, C.A.; Smith, M.L.; Iwase, S.; et al. Cardiovascular and sympathetic neural responses to handgrip and cold pressor stimuli in humans before, during and after spaceflight. J. Physiol. 2002, 544, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Maaß, H.; Raabe, W.; Wegmann, H.M. Effects of microgravity on glucose tolerance. In Proceedings of the Norderney Symposium on Scientific Results of the German Spacelab Mission D-2; Sahm, P.R., Keller, M.H., Schiewe, B., Eds.; Wissenschaftliche Projectführung D-2: Norderney, Germany, 1995; pp. 732–735. [Google Scholar]
- Stein, T.P.; Schulter, M.D.; Boden, G. Development of insulin resistance by astronauts during spaceflight. Aviat. Space Environ. Med. 1994, 65, 1091–1096. [Google Scholar] [PubMed]
- Biolo, G.; Ciocchi, B.; Stulle, M.; Piccoli, A.; Lorenzon, S.; Dal Mas, V.; Barazzoni, R.; Zanetti, M.; Guarnieri, G. Metabolic consequences of physical inactivity. J. Ren. Nutr. 2005, 15, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Vernikos-Danellis, J.; Leach, C.S.; Winget, C.M.; Goodwin, A.L.; Rambaut, P.C. Changes in glucose, insulin, and growth hormone levels associated with bedrest. Aviat. Space Environ. Med. 1976, 47, 583–587. [Google Scholar] [PubMed]
- Stuart, C.A.; Shangraw, R.E.; Prince, M.J.; Peters, E.J.; Wolfe, R.R. Bed-rest-induced insulin resistance occurs primarily in muscle. Metabolism 1988, 37, 802–806. [Google Scholar] [CrossRef]
- Clarkson, P.M.; Haymes, E.M. Exercise and mineral status of athletes: Calcium, magnesium, phosphorus, and iron. Med. Sci. Sports Exerc. 1995, 27, 831–843. [Google Scholar] [PubMed]
- Lutwak, L.; Whedon, G.D.; Lachance, P.A.; Reid, J.M.; Lipscomb, H.S. Mineral, electrolyte and nitrogen balance studies of the Gemini-VII fourteen-day orbital space flight. J. Clin. Endocrinol. Metab. 1969, 29, 1140–1156. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.L.; Skulan, J.L.; Gordon, G.W.; Romaniello, S.J.; Smith, S.M.; Anbar, A.D. Rapidly assessing changes in bone mineral balance using natural stable calcium isotopes. Proc. Natl. Acad. Sci. USA 2012, 109, 9989–9994. [Google Scholar] [CrossRef] [PubMed]
- Skulan, J.; Bullen, T.; Anbar, A.D.; Puzas, J.E.; Shackelford, L.; LeBlanc, A.; Smith, S.M. Natural calcium isotopic composition of urine as a marker of bone mineral balance. Clin. Chem. 2007, 53, 1155–1158. [Google Scholar] [CrossRef] [PubMed]
- Channon, M.B.; Gordon, G.W.; Morgan, J.L.; Skulan, J.L.; Smith, S.M.; Anbar, A.D. Using natural, stable calcium isotopes of human blood to detect and monitor changes in bone mineral balance. Bone 2015, 77, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Agus, Z.S. Hypomagnesemia. J. Am. Soc. Nephrol. 1999, 10, 1616–1622. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, S.M.; Zwart, S.R. Magnesium and Space Flight. Nutrients 2015, 7, 10209-10222. https://doi.org/10.3390/nu7125528
Smith SM, Zwart SR. Magnesium and Space Flight. Nutrients. 2015; 7(12):10209-10222. https://doi.org/10.3390/nu7125528
Chicago/Turabian StyleSmith, Scott M., and Sara R. Zwart. 2015. "Magnesium and Space Flight" Nutrients 7, no. 12: 10209-10222. https://doi.org/10.3390/nu7125528
APA StyleSmith, S. M., & Zwart, S. R. (2015). Magnesium and Space Flight. Nutrients, 7(12), 10209-10222. https://doi.org/10.3390/nu7125528



