Quantification of in Vivo Colonic Short Chain Fatty Acid Production from Inulin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Design
2.3. Analytical Procedures
2.3.1. Analysis of Breath Samples
2.3.2. Analysis of 13C Enrichment in Plasma Samples
2.3.3. Analysis of Butyrate Producing Capacity in Fecal Samples
| Standard Curve | DNA Source | Oligonucleotide | Sequence (5′–3′) |
|---|---|---|---|
| Clostridium Cluster IV | Butyricicoccus pullicaecorum | Forward primer | AGTACGGCCGCAAGGTTGAAA |
| Reverse primer | CTGCCATTGTAGTACGTGTG | ||
| Clostridium Cluster XIV | Butyricicoccus pullicaecorum | Forward primer | TGACCGGCCACATTGGGACTG |
| Reverse primer | TCATCCCCACCTTCCTCCAG | ||
| Butyryl-CoA:acetate-CoA transferase | Butyricicoccus pullicaecorum | Forward primer | AATCCGGAGACTGGGTAGAT |
| Reverse primer | GGACAGATAAGCTCCGAGC | ||
| Butyrate kinase | Clostridium perfringens | Forward primer | TGGGGGAGGAAAGTTATATGGC |
| Reverse primer | CTCCTACTGAAACTCCGCCC |
2.4. Calculations
2.5. Statistics
3. Results
3.1. Study Population
| Men | Women | p Value | |
|---|---|---|---|
| N | 5 | 7 | |
| Age (year) | 27 ± 8 | 24 ± 4 | 0.530 |
| Length (m) | 1.82 ± 0.09 | 1.65 ± 0.06 | 0.003 |
| Weight (kg) | 78 ± 8 | 57 ± 4 | 0.005 |
| Body mass index (kg/m2) | 24 ± 4 | 21 ± 2 | 0.149 |
| log butyrate kinase (copies/g feces) | 3.83 ± 0.79 | 3.97 ± 0.80 * | 0.792 |
| log butyryl-CoA:acetate-CoA transferase (copies/g feces) | 8.14 ± 0.49 | 7.85 ± 0.66 * | 0.429 |
| log Clostridium cluster IV (copies/g feces) | 8.80 ± 0.58 | 8.67 ± 0.60 * | 0.662 |
| log Clostridium cluster XIV (copies/g feces) | 9.72 ± 0.31 | 9.52 ± 0.47 * | 0.537 |
3.2. Steady-State Characteristics
| Acetate | Propionate | Butyrate | p-Value | ||
|---|---|---|---|---|---|
| Isotopic enrichment (APE) | Before inulin fermentation | 0.86 ± 0.36 a | 2.53 ± 0.74 b | 1.03 ± 0.47 a | < 0.001 |
| Minimum during fermentation | 0.48 ± 0.20 c | 1.60 ± 0.59 d | 0.61 ± 0.27 c | < 0.001 | |
| Ra (μmol·kg−1·min−1) | Before inulin fermentation | 13.26 ± 4.82 e | 0.27 ± 0.09 f | 0.28 ± 0.12 f | < 0.001 |
| Maximum during fermentation | 24.98 ± 10.70 g | 0.47 ± 0.23 h | 0.50 ± 0.29 h | < 0.001 |
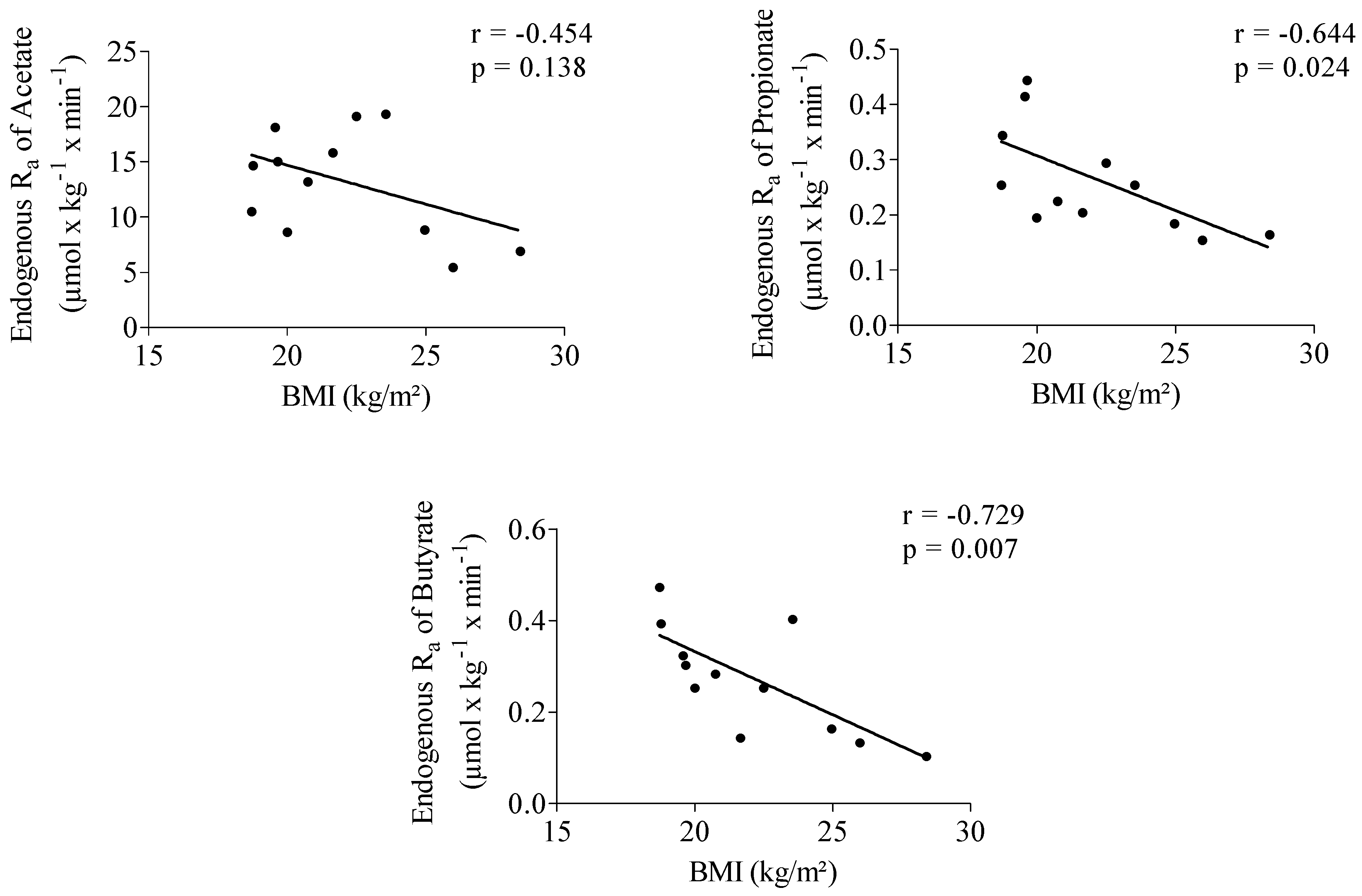
3.3. Impact of Inulin on Rate of Appearance of SCFA
3.3.1. Start of Fermentation
3.3.2. SCFA Enrichment in Plasma and Rate of SCFA Appearance
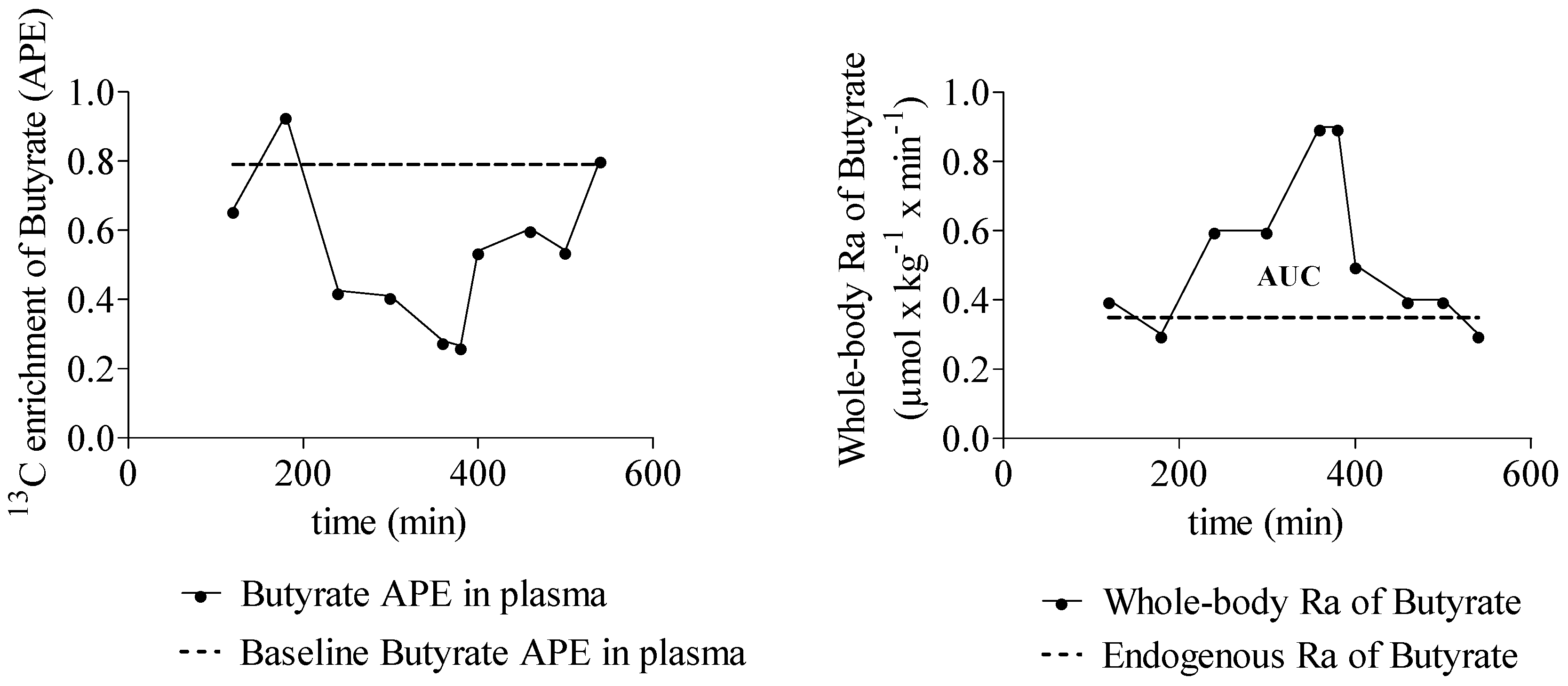
3.3.3. Quantification of SCFA Production from Inulin
| Acetate | Propionate | Butyrate | |
|---|---|---|---|
| Cumulative amount SCFA in plasma (μmol/kg) | 860 ± 497 | 17 ± 13 | 16 ± 15 |
| Peripheral SCFA (mmol) | 55 ± 30 | 1.1 ± 0.9 | 1.0 ± 0.9 |
| Colonic SCFA (mmol) * | 137 ± 75 | 11 ± 9 | 20 ± 17 |
| Ratio (%) ** | 82 | 6 | 12 |
3.3.4. Butyrate-Producing Capacity
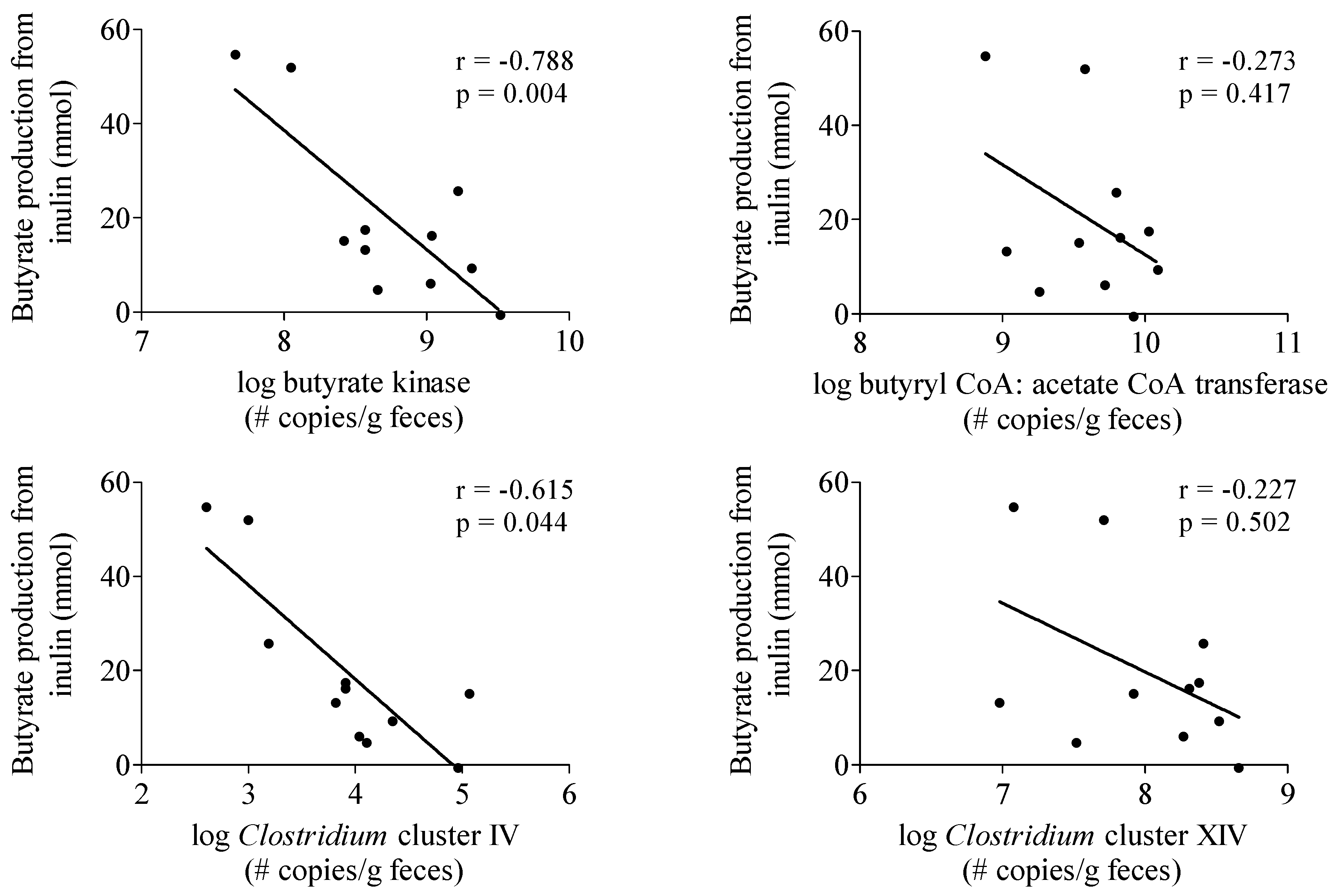
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cummings, J.H.; Pomare, E.W.; Branch, W.J.; Naylor, C.P.; Macfarlane, G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987, 28, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, N.; Duncan, S.H.; Young, P.; Belenguer, A.; McWilliam Leitch, C.; Scott, K.P.; Flint, H.J.; Louis, P. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014, 8, 1323–1335. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Duncan, S.H.; McCrae, S.I.; Millar, J.; Jackson, M.S.; Flint, H.J. Restricted distribution of the butyrate kinase pathway among butyrate-producing bacteria from the human colon. J. Bacteriol. 2004, 186, 2099–2106. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Roediger, W.E. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut 1980, 21, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Bloemen, J.G.; Venema, K.; van de Poll, M.C.; Olde Damink, S.W.; Buurman, W.A.; Dejong, C.H. Short chain fatty acids exchange across the gut and liver in humans measured at surgery. Clin. Nutr. 2009, 28, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Hellerstein, M.K.; Christiansen, M.; Kaempfer, S.; Kletke, C.; Wu, K.; Reid, J.S.; Mulligan, K.; Hellerstein, N.S.; Shackleton, C.H. Measurement of de novo hepatic lipogenesis in humans using stable isotopes. J. Clin. Investig. 1991, 87, 1841–1852. [Google Scholar] [CrossRef] [PubMed]
- Hellman, L.; Rosenfeld, R.S.; Gallagher, T.F. Cholesterol synthesis from C14-acetate in man. J. Clin. Investig. 1954, 33, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Raiford, R.L.; Wong, H.Y. In vivo incorporation of acetate-1-C14 into cholesterol and fatty acids following testosterone propionate administration. Circ. Res. 1962, 11, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M.; Spadafora, P.; Eshuis, H. Interaction between colonic acetate and propionate in humans. Am. J. Clin. Nutr. 1991, 53, 681–687. [Google Scholar] [PubMed]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [PubMed]
- Tedelind, S.; Westberg, F.; Kjerrulf, M.; Vidal, A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: A study with relevance to inflammatory bowel disease. World J. Gastroenterol. 2007, 13, 2826–2832. [Google Scholar] [PubMed]
- Hayden, M.S.; West, A.P.; Ghosh, S. NF-κB and the immune response. Oncogene 2006, 25, 6758–6780. [Google Scholar] [CrossRef] [PubMed]
- Meijer, K.; Vonk, R.J.; Priebe, M.G.; Roelofsen, H. Cell-based screening assay for anti-inflammatory activity of bioactive compounds. Food Chem. 2015, 166, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Gurav, A.; Sivaprakasam, S.; Bhutia, Y.D.; Boettger, T.; Singh, N.; Ganapathy, V. Slc5a8, a Na+-coupled high-affinity transporter for short-chain fatty acids, is a conditional tumor suppressor in colon that protects against colitis and colon cancer under low-fiber dietary conditions. Biochem. J. 2015, in press. [Google Scholar] [CrossRef] [PubMed]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Kashihara, D.; Ichimura, A.; Kimura, I.; Tsujimoto, G.; Hirasawa, A. Role of free fatty acid receptors in the regulation of energy metabolism. Biochim. Biophys. Acta 2014, 1841, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Backhed, F.; Mithieux, G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Leng, R.A.; Steel, J.W.; Luick, J.R. Contribution of propionate to glucose synthesis in sheep. Biochem. J. 1967, 103, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Den Besten, G.; Lange, K.; Havinga, R.; van Dijk, T.H.; Gerding, A.; van Eunen, K.; Muller, M.; Groen, A.K.; Hooiveld, G.J.; Bakker, B.M.; et al. Gut-derived short-chain fatty acids are vividly assimilated into host carbohydrates and lipids. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G900–G910. [Google Scholar] [CrossRef] [PubMed]
- Den Besten, G.; Bleeker, A.; Gerding, A.; van Eunen, K.; Havinga, R.; van Dijk, T.H.; Oosterveer, M.H.; Jonker, J.W.; Groen, A.K.; Reijngoud, D.J.; et al. Short-chain fatty acids protect against high-fat diet-induced obesity via a ppargamma-dependent switch from lipogenesis to fat oxidation. Diabetes 2015, 64, 2398–2408. [Google Scholar] [CrossRef] [PubMed]
- Geypens, B.; Bennink, R.; Peeters, M.; Evenepoel, P.; Mortelmans, L.; Maes, B.; Ghoos, Y.; Rutgeerts, P. Validation of the lactose-[13C]ureide breath test for determination of orocecal transit time by scintigraphy. J. Nucl. Med. 1999, 40, 1451–1455. [Google Scholar] [PubMed]
- Morrison, D.J.; Cooper, K.; Waldron, S.; Slater, C.; Weaver, L.T.; Preston, T. A streamlined approach to the analysis of volatile fatty acids and its application to the measurement of whole-body flux. Rapid Commun. Mass Spectrom. 2004, 18, 2593–2600. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, K.; Ferchaud-Roucher, V.; Preston, T.; Small, A.C.; Henckaerts, L.; Krempf, M.; Wang, H.; Vonk, R.J.; Priebe, M.G. Influence of the type of indigestible carbohydrate on plasma and urine short-chain fatty acid profiles in healthy human volunteers. Eur. J. Clin. Nutr. 2010, 64, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Matsuki, T.; Watanabe, K.; Fujimoto, J.; Takada, T.; Tanaka, R. Use of 16s rRNA gene-targeted group-specific primers for real-time PCR analysis of predominant bacteria in human feces. Appl. Environ. Microbiol. 2004, 70, 7220–7228. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J. Development of a semiquantitative degenerate real-time PCR-based assay for estimation of numbers of butyryl-coenzyme a (COA) COA transferase genes in complex bacterial samples. Appl. Environ. Microbiol. 2007, 73, 2009–2012. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Liu, C.; Finegold, S.M. Real-time PCR quantitation of clostridia in feces of autistic children. Appl. Environ. Microbiol. 2004, 70, 6459–6465. [Google Scholar] [CrossRef] [PubMed]
- Vital, M.; Penton, C.R.; Wang, Q.; Young, V.B.; Antonopoulos, D.A.; Sogin, M.L.; Morrison, H.G.; Raffals, L.; Chang, E.B.; Huffnagle, G.B.; et al. A gene-targeted approach to investigate the intestinal butyrate-producing bacterial community. Microbiome 2013, 1, 8. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, R.I.; Whiteley, A.S.; O’Donnell, A.G.; Bailey, M.J. Rapid method for coextraction of DNA and RNA from natural environments for analysis of ribosomal DNA- and rRNA-based microbial community composition. Appl. Environ. Microbiol. 2000, 66, 5488–5491. [Google Scholar] [CrossRef] [PubMed]
- Pouteau, E.; Piloquet, H.; Maugeais, P.; Champ, M.; Dumon, H.; Nguyen, P.; Krempf, M. Kinetic aspects of acetate metabolism in healthy humans using [1-13C]acetate. Am. J. Physiol. 1996, 271, E58–E64. [Google Scholar] [PubMed]
- Pouteau, E.; Vahedi, K.; Messing, B.; Flourie, B.; Nguyen, P.; Darmaun, D.; Krempf, M. Production rate of acetate during colonic fermentation of lactulose: A stable-isotope study in humans. Am. J. Clin. Nutr. 1998, 68, 1276–1283. [Google Scholar] [PubMed]
- Vogt, J.A.; Ishii-Schrade, K.B.; Pencharz, P.B.; Wolever, T.M. l-Rhamnose increases serum propionate after long-term supplementation, but lactulose does not raise serum acetate. Am. J. Clin. Nutr. 2004, 80, 1254–1261. [Google Scholar] [PubMed]
- Cook; Sellin. Review article: Short chain fatty acids in health and disease. Aliment. Pharmacol. Ther. 1998, 12, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Van der Beek, C.M.; Bloemen, J.G.; van den Broek, M.A.; Lenaerts, K.; Venema, K.; Buurman, W.A.; Dejong, C.H. Hepatic uptake of rectally administered butyrate prevents an increase in systemic butyrate concentrations in humans. J. Nutr. 2015, 145, 2019–2024. [Google Scholar] [CrossRef] [PubMed]
- De Preter, V.; Falony, G.; Windey, K.; Hamer, H.M.; De Vuyst, L.; Verbeke, K. The prebiotic, oligofructose-enriched inulin modulates the faecal metabolite profile: An in vitro analysis. Mol. Nutr. Food Res. 2010, 54, 1791–1801. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.L.; Timm, D.A.; Slavin, J.L. Fructooligosaccharides exhibit more rapid fermentation than long-chain inulin in an in vitro fermentation system. Nutr. Res. 2008, 28, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Marteau, P.; Pochart, P.; Dore, J.; Bera-Maillet, C.; Bernalier, A.; Corthier, G. Comparative study of bacterial groups within the human cecal and fecal microbiota. Appl. Environ. Microbiol. 2001, 67, 4939–4942. [Google Scholar] [PubMed]
- Possemiers, S.; Verthe, K.; Uyttendaele, S.; Verstraete, W. PCR-DGGE-based quantification of stability of the microbial community in a simulator of the human intestinal microbial ecosystem. FEMS Microbiol. Ecol. 2004, 49, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Grootaert, C.; Van den Abbeele, P.; Marzorati, M.; Broekaert, W.F.; Courtin, C.M.; Delcour, J.A.; Verstraete, W.; Van de Wiele, T. Comparison of prebiotic effects of arabinoxylan oligosaccharides and inulin in a simulator of the human intestinal microbial ecosystem. FEMS Microbiol. Ecol. 2009, 69, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Reimer, R.A.; Maathuis, A.J.; Venema, K.; Lyon, M.R.; Gahler, R.J.; Wood, S. Effect of the novel polysaccharide polyglycoplex(r) on short-chain fatty acid production in a computer-controlled in vitro model of the human large intestine. Nutrients 2014, 6, 1115–1127. [Google Scholar] [PubMed]
- McOrist, A.L.; Miller, R.B.; Bird, A.R.; Keogh, J.B.; Noakes, M.; Topping, D.L.; Conlon, M.A. Fecal butyrate levels vary widely among individuals but are usually increased by a diet high in resistant starch. J. Nutr. 2011, 141, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Bergman, E.N.; Wolff, J.E. Metabolism of volatile fatty acids by liver and portal-drained viscera in sheep. Am. J. Physiol. 1971, 221, 586–592. [Google Scholar] [PubMed]
- Alam, M.K.; Sasaki, M.; Al-Mamun, M.; Sano, H. Plasma acetate turnover rate and rumen fermentation characteristics in sheep fed rice straw supplemented with soybean meal. J. Animal Sci. Adv. 2013, 3, 65–73. [Google Scholar] [CrossRef]
- Wolfe, R.R. Radioactive and stable isotope tracers in biomedicine. In Principles and Practice of Kinetic Analysis; Wiley-Liss: New York, NY, USA, 1992. [Google Scholar]
- Ferchaud-Roucher, V.; Pouteau, E.; Piloquet, H.; Zair, Y.; Krempf, M. Colonic fermentation from lactulose inhibits lipolysis in overweight subjects. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E716–E720. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Walter, J.H.; Thompson, G.N.; Leonard, J.V.; Heatherington, C.S.; Bartlett, K. Measurement of propionate turnover in vivo using sodium [2H5]propionate and sodium [13C]propionate. Clin. Chim. Acta Int. J. Clin. Chem. 1989, 182, 141–150. [Google Scholar] [CrossRef]
- Pouteau, E.; Rochat, F.; Jann, A.; Meirim, I.; Sanchez-Garcia, J.L.; Ornstein, K.; German, B.; Ballevre, O. Chicory increases acetate turnover, but not propionate and butyrate peripheral turnovers in rats. Br. J. Nutr. 2008, 99, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.A.; Shewry, P.R.; Li, L.; Gibson, G.R.; Sanz, M.L.; Rastall, R.A. In vitro fermentation by human fecal microflora of wheat arabinoxylans. J. Agric. Food Chem. 2007, 55, 4589–4595. [Google Scholar] [CrossRef] [PubMed]
- Velázquez, M.; Davies, C.; Marett, R.; Slavin, J.L.; Feirtag, J.M. Effect of oligosaccharides and fibre substitutes on short-chain fatty acid production by human faecal microflora. Anaerobe 2000, 6, 87–92. [Google Scholar] [CrossRef]
- Timm, D.A.; Stewart, M.L.; Hospattankar, A.; Slavin, J.L. Wheat dextrin, psyllium, and inulin produce distinct fermentation patterns, gas volumes, and short-chain fatty acid profiles in vitro. J. Med. Food 2010, 13, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Rycroft, C.E.; Jones, M.R.; Gibson, G.R.; Rastall, R.A. A comparative in vitro evaluation of the fermentation properties of prebiotic oligosaccharides. J. Appl. Microbiol. 2001, 91, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Gomez, E.; Tuohy, K.M.; Gibson, G.R.; Klinder, A.; Costabile, A. In vitro evaluation of the fermentation properties and potential prebiotic activity of agave fructans. J. Appl. Microbiol. 2010, 108, 2114–2121. [Google Scholar] [PubMed]
- Noack, J.; Timm, D.; Hospattankar, A.; Slavin, J. Fermentation profiles of wheat dextrin, inulin and partially hydrolyzed guar gum using an in vitro digestion pretreatment and in vitro batch fermentation system model. Nutrients 2013, 5, 1500–1510. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gibson, G.R. Effects of the in vitro fermentation of oligofructose and inulin by bacteria growing in the human large intestine. J. Appl. Bacteriol. 1993, 75, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Van den Abbeele, P.; Venema, K.; Van de Wiele, T.; Verstraete, W.; Possemiers, S. Different human gut models reveal the distinct fermentation patterns of arabinoxylan versus inulin. J. Agric. Food Chem. 2013, 61, 9819–9827. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.G.; Pomare, E.W.; Fisher, C.A. Portal and peripheral blood short chain fatty acid concentrations after caecal lactulose instillation at surgery. Gut 1992, 33, 1249–1252. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schwiertz, A.; Taras, D.; Schafer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 2010, 18, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Rahat-Rozenbloom, S.; Fernandes, J.; Gloor, G.B.; Wolever, T.M. Evidence for greater production of colonic short-chain fatty acids in overweight than lean humans. Int. J. Obes. (Lond.) 2014, 38, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.; Su, W.; Rahat-Rozenbloom, S.; Wolever, T.M.; Comelli, E.M. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr. Diabetes 2014, 4, e121. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.V.; Frassetto, A.; Kowalik, E.J., Jr.; Nawrocki, A.R.; Lu, M.M.; Kosinski, J.R.; Hubert, J.A.; Szeto, D.; Yao, X.; Forrest, G.; et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS ONE 2012, 7, e35240. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boets, E.; Deroover, L.; Houben, E.; Vermeulen, K.; Gomand, S.V.; Delcour, J.A.; Verbeke, K. Quantification of in Vivo Colonic Short Chain Fatty Acid Production from Inulin. Nutrients 2015, 7, 8916-8929. https://doi.org/10.3390/nu7115440
Boets E, Deroover L, Houben E, Vermeulen K, Gomand SV, Delcour JA, Verbeke K. Quantification of in Vivo Colonic Short Chain Fatty Acid Production from Inulin. Nutrients. 2015; 7(11):8916-8929. https://doi.org/10.3390/nu7115440
Chicago/Turabian StyleBoets, Eef, Lise Deroover, Els Houben, Karen Vermeulen, Sara V. Gomand, Jan A. Delcour, and Kristin Verbeke. 2015. "Quantification of in Vivo Colonic Short Chain Fatty Acid Production from Inulin" Nutrients 7, no. 11: 8916-8929. https://doi.org/10.3390/nu7115440
APA StyleBoets, E., Deroover, L., Houben, E., Vermeulen, K., Gomand, S. V., Delcour, J. A., & Verbeke, K. (2015). Quantification of in Vivo Colonic Short Chain Fatty Acid Production from Inulin. Nutrients, 7(11), 8916-8929. https://doi.org/10.3390/nu7115440






