Effect of Probiotic Supplement on Cytokine Levels in HIV-Infected Individuals: A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Design
2.3. Preparation of Serum Samples
2.4. Biochemical Analyses
2.5. Virologic and Immunologic Markers
2.6. Preparation of PBMC
2.7. mRNA Extraction and Reverse Transcription-Polymerase Chain Reaction Analysis
2.8. Cytokine Measurements
2.9. Statistical Analysis
3. Results
3.1. Study Population
| Patients characteristics | |
|---|---|
| Age (year), mean ± SD | 42.3 ± 11.3 |
| CDC stage, n (%) | |
| A | 20 (66.7) |
| B | 8 (26.7) |
| C | 2 (6.6) |
| Risk factor, n (%) | |
| Heterosexual | 12 (40.0) |
| Homosexual | 11 (36.7) |
| IDU | 7 (23.3) |
| Time from diagnosis (months), median (IQR) | 69 (42–110) |
| Time of treatment (months), median (IQR) | 47 (13–87) |
3.2. Effects on Blood Metabolic Markers
3.3. Effects of LcS on Circulating T Lymphocyte Subtype Counts
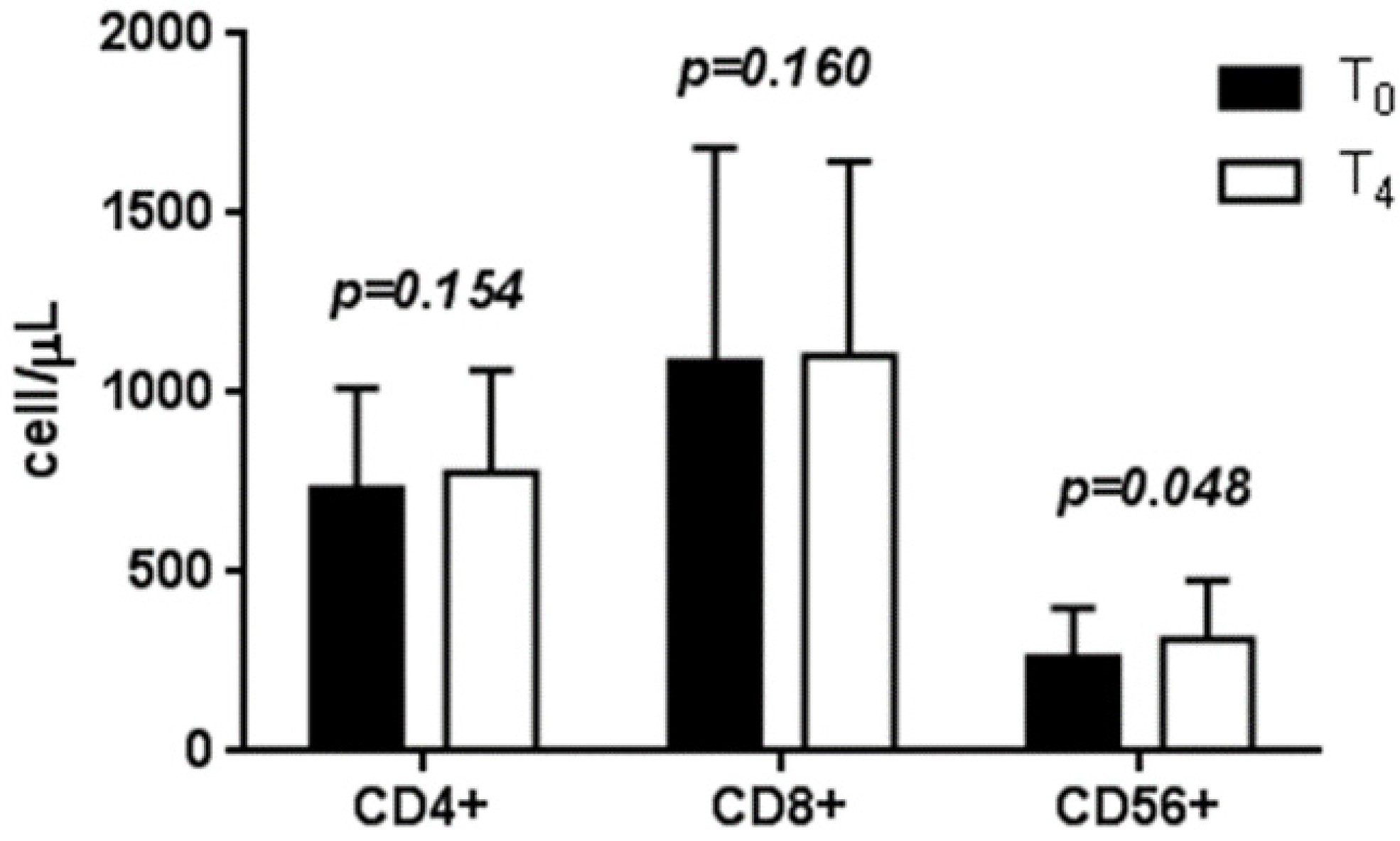
3.4. Serum Cytokine Levels before and after LcS Intake
| Cytokines (pg/mL) | T0 | T4 | (T4-T0) | p-value a |
|---|---|---|---|---|
| TGF-β | 4162.1 ± 2325.9 | 1345.4 ± 290.5 | −2816.7 ± 2097.5 | 0.417 |
| IL-23 | 9.0 ± 0.7 | 9.8 ± 1.1 | 0.8 ± 1.5 | 0.037 |
| IL-4 | 3.6 ± 1.0 | 5.6 ± 2.1 | 1.6 ± 1.9 | 0.433 |
| IL-10 | 4.7 ± 1.9 | 4.4 ± 0.9 | −0.4 ± 1.7 | 0.619 |
| IL-12 | 938.1 ± 259.6 | 874.1 ± 233.3 | −44.5 ± 75.5 | 0.104 |
3.5. Effects on Cytokine Expression
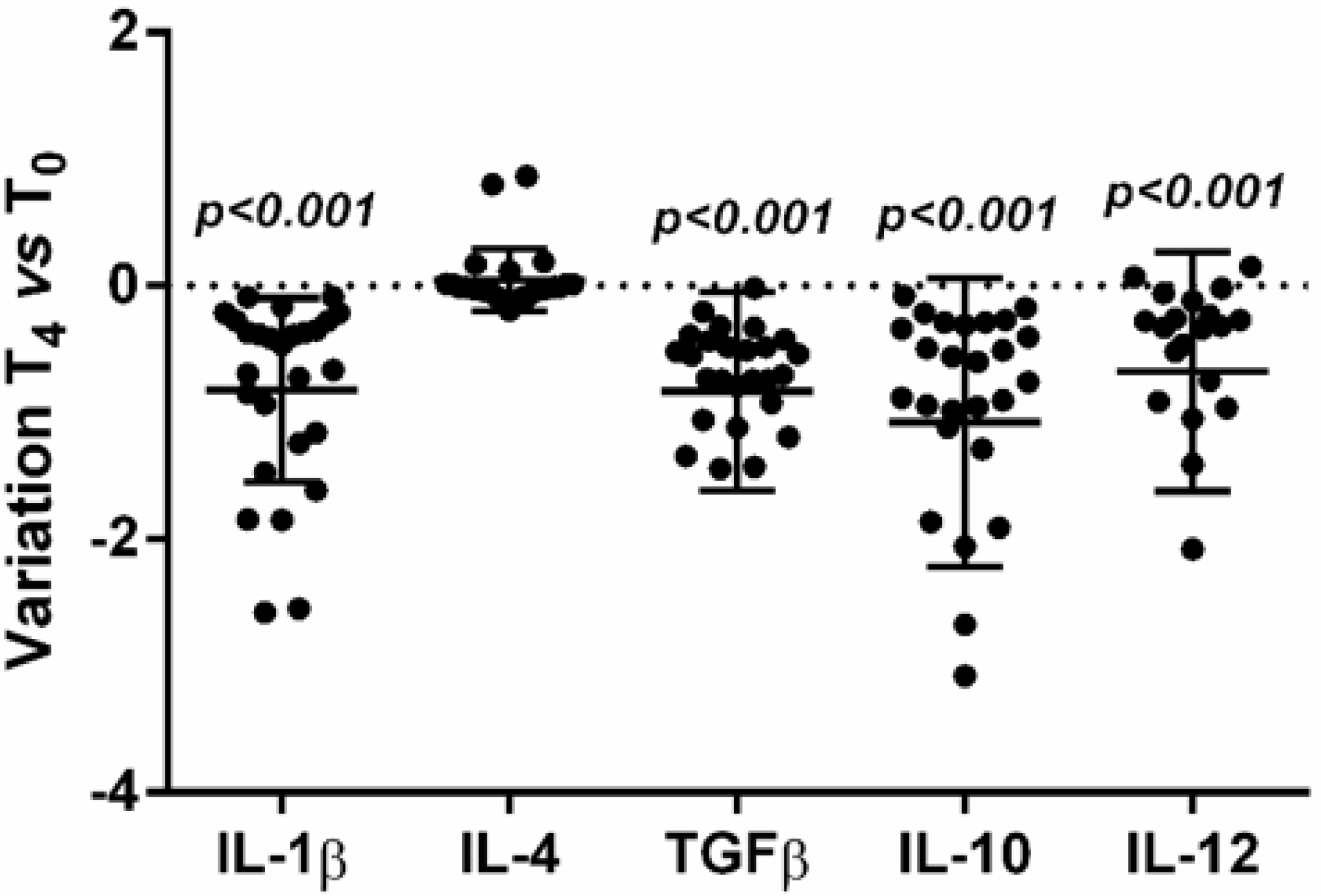
3.6. Effects of LcS on Cytokine Production by PBMC
| Cytokines (pg/mL) | T0 | T4 | (T4-T0) | p-value a |
|---|---|---|---|---|
| TGF-β | 25,042.2 ± 1614.2 | 25,984.0 ± 1924.6 | 941.8 ± 1438.2 | 0.004 |
| IL-23 | 11.5 ± 0.8 | 10.1 ± 1.0 | −1.4 ± 0.6 | 0.989 |
| IL-4 | 33.4 ± 25.1 | 35.6 ± 25.3 | 2.2 ± 2.4 | 0.398 |
| IL-10 | 14.4 ± 6.5 | 16.6 ± 5.7 | 2.2 ± 4.5 | 0.424 |
3.7. Th1/Th2 Ratio
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pirs, M.; Jug, B.; Erzen, B.; Sabovic, M.; Karner, P.; Poljak, M.; Tomazic, J. Cardiovascular risk assessment in HIV-infected male patients: A comparison of framingham, score, procam and dad risk equations. Acta Dermatovenerol. Alp. Pannonica Adriat. 2014, 23, 43–47. [Google Scholar] [PubMed]
- Falasca, K.; Ucciferri, C.; Manzoli, L.; Mancino, P.; Pizzigallo, E.; Conti, P.; Vecchiet, J. Metabolic syndrome and cardiovascular risk in HIV-infected patients with lipodystrophy. Int. J. Immunopathol. Pharmacol. 2007, 20, 519–527. [Google Scholar] [PubMed]
- Teofili, L.; Iachininoto, M.G.; Capodimonti, S.; Ucciferri, C.; Nuzzolo, E.R.; Martini, M.; Torti, L.; Falasca, K.; Vecchiet, J.; Leone, G.; et al. Endothelial progenitor cell trafficking in human immunodeficiency virus-infected persons. AIDS 2010, 24, 2443–2450. [Google Scholar] [CrossRef] [PubMed]
- Maldarelli, F.; Palmer, S.; King, M.S.; Wiegand, A.; Polis, M.A.; Mican, J.; Kovacs, J.A.; Davey, R.T.; Rock-Kress, D.; Dewar, R.; et al. ART suppresses plasma HIV-1 RNA to a stable set point predicted by pretherapy viremia. PLoS Pathog. 2007, 3, e46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaaqoq, A.M.; Khasawneh, F.A.; Smalligan, R.D. Cardiovascular complications of HIV-associated immune dysfunction. Cardiol. Res. Pract. 2015, 2015, 302638. [Google Scholar] [CrossRef] [PubMed]
- Brenchley, J.M.; Price, D.A.; Schacker, T.W.; Asher, T.E.; Silvestri, G.; Rao, S.; Kazzaz, Z.; Bornstein, E.; Lambotte, O.; Altmann, D.; et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006, 12, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Mavigner, M.; Cazabat, M.; Dubois, M.; L’Faqihi, F.E.; Requena, M.; Pasquier, C.; Klopp, P.; Amar, J.; Alric, L.; Barange, K.; et al. Altered CD4+ T cell homing to the gut impairs mucosal immune reconstitution in treated HIV-infected individuals. J. Clin. Investig. 2012, 122, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Katsikis, P.D.; Mueller, Y.M.; Villinger, F. The cytokine network of acute HIV infection: A promising target for vaccines and therapy to reduce viral set-point? PLoS Pathog. 2011, 7, e1002055. [Google Scholar] [CrossRef] [PubMed]
- Veazey, R.S.; DeMaria, M.; Chalifoux, L.V.; Shvetz, D.E.; Pauley, D.R.; Knight, H.L.; Rosenzweig, M.; Johnson, R.P.; Desrosiers, R.C.; Lackner, A.A. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 1998, 280, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Hummelen, R.; Vos, A.P.; van’t Land, B.; van Norren, K.; Reid, G. Altered host-microbe interaction in HIV: A target for intervention with pro- and prebiotics. Int. Rev. Immunol. 2010, 29, 485–513. [Google Scholar] [CrossRef] [PubMed]
- Cassol, E.; Malfeld, S.; Mahasha, P.; van der Merwe, S.; Cassol, S.; Seebregts, C.; Alfano, M.; Poli, G.; Rossouw, T. Persistent microbial translocation and immune activation in HIV-1-infected south africans receiving combination antiretroviral therapy. J. Infect. Dis. 2010, 202, 723–733. [Google Scholar] [PubMed]
- Buzon, M.J.; Massanella, M.; Llibre, J.M.; Esteve, A.; Dahl, V.; Puertas, M.C.; Gatell, J.M.; Domingo, P.; Paredes, R.; Sharkey, M.; et al. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat. Med. 2010, 16, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Sylwester, A.W.; Mitchell, B.L.; Edgar, J.B.; Taormina, C.; Pelte, C.; Ruchti, F.; Sleath, P.R.; Grabstein, K.H.; Hosken, N.A.; Kern, F.; et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J. Exp. Med. 2005, 202, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Gori, A.; Rizzardini, G.; Van’t Land, B.; Amor, K.B.; van Schaik, J.; Torti, C.; Quirino, T.; Tincati, C.; Bandera, A.; Knol, J.; et al. Specific prebiotics modulate gut microbiota and immune activation in HAART-naive HIV-infected adults: Results of the “copa” pilot randomized trial. Mucosal Immunol. 2011, 4, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Kekkonen, R.A.; Lummela, N.; Karjalainen, H.; Latvala, S.; Tynkkynen, S.; Jarvenpaa, S.; Kautiainen, H.; Julkunen, I.; Vapaatalo, H.; Korpela, R. Probiotic intervention has strain-specific anti-inflammatory effects in healthy adults. World J. Gastroenterol. 2008, 14, 2029–2036. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Takada, T.; Shimizu, K.; Moriyama, K.; Kawakami, K.; Hirano, K.; Kajimoto, O.; Nomoto, K. Effects of a probiotic fermented milk beverage containing lactobacillus casei strain shirota on defecation frequency, intestinal microbiota, and the intestinal environment of healthy individuals with soft stools. J. Biosci. Bioeng. 2010, 110, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Reale, M.; Boscolo, P.; Bellante, V.; Tarantelli, C.; Di Nicola, M.; Forcella, L.; Li, Q.; Morimoto, K.; Muraro, R. Daily intake of lactobacillus casei shirota increases natural killer cell activity in smokers. Br. J. Nutr. 2012, 108, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Rowland, I.; Thomas, L.V.; Yaqoob, P. Immunomodulatory effects of a probiotic drink containing Lactobacillus casei Shirota in healthy older volunteers. Eur. J. Nutr. 2013, 52, 1853–1863. [Google Scholar] [CrossRef] [PubMed]
- Shida, K.; Nanno, M.; Nagata, S. Flexible cytokine production by macrophages and T cells in response to probiotic bacteria: A possible mechanism by which probiotics exert multifunctional immune regulatory activities. Gut Microbes 2011, 2, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Suzuki, T.; Shimada, S.I.; Shida, K.; Nanno, M.; Okumura, K. Interleukin-12 is involved in the enhancement of human natural killer cell activity by Lactobacillus casei shirota. Clin. Exp. Immunol. 2006, 146, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Osborn, L.; Kunkel, S.; Nabel, G.J. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor κB. Proc. Natl. Acad. Sci. USA 1989, 86, 2336–2340. [Google Scholar] [CrossRef] [PubMed]
- Schnittman, S.M.; Singer, K.H.; Greenhouse, J.J.; Stanley, S.K.; Whichard, L.P.; Le, P.T.; Haynes, B.F.; Fauci, A.S. Thymic microenvironment induces HIV expression. Physiologic secretion of IL-6 by thymic epithelial cells up-regulates virus expression in chronically infected cells. J. Immunol. 1991, 147, 2553–2558. [Google Scholar] [PubMed]
- Amadori, A.; Zamarchi, R.; Veronese, M.L.; Panozzo, M.; Barelli, A.; Borri, A.; Sironi, M.; Colotta, F.; Mantovani, A.; Chieco-Bianchi, L. B cell activation during HIV-1 infection. II. Cell-to-cell interactions and cytokine requirement. J. Immunol. 1991, 146, 57–62. [Google Scholar] [PubMed]
- Barqasho, B.; Nowak, P.; Tjernlund, A.; Kinloch, S.; Goh, L.E.; Lampe, F.; Fisher, M.; Andersson, J.; Sonnerborg, A. Kinetics of plasma cytokines and chemokines during primary HIV-1 infection and after analytical treatment interruption. HIV Med. 2009, 10, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Resino, S.; Bellon, J.M.; Sanchez-Ramon, S.; Gurbindo, D.; Munoz-Fernandez, M.A. Clinical relevance of cytokine production in HIV-1 infection in children on antiretroviral therapy. Scand. J. Immunol. 2000, 52, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Appay, V.; Sauce, D. Immune activation and inflammation in HIV-1 infection: Causes and consequences. J. Pathol. 2008, 214, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Ucciferri, C.; Falasca, K.; Vignale, F.; Di Nicola, M.; Pizzigallo, E.; Vecchiet, J. Improved metabolic profile after switch to darunavir/ritonavir in HIV positive patients previously on protease inhibitor therapy. J. Med. Virol. 2013, 85, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Vecchiet, J.; Ucciferri, C.; Falasca, K.; Mancino, P.; Di Iorio, A.; De Caterina, R. Antihypertensive and metabolic effects of telmisartan in hypertensive HIV-positive patients. Antivir. Ther. 2011, 16, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Falasca, K.; Ucciferri, C.; Mancino, P.; Di Iorio, A.; Vignale, F.; Pizzigallo, E.; Vecchiet, J. Cystatin C, adipokines and cardiovascular risk in HIV infected patients. Curr. HIV Res. 2010, 8, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Ciccarelli, N.; Fabbiani, M.; Grima, P.; Falasca, K.; Tana, M.; Baldonero, E.; Colafigli, M.; Silveri, M.C.; Vecchiet, J.; Cauda, R.; et al. Comparison of cognitive performance in HIV or HCV mono-infected and HIV-HCV co-infected patients. Infection 2013, 41, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Falasca, K.; Ucciferri, C.; Mancino, P.; Pizzigallo, E.; Calza, L.; Vecchiet, J. Severe HIV-associated hypertriglyceridaemia treated with rosuvastatin plus omega-3 fatty acids. Int. J. STD AIDS 2009, 20, 580–581. [Google Scholar] [CrossRef] [PubMed]
- Ucciferri, C.; Falasca, K.; Mancino, P.; Di Iorio, A.; Vecchiet, J. Microalbuminuria and hypertension in HIV-infected patients: A preliminary study of telmisartan. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 491–498. [Google Scholar] [PubMed]
- Falasca, K.; Ucciferri, C.; Di Nicola, M.; Vignale, F.; Di Biase, J.; Vecchiet, J. Different strategies of 25OH vitamin D supplementation in HIV-positive subjects. Int. J. STD AIDS 2014, 25, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Schacker, T.; Zeh, J.; Hu, H.; Shaughnessy, M.; Corey, L. Changes in plasma human immunodeficiency virus type 1 RNA associated with herpes simplex virus reactivation and suppression. J. Infect. Dis. 2002, 186, 1718–1725. [Google Scholar] [CrossRef]
- Boulware, D.R.; Hullsiek, K.H.; Puronen, C.E.; Rupert, A.; Baker, J.V.; French, M.A.; Bohjanen, P.R.; Novak, R.M.; Neaton, J.D.; Sereti, I.; et al. Higher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J. Infect. Dis. 2011, 203, 1637–1646. [Google Scholar] [CrossRef] [PubMed]
- Hummelen, R.; Changalucha, J.; Butamanya, N.L.; Koyama, T.E.; Cook, A.; Habbema, J.D.; Reid, G. Effect of 25 weeks probiotic supplementation on immune function of HIV patients. Gut Microbes 2011, 2, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Helwig, U.; Lammers, K.M.; Rizzello, F.; Brigidi, P.; Rohleder, V.; Caramelli, E.; Gionchetti, P.; Schrezenmeir, J.; Foelsch, U.R.; Schreiber, S.; et al. Lactobacilli, bifidobacteria and E. coli nissle induce pro- and anti-inflammatory cytokines in peripheral blood mononuclear cells. World J. Gastroenterol. 2006, 12, 5978–5986. [Google Scholar] [PubMed]
- Klein, A.; Friedrich, U.; Vogelsang, H.; Jahreis, G. Lactobacillus acidophilus 74-2 and Bifidobacterium animalis subsp lactis DGCC 420 modulate unspecific cellular immune response in healthy adults. Eur. J. Clin. Nutr. 2008, 62, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.E.; Zhang, Y.; Zhang, J.; Dong, P.L.; Chen, M.; Duan, Z.P. Probiotic yogurt effects on intestinal flora of patients with chronic liver disease. Nurs. Res. 2010, 59, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Baken, K.A.; Pennings, J.L.; de Vries, A.; Breit, T.M.; van Steeg, H.; van Loveren, H. Gene expression profiling of Bis(tri-n-butyltin)oxide (TBTO)-induced immunotoxicity in mice and rats. J. Immunotoxicol. 2006, 3, 227–244. [Google Scholar] [CrossRef] [PubMed]
- Kekow, J.; Wachsman, W.; McCutchan, J.A.; Gross, W.L.; Zachariah, M.; Carson, D.A.; Lotz, M. Transforming growth factor-beta and suppression of humoral immune responses in HIV infection. J. Clin. Investig. 1991, 87, 1010–1016. [Google Scholar] [CrossRef]
- Futh, R.; Herder, C.; Forster, S.; Muller-Scholze, S.; Kruse, N.; Rieckmann, P.; Heinig, A.; Koenig, W.; Scherbaum, W.A.; Kolb, H.; et al. Evaluation of diagnostic relevance of mrna levels in peripheral blood: Predictive value for mortality in hemodialysis patients. Cytokine 2004, 27, 166–172. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, R.W.; Kay, T.; Lyle, E.A.; Traxler, S.A.; Deveney, C.W.; Jobe, B.A.; Roberts, C.T., Jr.; Marks, D.; Rosenbaum, J.T. Alterations in peripheral blood lymphocyte cytokine expression in obesity. Clin. Exp. Immunol. 2006, 146, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Brockman, M.A.; Kwon, D.S.; Tighe, D.P.; Pavlik, D.F.; Rosato, P.C.; Sela, J.; Porichis, F.; Le Gall, S.; Waring, M.T.; Moss, K.; et al. IL-10 is up-regulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood 2009, 114, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, E.; Aukrust, P.; Kvale, D.; Muller, F.; Froland, S.S. IL-10 in HIV infection: Increasing serum IL-10 levels with disease progression—Down-regulatory effect of potent anti-retroviral therapy. Clin. Exp. Immunol. 1999, 116, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Clerici, M.; Wynn, T.A.; Berzofsky, J.A.; Blatt, S.P.; Hendrix, C.W.; Sher, A.; Coffman, R.L.; Shearer, G.M. Role of interleukin-10 in T helper cell dysfunction in asymptomatic individuals infected with the human immunodeficiency virus. J. Clin. Investig. 1994, 93, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; French, M.A.; Price, P. IL-23 and IFN-gamma deficiency in immunodeficient HIV patients who achieved a long-term increase in CD4 T-cell counts on highly active antiretroviral therapy. AIDS 2004, 18, 1337–1340. [Google Scholar] [CrossRef] [PubMed]
- Hummelen, R.; Hemsworth, J.; Changalucha, J.; Butamanya, N.L.; Hekmat, S.; Habbema, J.D.; Reid, G. Effect of micronutrient and probiotic fortified yogurt on immune-function of anti-retroviral therapy naive HIV patients. Nutrients 2011, 3, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Buisson, S.; Benlahrech, A.; Gazzard, B.; Gotch, F.; Kelleher, P.; Patterson, S. Monocyte-derived dendritic cells from HIV type 1-infected individuals show reduced ability to stimulate T cells and have altered production of interleukin (IL)-12 and IL-10. J. Infect. Dis. 2009, 199, 1862–1871. [Google Scholar] [CrossRef] [PubMed]
- Vanham, G.; Penne, L.; Fransen, K.; Kestens, L.; De Brabander, M. HIV-associated dysfunction of in vitro IL-12 production depends on the nature of the stimulus and on the CD4 T-cell count of the patient. Blood 2000, 95, 2185–2187. [Google Scholar] [PubMed]
- Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003, 3, 133–146. [Google Scholar] [CrossRef] [PubMed]
- El Hed, A.; Khaitan, A.; Kozhaya, L.; Manel, N.; Daskalakis, D.; Borkowsky, W.; Valentine, F.; Littman, D.R.; Unutmaz, D. Susceptibility of human Th17 cells to human immunodeficiency virus and their perturbation during infection. J. Infect. Dis. 2010, 201, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Cunningham-Rundles, S.; Ahrne, S.; Johann-Liang, R.; Abuav, R.; Dunn-Navarra, A.M.; Grassey, C.; Bengmark, S.; Cervia, J.S. Effect of probiotic bacteria on microbial host defense, growth, and immune function in human immunodeficiency virus type-1 infection. Nutrients 2011, 3, 1042–1070. [Google Scholar] [PubMed]
- O’Garra, A.; Murphy, K.M. From IL-10 to IL-12: How pathogens and their products stimulate APCs to induce T(H)1 development. Nat. Immunol. 2009, 10, 929–932. [Google Scholar] [CrossRef] [PubMed]
- Van Hemert, S.; Meijerink, M.; Molenaar, D.; Bron, P.A.; de Vos, P.; Kleerebezem, M.; Wells, J.M.; Marco, M.L. Identification of Lactobacillus plantarum genes modulating the cytokine response of human peripheral blood mononuclear cells. BMC Microbiol. 2010, 10, 293. [Google Scholar] [CrossRef] [PubMed]
- Amirayan-Chevillard, N.; Tissot-Dupont, H.; Capo, C.; Brunet, C.; Dignat-George, F.; Obadia, Y.; Gallais, H.; Mege, J.L. Impact of highly active anti-retroviral therapy (HAART) on cytokine production and monocyte subsets in HIV-infected patients. Clin. Exp. Immunol. 2000, 120, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.C.; Orekhova, T.; Meadows, M.J.; Heidorn, S.M.; Yang, J.; Finkelman, F.D. IL-4 induces in vivo production of IFN-gamma by NK and NKT cells. J. Immunol. 2006, 176, 5299–5305. [Google Scholar] [CrossRef] [PubMed]
- Leite-De-Moraes, M.C.; Hameg, A.; Pacilio, M.; Koezuka, Y.; Taniguchi, M.; Van Kaer, L.; Schneider, E.; Dy, M.; Herbelin, A. IL-18 enhances IL-4 production by ligand-activated NKT lymphocytes: A pro-Th2 effect of IL-18 exerted through NKT cells. J. Immunol. 2001, 166, 945–951. [Google Scholar] [CrossRef]
- Hemsworth, J.C.; Hekmat, S.; Reid, G. Micronutrient supplemented probiotic yogurt for HIV-infected adults taking HAART in London, Canada. Gut Microbes 2012, 3, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, A.; Gupta, A. Nutrition and disease progression pre-highly active antiretroviral therapy (HAART) and post-HAART: Can good nutrition delay time to HAART and affect response to HAART? Am. J. Clin. Nutr. 2011, 94, 1703S–1715S. [Google Scholar] [CrossRef] [PubMed]
- Odden, M.C.; Scherzer, R.; Bacchetti, P.; Szczech, L.A.; Sidney, S.; Grunfeld, C.; Shlipak, M.G. Cystatin C level as a marker of kidney function in human immunodeficiency virus infection: The FRAM study. Arch. Intern. Med. 2007, 167, 2213–2219. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, G.; MacDonald, K.; Reid, G.; Burton, J.P. The influence of the human microbiome and probiotics on cardiovascular health. Gut Microbes 2014, 5, 719–728. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falasca, K.; Vecchiet, J.; Ucciferri, C.; Di Nicola, M.; D'Angelo, C.; Reale, M. Effect of Probiotic Supplement on Cytokine Levels in HIV-Infected Individuals: A Preliminary Study. Nutrients 2015, 7, 8335-8347. https://doi.org/10.3390/nu7105396
Falasca K, Vecchiet J, Ucciferri C, Di Nicola M, D'Angelo C, Reale M. Effect of Probiotic Supplement on Cytokine Levels in HIV-Infected Individuals: A Preliminary Study. Nutrients. 2015; 7(10):8335-8347. https://doi.org/10.3390/nu7105396
Chicago/Turabian StyleFalasca, Katia, Jacopo Vecchiet, Claudio Ucciferri, Marta Di Nicola, Chiara D'Angelo, and Marcella Reale. 2015. "Effect of Probiotic Supplement on Cytokine Levels in HIV-Infected Individuals: A Preliminary Study" Nutrients 7, no. 10: 8335-8347. https://doi.org/10.3390/nu7105396
APA StyleFalasca, K., Vecchiet, J., Ucciferri, C., Di Nicola, M., D'Angelo, C., & Reale, M. (2015). Effect of Probiotic Supplement on Cytokine Levels in HIV-Infected Individuals: A Preliminary Study. Nutrients, 7(10), 8335-8347. https://doi.org/10.3390/nu7105396






