The Spectrum of Differences between Childhood and Adulthood Celiac Disease
Abstract
:1. Introduction
2. Epidemiology and Genetics
3. Serologic and Histologic Differences between CD in Children and Adults
4. Associated Diseases
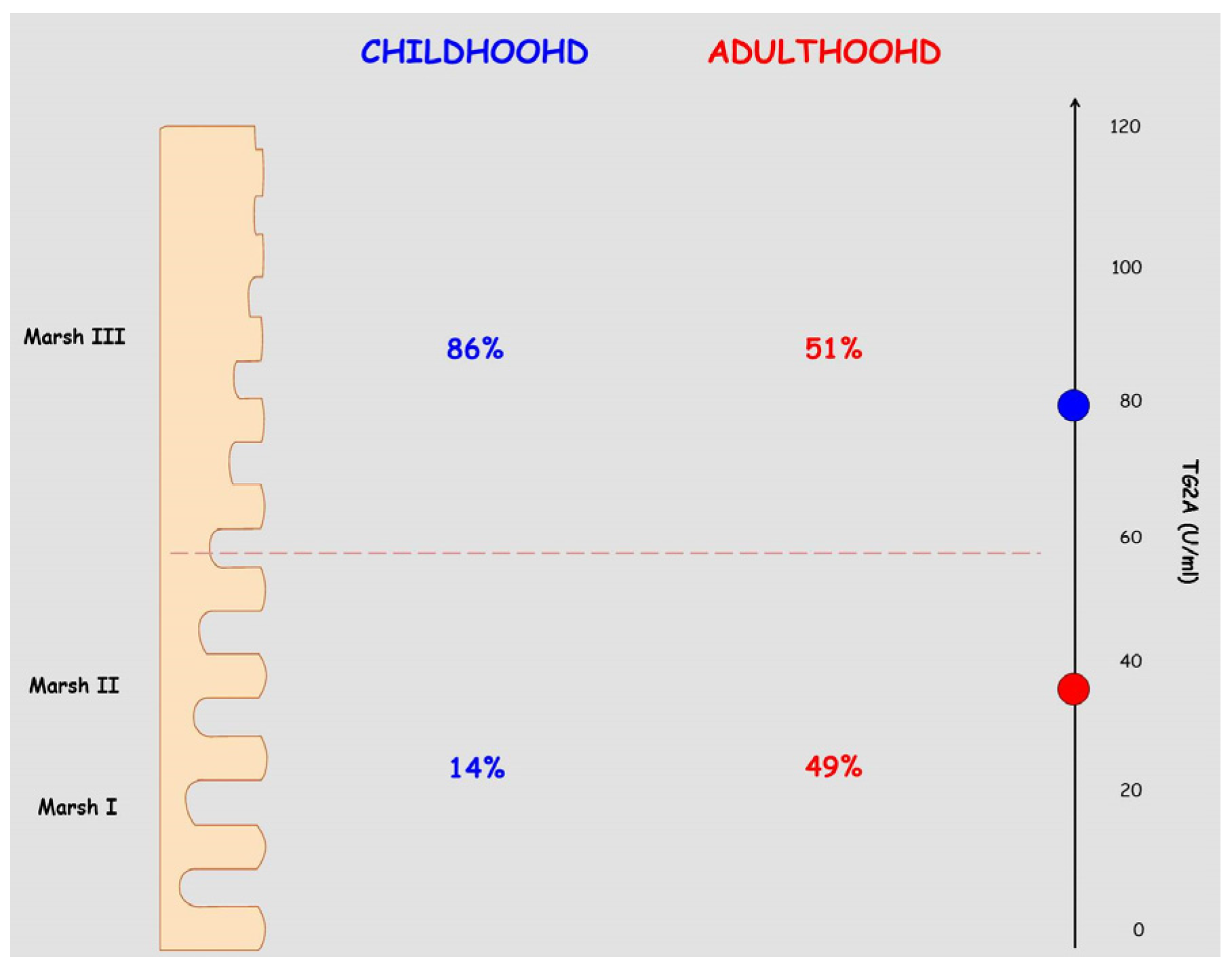
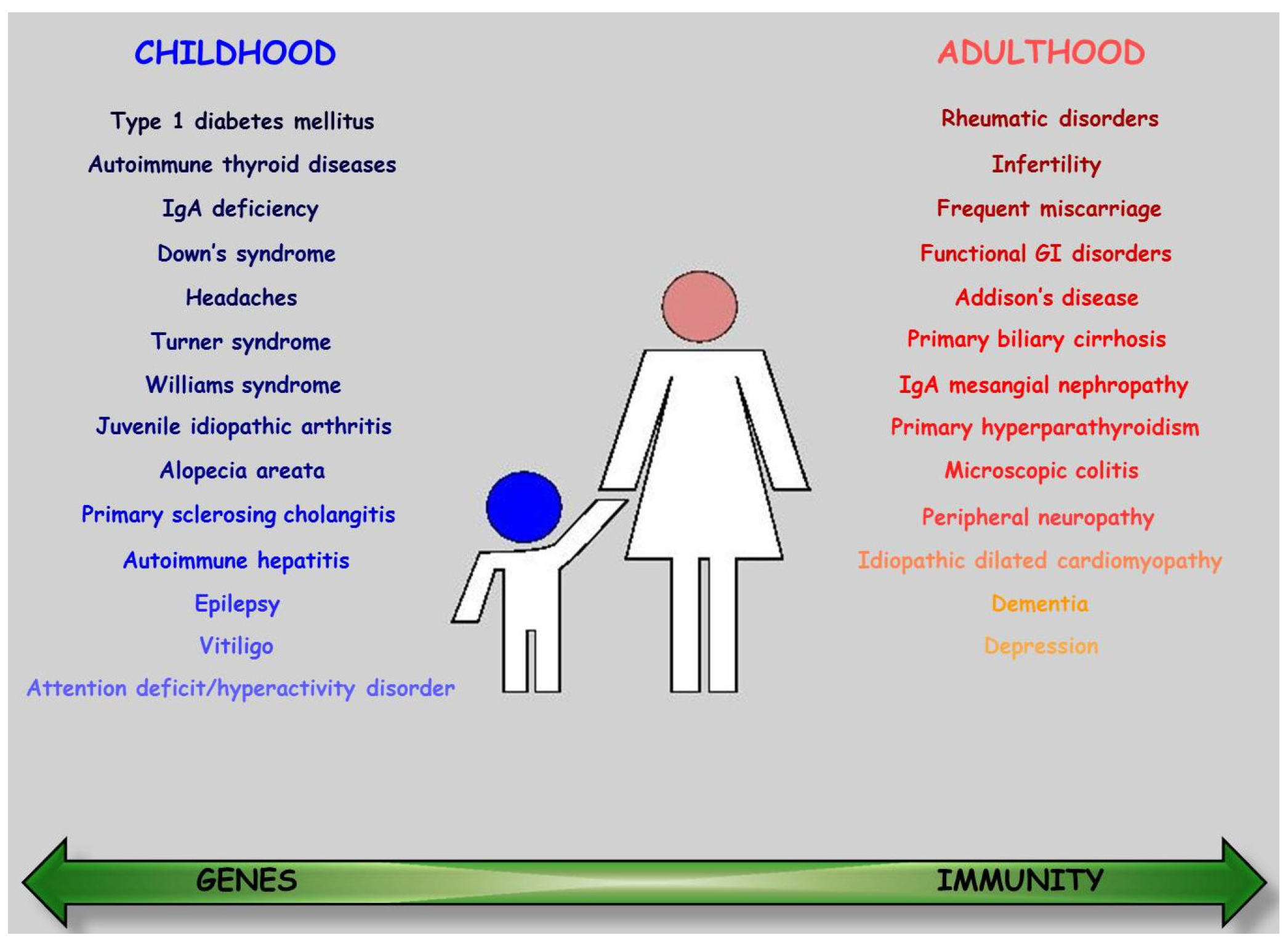
4.1. Type 1 Diabetes Mellitus
4.2. Autoimmune Thyroid Disease
4.3. Other Immune-Mediated Disorders
4.4. Rheumatic Disorders
4.5. Selective IgA Deficiency
4.6. Neuro-Psychiatric Conditions
4.7. Skin and Annexes
4.8. Liver
4.9. Reproduction
4.10. Genetic Disorders
5. Response to GFD and Prognosis
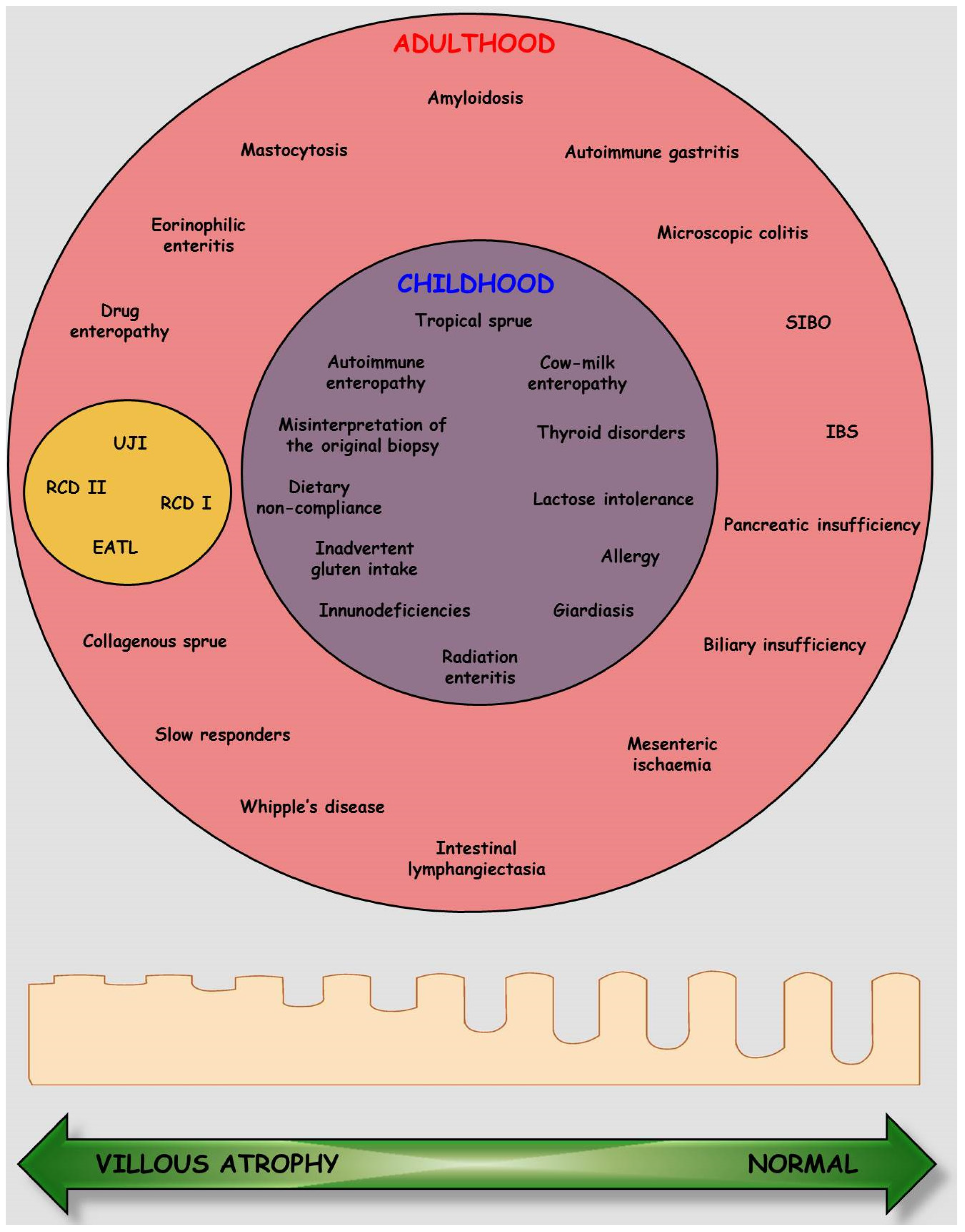
5.1. Complicated Celiac Disease
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Green, P.H.; Cellier, C. Celiac disease. N. Engl. J. Med. 2007, 357, 1731–1743. [Google Scholar] [CrossRef] [PubMed]
- Di Sabatino, A.; Corazza, G.R. Coeliac disease. Lancet 2009, 373, 1480–1493. [Google Scholar] [CrossRef]
- Wright, D.H. The major complications of coeliac disease. Baillieres Clin. Gastroenterol. 1995, 9, 351–369. [Google Scholar] [CrossRef]
- Sainsbury, K.; Mullan, B.; Sharpe, L. Reduced quality of life in coeliac disease is more strongly associated with depression than gastrointestinal symptoms. J. Psychosom. Res. 2013, 75, 135–141. [Google Scholar] [PubMed]
- Wierdsma, N.J.; van Bokhorst-de van der Schueren, M.A.; Berkenpas, M.; Mulder, C.J.; van Bodegraven, A.A. Vitamin and mineral deficiencies are highly prevalent in newly diagnosed celiac disease patients. Nutrients 2013, 5, 3975–3992. [Google Scholar] [CrossRef] [PubMed]
- Mazure, R.; Vazquez, H.; Gonzalez, D.; Mautalen, C.; Pedreira, S.; Boerr, L.; Bai, J.C. Bone mineral affection in asymptomatic adult patients with celiac disease. Am. J. Gastroenterol. 1994, 89, 2130–2134. [Google Scholar] [PubMed]
- Stein, E.M.; Rogers, H.; Leib, A.; McMahon, D.J.; Young, P.; Nishiyama, K.; Guo, X.E.; Lewis, S.; Green, P.H.; Shane, E. Abnormal skeletal strength and microarchitecture in women with celiac disease. J. Clin. Endocrinol. Metable 2015, 100, 2347–2353. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.; MacDonald, T.T.; Diss, T.C.; Walker-Smith, J.A.; Ciclitira, P.J.; Isaacson, P.G. Changes in intraepithelial lymphocyte subpopulations in coeliac disease and enteropathy associated T cell lymphoma (malignant histiocytosis of the intestine). Gut 1989, 30, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Grigg-Gutierrez, N.M.; Estremera-Marcial, R.; Caceres, W.W.; Toro, D.H. Primary enteropathy-associated t-cell lymphoma type 2: An emerging entity? Cancer Control. 2015, 22, 242–247. [Google Scholar] [PubMed]
- Hernandez, L.; Green, P.H. Extraintestinal manifestations of celiac disease. Curr. Gastroenterol. Rep. 2006, 8, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Trigoni, E.; Tsirogianni, A.; Pipi, E.; Mantzaris, G.; Papasteriades, C. Celiac disease in adult patients: Specific autoantibodies in the diagnosis, monitoring, and screening. Autoimmun. Dis. 2014, 2014, 623514. [Google Scholar] [CrossRef] [PubMed]
- Kakleas, K.; Soldatou, A.; Karachaliou, F.; Karavanaki, K. Associated autoimmune diseases in children and adolescents with type 1 diabetes mellitus (T1DM). Autoimmun. Rev. 2015, 14, 781–797. [Google Scholar] [CrossRef] [PubMed]
- Berti, I.; Trevisiol, C.; Tommasini, A.; Città, A.; Neri, E.; Geatti, O.; Giammarini, A.; Ventura, A.; Not, T. Usefulness of screening program for celiac disease in autoimmune thyroiditis. Dig. Dis. Sci. 2000, 45, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Molberg, O.; Mcadam, S.N.; Körner, R.; Quarsten, H.; Kristiansen, C.; Madsen, L.; Fugger, L.; Scott, H.; Norén, O.; Roepstorff, P.; et al. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat. Med. 1998, 4, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Di Sabatino, A.; Ciccocioppo, R.; Cupelli, F.; Cinque, B.; Millimaggi, D.; Clarkson, M.M.; Paulli, M.; Cifone, M.G.; Corazza, G.R. Epithelium derived interleukin 15 regulates intraepithelial lymphocyte Th1 cytokine production, cytotoxicity, and survival in coeliac disease. Gut 2006, 55, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, G.; Pender, S.L.; Alstead, E.; Hauer, A.C.; Lionetti, P.; McKenzie, C.; MacDonald, T.T. Role of interferon alpha in promoting T helper cell type 1 responses in the small intestine in coeliac disease. Gut 2001, 48, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Catassi, C. Current approaches to diagnosis and treatment of celiac disease: An evolving spectrum. Gastroenterology 2001, 120, 636–651. [Google Scholar] [CrossRef] [PubMed]
- Du Pre, M.F.; Sollid, L.M. T-cell and B-cell immunity in celiac disease. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Haines, M.L.; Anderson, R.P.; Gibson, P.R. Systematic review: The evidence base for long-term management of coeliac disease. Aliment. Pharmacol. Ther. 2008, 28, 1042–1066. [Google Scholar] [CrossRef] [PubMed]
- Smecuol, E.; Gonzalez, D.; Mautalen, C.; Siccardi, A.; Cataldi, M.; Niveloni, S.; Mazure, R.; Vazquez, H.; Pedreira, S.; Soifer, G.; et al. Longitudinal study on the effect of treatment on body composition and anthropometry of celiac disease patients. Am. J. Gastroenterol. 1997, 92, 639–643. [Google Scholar] [PubMed]
- Bardella, M.T.; Fredella, C.; Prampolini, L.; Molteni, N.; Giunta, A.M.; Bianchi, P.A. Body composition and dietary intakes in adult celiac disease patients consuming a strict gluten-free diet. Am. J. Clin. Nutr. 2000, 72, 937–939. [Google Scholar] [PubMed]
- Kang, J.Y.; Kang, A.H.Y.; Green, A.; Gwee, K.A.; Ho, K.Y. Systematic review: Worldwide variation in the frequency of coeliac disease and changes over time. Aliment. Pharmacol. Ther. 2013, 38, 226–245. [Google Scholar] [CrossRef] [PubMed]
- Elson, C.O.; Ballew, M.; Banard, J.A. NIH Consensus Development Conference on Celiac Disease. NIH Consens. State Sci. Statements 2004, 21, 1–23. [Google Scholar]
- Lionetti, E.; Gatti, S.; Pulvirenti, A.; Catassi, C. Celiac disease from a global perspective. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Gatti, S.; Lionetti, E. World perspective and celiac disease epidemiology. Dig. Dis. 2015, 33, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.J.; Ahmad, T.; Rajaguru, C.; Barnardo, M.; Warren, B.F.; Jewell, D.P. Contribution of histological, serological, and genetic factors to the clinical heterogeneity of adult-onset coeliac disease. Scand. J. Gastroenterol. 2009, 44, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Fabiani, E.; Ratsch, I.M.; Coppa, G.V.; Giorgi, P.L.; Pierdomenico, R.; Alessandrini, S.; Iwanejko, G.; Domenici, R.; Mei, E.; et al. The coeliac iceberg in Italy. A multicentre antigliadin antibodies screening for coeliac disease in school-age subjects. Acta Paediatr. Suppl. 1996, 412, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, A.; Skaaby, T.; Kårhus, L.L.; Schwarz, P.; Jørgensen, T.; Rumessen, J.J.; Linneberg, A. Screening for celiac disease in Danish adults. Scand. J. Gastroenterol. 2015, 50, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Sollid, L.M.; Markussen, G.; Ek, J.; Gjerde, H.; Vartdal, F.; Thorsby, E. Evidence for a primary association of celiac disease to a particular HLA-DQ alpha/beta heterodimer. J. Exp. Med. 1989, 169, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Tjon, J.M.; van Bergen, J.; Koning, F. Celiac disease: How complicated can it get? Immunogenetics 2010, 62, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Hadithi, M.; von Blomberg, B.M.; Crusius, J.B.; Bloemena, E.; Kostense, P.J.; Meijer, J.W.; Mulder, C.J.; Stehouwer, C.D.; Peña, A.S. Accuracy of serologic tests and HLA-DQ typing for diagnosing celiac disease. Ann. Intern. Med. 2007, 147, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Malamut, G.; Meresse, B.; Cellier, C.; Cerf-Bensussan, N. Refractory celiac disease: From bench to bedside. Semin. Immunopathol. 2012, 34, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Polvi, A.; Eland, C.; Koskimies, S.; Mäki, M.; Partanen, J. HLA DQ and DP in Finnish families with celiac disease. Eur. J. Immunogenet. 1996, 23, 221–234. [Google Scholar] [CrossRef]
- Koskinen, L.L.; Einarsdottir, E.; Korponay-Szabo, I.R.; Kurppa, K.; Kaukinen, K.; Sistonen, P.; Pocsai, Z.; Széles, G.; Adány, R.; Mäki, M.; et al. Fine mapping of the CELIAC2 locus on chromosome 5q31-q33 in the Finnish and Hungarian populations. Tissue Antigens 2009, 74, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Holopainen, P.; Naluai, A.T.; Moodie, S.; Percopo, S.; Coto, I.; Clot, F.; Ascher, H.; Sollid, L.; Ciclitira, P.; Greco, L.; et al. Candidate gene region 2q33 in European families with coeliac disease. Tissue Antigens 2004, 63, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Van Belzen, M.J.; Meijer, J.W.; Sandkuijl, L.A.; Bardoel, A.F.; Mulder, C.J.; Pearson, P.L.; Houwen, R.H.; Wijmenga, C. A major non-HLA locus in celiac disease maps to chromosome 19. Gastroenterology 2003, 125, 1032–1041. [Google Scholar] [CrossRef]
- Hill, I.D.; Dirks, M.H.; Liptak, G.D.; Colletti, R.B.; Fasano, A.; Guandalini, S.; Hoffenberg, E.J.; Horvath, K.; Murray, J.A.; Pivor, M.; et al. Guideline for the diagnosis and treatment of celiac disease in children: Recommendations of the North American Society for Pediatric Gastroenterology Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2005, 40, 1–19. [Google Scholar] [CrossRef] [PubMed]
- James, S.P. National Institutes of Health Consensus Development Conference Statement on Celiac Disease. Gastroenterology 2005, 128, 1–9. [Google Scholar] [CrossRef]
- Husby, S.; Koletzko, S.; Korponay-Szabo, I.R.; Mearin, M.L.; Phillips, A.; Shami, R.; Troncone, R.; Giersiepen, K.; Branski, D.; Catassi, C.; et al. European Society for Pediatric Gastroenterology, Hepatology and Nutrition Guidelines for the diagnosis of Coeliac disease. JPGN 2012, 54, 136–160. [Google Scholar] [CrossRef] [PubMed]
- Dickson, B.C.; Streutker, C.J.; Chetty, R. Coeliac disease: An update for pathologists. Rev. J. Clin. Path. 2006, 59, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, B.M.; Feighery, C.; Coates, C.; O’Shea, U.; Delaney, D.; O’Briain, S.; Kelly, J.; Abuzakouk, M. The absence of a mucosal lesion on standard histological examination does not exclude diagnosis of celiac disease. Dig. Dis. Sci. 2008, 53, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Kaukinen, K.; Collin, P.; Mäki, M. Latent coeliac disease or coeliac disease beyond villous atrophy? Gut 2007, 56, 1339–1340. [Google Scholar] [CrossRef] [PubMed]
- Mubarak, A.; Nikkels, P.; Houwen, R.; Kate, F.T. Reproducibility of the histological diagnosis of celiac disease. Scand. J. Gastroenterol. 2011, 46, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Barker, C.C.; Mitton, C.; Jevon, G.; Mock, T. Can tissue transglutaminase antibody titers replace small-bowel biopsy to diagnose celiac disease in select pediatric populations? Pediatrics 2005, 115, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, M.R.; Book, L.S.; Leiferman, K.M.; Zone, J.J.; Neuhausen, S.L. Strongly positive tissue transglutaminaseantibodies are associated with Marsh 3 histopathology in adult and pediatric celiac disease. J. Clin. Gastroenterol. 2008, 42, 256–260. [Google Scholar] [PubMed]
- Vivas, S.; de Morales, J.G.R.; Riestra, S.; Arias, L.; Fuentes, D.; Alvarez, N.; Calleja, S.; Hernando, M.; Herrero, B.; Casqueiro, J.; et al. Duodenal biopsy may be avoided when high transglutaminase antibody titers are present. World J. Gastroenterol. 2009, 15, 4775–4780. [Google Scholar] [CrossRef] [PubMed]
- Tortora, R.; Imperatore, N.; Capone, P.; de Palma, G.D.; de Stefano, G.; Gerbino, N.; Caporaso, N.; Rispo, A. The presence of anti-endomysial antibodies and the level of anti-tissue transglutainase can be used to diagnose adult coeliac disease without duodenal biopsy. Aliment. Pharmacol. Ther. 2014, 40, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Misak, Z.; Hojsak, I.; Jadresin, O.; Kekez, A.J.; Abdovic, S.; Kolacek, S. Diagnosis of coeliac disease in children younger than 2 years. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Vermeersch, P.; Geboes, K.; Marien, G.; Hoffman, I.; Hiele, M.; Bossuyt, X. Serological diagnosis of celiac disease: Comparative analysis of different strategies. Clin. Chimic. Acta 2012, 413, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Bürgin-Wolff, A.; Mauro, B.; Faruk, H. Intestinal biopsy is not always required to diagnose celiac disease: A retrospective analysis of combined antibody tests. BMC Gastroenterol. 2013, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Bottaro, G.; Cataldo, F.; Rotolo, N.; Spina, M.; Corazza, G.R. The clinical pattern of subclinical/silent celiac disease: An analysis on 1026 consecutive cases. Am. J. Gastroenterol. 1999, 94, 691–696. [Google Scholar] [PubMed]
- Ventura, A.; Magazzu, G.; Greco, L. Duration of exposure to gluten and risk for autoimmune disorders in patients with celiac disease. Gastroenterology 1999, 117, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Cosnes, J.; Cellier, C.; Viola, S.; Colombel, J.F.; Michaud, L.; Sarles, J.; Hugot, J.P.; Ginies, J.L.; Dabadie, A.; Mouterde, O.; et al. Nion-Larmurier IIncidence of autoimmune diseases in celiacdisease: Protective effect of the gluten-free diet. Clin. Gastroenterol. Hepatol. 2008, 6, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Sategna-Guidetti, C.; Solerio, E.; Scaglione, N.; Aimo, G.; Mengozzi, G. Duration of gluten exposure in adult coeliac disease does not correlate with the risk for autoimmune disorders. Gut 2001, 49, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Viljamaa, M.; Kaukinen, K.; Huhtala, H.; Kyrönpalo, S.; Rasmussen, M.; Collin, P. Coeliac disease, autoimmune diseases and gluten exposure. Scand. J. Gastroenterol. 2005, 40, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Ott, C.; Schölmerich, J. Extraintestinal manifestations and complications in IBD. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Denham, J.M.; Hill, D. Celiac disease and autoimmunity: Review and controversies. Curr. Allergy Asthma Rep. 2013, 13, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Achury, J.; de Almeida, R.C.; Wijmenga, C. Shared genetics in coeliac disease and other immune-mediated diseases. J. Intern. Med. 2011, 269, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Lammers, K.M.; Lu, R.; Brownley, J.; Lu, B.; Gerard, C.; Thomas, K.; Rallabhandi, P.; Shea-Donohue, T.; Tamiz, A.; Alkan, S.; et al. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology 2008, 135, 194–204. [Google Scholar] [CrossRef] [PubMed]
- McLean, M.H.; Dieguez, D., Jr.; Miller, L.M.; Young, H.A. Does the microbiota play a role in the pathogenesis of autoimmune diseases? Gut 2015, 64, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Koning, F.; Thomas, R.; Rossjohn, J.; Toes, R.E. Coeliac disease and rheumatoid arthritis: Similar mechanisms, different antigens. Nat. Rev. Rheumatol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Guandalini, S.; Assiri, A. Celiac disease: A review. JAMA Pediatr. 2014, 168, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Volta, U.; Caio, G.; Stanghellini, V.; de Giorgio, R. The changing clinical profile of celiac disease: A 15-year experience (1998–2012) in an Italian referral center. BMC Gastroenterol. 2014, 14, 194. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Singh, P.; Agnihotri, A.; Das, P.; Mishra, A; Verma, A.K.; Ahuja, A.; Sreenivas, V.; Khadgawat, R.; Gupta, S.D.; et al. Celiac disease: A disease with varied manifestations in adults and adolescents. J. Dig. Dis. 2013, 14, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Holmes, G.K. Coeliac disease and type 1 diabetes mellitus: An important association. J. Diab. Nurs. 2015, 19, 111–116. [Google Scholar]
- Elfström, P.; Sundström, J.; Ludvigsson, J.F. Systematic review with meta-analysis: Associations between coeliac disease and type 1 diabetes. Alim. Pharmacol. Ther. 2014, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, F.; Bruno, G.; Chiarelli, F.; Lorini, R.; Meschi, F.; Sacchetti, C. Younger age at onset and sex predict celiac disease in children and adolescents with type 1diabetes: An Italian multicenter study. Diabetes Care 2004, 27, 1294–1298. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Ludvigsson, J.; Ekbom, A.; Montgomery, S.M. Celiac disease and risk of subsequent type 1 diabetes: A general population cohort study of children and adolescents. Diabetes Care 2006, 29, 2483–2488. [Google Scholar] [CrossRef] [PubMed]
- Pocecco, M.; Ventura, A. Coeliac disease and insulin-dependent diabetes mellitus: A causal association? Acta. Paediatr. 1995, 84, 1432–1433. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.; Brock-Jacobsen, B.; Lund, E.; Bjorn, C.; Hansen, L.P.; Nielsen, C.; Fenger, C.; Lillevang, S.T.; Husby, S. Clinical benefit of a gluten-free diet in type 1 diabetic children with screening-detected celiac disease: A population-based screening study with 2 years’ follow-up. Diabetes Care 2006, 29, 2452–2456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, R.; Murphy, N.; Edge, J.; Ahmed, M.L.; Acerini, C.L.; Dunger, D.B. A longitudinal study of the effects of a gluten-free diet on glycemic control and weight gain in subjects with type 1 diabetes and celiac disease. Diabetes Care 2002, 25, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Albisua, I.; Wolf, J.; Neu, A.; Geiger, H.; Wascher, I.; Stern, M. Coeliac disease in children with type 1 diabetes mellitus: The effect of the gluten-free diet. Diabet. Med. 2005, 22, 1079–1082. [Google Scholar] [CrossRef] [PubMed]
- Mohn, A.; Cerruto, M.; Iafusco, D.; Prisco, F.; Tumini, S.; Stoppoloni, O.; Chiarelli, F. Celiac disease in children and adolescents with type I diabetes: Importance of hypoglycemia. J. Pediatr. Gastroenterol. Nutr. 2001, 32, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Penagini, F.; Dilillo, D.; Meneghin, F.; Mameli, C.; Fabiano, V.; Zuccotti, G.V. Gluten-free diet in children: an approach to a nutritionally adequate and balanced diet. Nutrients 2013, 5, 4553–4565. [Google Scholar] [CrossRef] [PubMed]
- Troncone, R.; Discepolo, V. Celiac disease and autoimmunity. J. Pediatr. Gastroenterol. Nutr. 2014, 59, S9–S11. [Google Scholar] [CrossRef] [PubMed]
- Marietta, E.V.; Gomez, A.M.; Yeoman, C.; Tilahun, A.Y.; Clark, C.R.; Luckey, D.H.; Murray, J.A.; White, B.A.; Kudva, Y.C.; Rajagopalan, G. Low incidence of spontaneous type 1 diabetes in non-obese diabetic mice raised on gluten-free diets is associated with changes in the intestinal microbiome. PLoS ONE 2013, 8, e78687. [Google Scholar] [CrossRef] [PubMed]
- Auricchio, R.; Paparo, F.; Maglio, M.; Franzese, A.; Lombardi, F.; Valerio, G.; Nardone, G.; Percopo, S.; Greco, L.; Troncone, R. In vitro-deranged intestinal immune response to gliadin in type 1 diabetes. Diabetes 2004, 53, 1680–1683. [Google Scholar] [CrossRef] [PubMed]
- Maglio, M.; Florian, F.; Vecchiet, M.; Auricchio, R.; Paparo, F.; Spadaro, R.; Zanzi, D.; Rapacciuolo, L; Franzese, A.; Sblattero, D.; et al. Majority of children with type 1 diabetes produce and deposit anti-tissue transglutaminase antibodies in the small intestine. Diabetes 2009, 58, 1578–1584. [Google Scholar] [CrossRef] [PubMed]
- Sategna-Guidetti, C.; Volta, U.; Ciacci, C.; Usai, P.; Carlino, A.; de Franceschi, L.; Camera, A.; Pelli, A.; Brossa, C. Prevalence of thyroid disorders in untreated adult celiac disease patients and effect of gluten withdrawal: An Italian multicenter study. Am. J. Gastroenterol. 2001, 96, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Larizza, D.; Calcaterra, V.; de Giacomo, C.; de Silvestri, A.; Asti, M.; Badulli, C.; Autelli, M.; Coslovich, E.; Martinetti, M. Celiac disease in children with autoimmune thyroid disease. J. Pediatr. 2001, 139, 738–740. [Google Scholar] [CrossRef] [PubMed]
- Diamanti, A.; Ferretti, F.; Guglielmi, R.; Panetta, F.; Colistro, F.; Cappa, M.; Daniele, A.; Sole Basso, M.; Noto, C.; Crisogianni, M.; et al. Thyroid autoimmunity in children with coeliac disease: A prospective survey. Arch. Dis. Child. 2011, 96, 1038–1041. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; Lazare, F.; Kacer, M.; Aguayo-Figueroa, L.; Desikan, V.; Garcia, M.; Lane, A.; Chawla, A.; Wilson, T. Celiac disease in children, adolescents, and young adults with autoimmune thyroid disease. J. Pediatr. 2011, 158, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Van der Pals, M.; Ivarsson, A.; Norström, F.; Högberg, L.; Svensson, J.; Carlsson, A. Prevalence of thyroid autoimmunity in children with celiac disease compared to healthy 12-year olds. Autoimmun. Dis. 2014. [Google Scholar] [CrossRef] [PubMed]
- Metso, S.; Hyytia-Ilmonen, H.; Kaukinen, K.; Huhtala, H.; Jaatinen, P.; Salmi, J.; Taurio, J.; Collin, P. Gluten-free diet and autoimmune thyroiditis in patients with celiac disease. A prospective controlled study. Scand. J. Gastroenterol. 2012, 47, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Kalyoncu, D.; Urganci, N. Antithyroid antibodies and thyroid function in pediatric patients with celiac disease. Inter. J. Endocrinol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Ergür, A.T.; Öçal, G.; Berberoglu, M.; Adıyaman, P.; Sıklar, Z.; Aycan, Z.; Evliyaoğlu, O.; Kansu, A.; Girgin, N.; Ensari, A. Celiac disease and autoimmune thyroid disease in children with type 1 diabetes mellitus: Clinical and HLA-genotyping results. J. Clin. Res. Ped. Endocrinol. 2010, 2, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Biagi, F.; Campanella, J.; Soriani, A.; Vailati, A.; Corazza, G.R. Prevalence of coeliac disease in Italian patients affected by Addison’s disease. Scand. J. Gastroenterol. 2006, 41, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Kämpe, O.; Lebwohl, B.; Green, P.H.; Silverberg, S.J.; Ekbom, A. Primary hyperparathyroidism and celiac disease: A population-based cohort study. J. Clin. Endocrinol. Metable 2012, 97, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Lauret, E.; Rodrigo, L. Celiac disease and autoimmune-associated conditions. Biol. Med. Res. Int. 2013. [Google Scholar] [CrossRef] [PubMed]
- Roblin, X.; Helluwaert, F.; Bonaz, B. Celiac disease must be evaluated in patients with Sjögren syndrome. Arch. Intern. Med. 2004, 164, 2387. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Rubio-Tapia, A.; Chowdhary, V.; Murray, J.A.; Simard, J.F. Increased risk of systemic lupus erythematosus in 29,000 patients with biopsy-verified celiac disease. J. Rheumatol. 2012, 39, 1964–1970. [Google Scholar] [CrossRef] [PubMed]
- De Maddi, F.; Pellegrini, F.; Raffaele, C.G.; Tarantino, G.; Rigante, D. Celiac disease and juvenile idiopathic arthritis: A still enigmatic crossover. Scand. J. Gastroenterol. 2013, 48, 511–512. [Google Scholar] [CrossRef] [PubMed]
- Abenaoli, L.; Proietti, I.; Leggio, L.; Ferrulli, A.; Vonghia, L.; Capizzi, R.; Rotoli, M.; Amerio, P.L.; Gasbarrini, G.; Addolorato, G. Cutaneous manifestations in celiac disease. World J. Gastroenterol. 2006, 12, 843–852. [Google Scholar]
- Rubio-Tapia, A.; Murray, J.A. The liver in celiac disease. Hepatology 2007, 46, 1650–1658. [Google Scholar] [CrossRef] [PubMed]
- Grossman, G. Neurological complications of coeliac disease: What is the evidence? Pract. Neurol. 2008, 8, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Frustaci, A.; Cuoco, L.; Chimenti, C.; Pieroni, M.; Fioravanti, G.; Gentiloni, N.; Maseri, A.; Gasbarrini, G. Celiac disease associated with autoimmune myocarditis. Circulation 2002, 105, 2611–2618. [Google Scholar] [CrossRef] [PubMed]
- Curione, M.; Barbato, M.; de Biase, L.; Viola, F.; lo Russo, L.; Cardi, E. Prevalence of coeliac disease in idiopathic dilated cardiomyopathy. Lancet 1999, 354, 222–223. [Google Scholar] [CrossRef]
- Emilsson, L.; Andersson, B.; Elfström, P.; Green, P.H.R.; Ludvigsson, J.F. Risk of idiopathic dilated cardiomyopathy in 29,000 patients with celiac disease. J. Am. Heart Assoc. 2012, 1, e001594. [Google Scholar] [CrossRef] [PubMed]
- Welander, A.; Sundelin, B.; Fored, M.; Ludvigsson, J.F. Increased risk of IgA nephropathy among individuals with celiac disease. J. Clin. Gastroenterol. 2013, 47, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Moeller, S.; Canetta, P.A.; Taylor, A.K.; Arguelles-Grande, C.; Snyder, H.; Green, P.H.; Kiryluk, K.; Alaedini, A. Lack of serologic evidence to link IgA nephropathy with celiac disease or immune reactivity to gluten. PLoS ONE 2014, 9, e94677. [Google Scholar] [CrossRef] [PubMed]
- Pittman, F.E.; Holub, D.A. Sjoegren’s syndrome and adult celiac disease. Gastroenterology 1965, 48, 869–876. [Google Scholar] [PubMed]
- Luft, L.M.; Barr, S.G.; Martin, L.O.; Chan, E.K.; Fritzler, M.J. Autoantibodies to tissue transglutaminase in Sjogren’s syndrome and related rheumatic diseases. J. Rheumatol. 2003, 30, 2613–2619. [Google Scholar] [PubMed]
- Iltanen, S.; Collin, P.; Korpela, M.; Holm, K.; Partanen, J.; Polvi, A.; Mäki, M. Celiac disease and markers of celiac disease latency in patients with primary Sjogren’s syndrome. Am. J. Gastroenterol. 1999, 94, 1042–1046. [Google Scholar] [PubMed]
- Shamir, R.; Shoenfeld, Y.; Blank, M.; Eliakim, R.; Lahat, N.; Sobel, E.; Shinar, E.; Lerner, A. The prevalence of coeliac disease antibodies in patients with the antiphospholipid syndrome. Lupus 2003, 12, 394–399. [Google Scholar] [CrossRef] [PubMed]
- George, E.K.; Hertzberger-ten Cate, R.; van Suijlekom-Smit, L.W.; von Blomberg, B.M.; Stapel, S.O.; van Elburg, R.M.; Mearin, M.L. Juvenile chronic arthritis and coeliac disease in The Netherlands. Clin. Exp. Rheumatol. 1996, 14, 571–575. [Google Scholar] [PubMed]
- Lepore, L.; Martelossi, S.; Pennesi, M.; Falcini, F.; Ermini, M.L.; Ferrari, R.; Perticarari, S.; Presani, G.; Lucchesi, A.; Lapini, M.; et al. Prevalence of celiac disease in patients with juvenile chronic arthritis. J. Pediatr. 1996, 129, 311–313. [Google Scholar] [CrossRef]
- Rensch, M.J.; Szyjkowski, R.; Shaffer, R.T.; Fink, S.; Kopecky, C.; Grissmer, L.; Enzenhauer, R.; Kadakia, S. The prevalence of celiac disease autoantibodies in patients with systemic lupus erythematosus. Am. J. Gastroenterol. 2001, 96, 1113–1115. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Shen, N.; Vyse, T.J.; Anand, V.; Gunnarson, I.; Sturfelt, G.; Rantapaa-Dahlqvist, S.; Elvin, K.; Truedsson, L.; Andersson, B.A.; et al. Selective IgA deficiency in autoimmune diseases. Mol. Med. 2011, 17, 1383–1396. [Google Scholar] [PubMed]
- Pan-Hammarstrom, Q.; Hammarstrom, L. Antibody deficiency diseases. Eur. J. Immunol. 2008, 38, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Meini, A.; Pillan, N.M.; Villanacci, V.; Monafo, V.; Ugazio, A.G.; Plebani, A. Prevalence and diagnosis of celiac disease in IgA-deficient children. Ann. Allergy Asthma Immunol. 1996, 77, 333–336. [Google Scholar] [CrossRef]
- Fiore, M.; Pera, C.; Delfino, L.; Scotese, I.; Ferrara, G.B.; Pignata, C. DNA typing of DQ and DR alleles in IgA-deficient subjects. Eur. J. Immunogenet. 1995, 22, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, H.A.; de Rosa, S.; Ruggieri, V.; de Davila, M.T.; Fejerman, N. Epilepsy, occipital calcifications, and oligosymptomatic celiac disease in childhood. J. Child. Neurol. 2002, 17, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, E.; Francavilla, R.; Pavone, P.; Pavone, L.; Francavilla, T.; Pulvirenti, A.; Giugno, R.; Ruggieri, M. The neurology of coeliac disease in childhood: What is the evidence? A systematic review and meta-analysis. Dev. Med. Child. Neurol. 2010, 52, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Buie, T. The relationship of autism and gluten. Clin. Ther. 2013, 35, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Millward, C.; Ferriter, M.; Calver, S.; Connell-Jones, G. Gluten- and casein-free diets for autistic spectrum disorder. Cochrane Database Syst. Rev. 2004. [Google Scholar] [CrossRef]
- Batista, I.C.; Gandolfi, L.; Nobrega, Y.K.; Almeida, R.C.; Almeida, L.M.; Campos Junior, D.; Pratesi, R. Autism spectrum disorder and celiac disease: No evidence for a link. Arq. Neuropsiquiatr. 2012, 70, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Zelnik, N.; Pacht, A.; Obeid, R.; Lerner, A. Range of neurologic disorders in patients with celiac disease. Pediatrics 2004, 113, 1672–1676. [Google Scholar] [CrossRef] [PubMed]
- Falcini, F.; Porfirio, B.; Lionetti, P. Juvenile dermatomyositis and celiac disease. J. Rheumatol. 1999, 26, 1419–1420. [Google Scholar] [PubMed]
- Marie, I.; Lecomte, F.; Hachulla, E.; Antonietti, M.; François, A.; Levesque, H.; Courtois, H. An uncommon association: Celiac disease and dermatomyositis in adults. Clin. Exper. Rheumatol. 2001, 19, 201–203. [Google Scholar]
- Song, M.S.; Farber, D.; Bitton, A.; Jass, J.; Singer, M.; Karpati, G. Dermatomyositis associated with celiac disease: Response to a gluten-free diet. Canadian J. Gastroenterol. 2006, 20, 433–435. [Google Scholar]
- Reed, A.M.; Pachman, L.; Ober, C. Molecular genetic studies of major histocompatibility complex genes in children with juvenile dermatomyositis: Increased risk associated with HLADQA1*0501. Hum. Immunol. 1991, 32, 235–240. [Google Scholar] [CrossRef]
- Rodríguez-García, C.; González-Hernández, S.; Pérez-Robayna, N.; Guimerá, F.; Fagundo, E.; Sánchez, R. Repigmentation of vitiligo lesions in a child with celiac disease after a gluten-free diet. Pediatr. Dermatol. 2011, 28, 209–210. [Google Scholar] [CrossRef] [PubMed]
- Corazza, G.R.; Andreani, M.L.; Venturo, N.; Bernardi, M.; Tosti, A.; Gasbarrini, G. Celiac disease and alopecia areata: Report of a new association. Gastroenterology 1995, 109, 1333–1337. [Google Scholar] [CrossRef]
- Bardella, M.T.; Marino, R.; Barbareschi, M.; Bianchi, F.; Faglia, G.; Bianchi, P. Alopecia areata and coeliac disease: No effect of a gluten-free diet on hair growth. Dermatology 2000, 200, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Fessatou, S.; Kostaki, M.; Karpathios, T. Coeliac disease and alopecia areata in childhood. J. Paediatr. Child. Health 2003, 39, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Caprai, S.; Vajro, P.; Ventura, A.; Sciveres, M.; Maggiore, G.; SIGENP Study Group for Autoimmune Liver Disorders in Celiac Disease. Autoimmune liver disease associated with celiac disease in childhood: A multicenter study. Clin. Gastroenterol. Hepatol. 2008, 6, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Van Gerven, N.M.; Bakker, S.F.; de Boer, Y.S.; Witte, B.I.; Bontkes, H.; van Nieuwkerk, C.M.; Mulder, C.J.; Bouma, G.; Dutch AIH working group. Seroprevalence of celiac disease in patients with autoimmune hepatitis. Eur. J. Gastroenterol. Hepatol. 2014, 26, 1104–1107. [Google Scholar] [CrossRef] [PubMed]
- Rostami, K.; Steegers, E.A.; Wong, W.Y.; Braat, D.D.; Steegers-Theunissen, R.P. Coeliac disease and reproductive disorders: A neglected association. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 96, 146–149. [Google Scholar] [CrossRef]
- Choi, J.M.; Lebwohl, B.; Wang, J.; Lee, S.K.; Murray, J.A.; Sauer, M.V.; Green, P.H. Increased prevalence of celiac disease in patients with unexplained infertility in the United States. J. Reprod. Med. 2011, 56, 199–203. [Google Scholar] [PubMed]
- Zugna, D.; Richiardi, L.; Akre, O.; Stephansson, O.; Ludvigsson, J.F. A nationwide population-based study to determine whether coeliac disease is associated with infertility. Gut 2010, 59, 1471–1475. [Google Scholar] [CrossRef] [PubMed]
- Santonicola, A.; Iovino, P.; Cappello, C.; Capone, P.; Andreozzi, P.; Ciacci, C. From menarche to menopause: The fertile life span of celiac women. Menopause 2011, 18, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Dhalwani, N.N.; West, J.; Sultan, A.A.; Ban, L.; Tata, L.J. Women with celiac disease present with fertility problems no more often than women in the general population. Gastroenterology 2014, 147, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Gale, L.; Wimalaratna, H.; Brotodiharjo, A.; Duggan, J.M. Down’s syndrome is strongly associated with coeliac disease. Gut 1997, 40, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Zachor, D.A.; Mroczek-Musulman, E.; Brown, P. Prevalence of celiac disease in Down syndrome in the United States. J. Pediatr. Gastroenterol. Nutr. 2000, 31, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Bonamico, M.; Mariani, P.; Danesi, H.M.; Crisogianni, M.; Failla, P.; Gemme, G.; Quartino, A.R.; Giannotti, A.; Castro, M.; Balli, F.; et al. Prevalence and clinical picture of celiac disease in Italian Down syndrome patients: A multicenter study. J. Pediatr. Gastroenterol. Nutr. 2001, 33, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Henderson, A.; Lynch, S.A.; Wilkinson, S.; Hunter, M. Adults with Down’s syndrome: The prevalence of complications and health care in the community. Br. J. Gen. Pract. 2007, 57, 50–55. [Google Scholar] [PubMed]
- Ivarsson, S.A.; Carlsson, A.; Bredberg, A.; Alm, J.; Aronsson, S.; Gustafsson, J.; Hagenas, L.; Hager, A.; Kristrom, B.; Marcus, C.; et al. Prevalence of coeliac disease in Turner syndrome. Acta. Paediatr. 1999, 88, 933–936. [Google Scholar] [CrossRef] [PubMed]
- Bonamico, M.; Pasquino, A.M.; Mariani, P.; Danesi, H.M.; Culasso, F.; Mazzanti, L.; Petri, A.; Bona, G. Prevalence and clinical picture of celiac disease in Turner syndrome. J. Clin. Endocrinol. MeTable 2002, 87, 5495–5498. [Google Scholar] [CrossRef] [PubMed]
- Giannotti, A.; Tiberio, G.; Castro, M.; Virgili, F.; Colistro, F.; Ferretti, F.; Digilio, M.C.; Gambarara, M.; Dallapiccola, B. Coeliac disease in Williams syndrome. J. Med. Genet. 2001, 38, 767–768. [Google Scholar] [CrossRef] [PubMed]
- Abadie, V.; Sollid, L.M.; Barreiro, L.B.; Jabri, B. Integration of genetic and immunological insights into a model of celiac disease pathogenesis. Annu. Rev. Immunol. 2011, 29, 493–525. [Google Scholar] [CrossRef] [PubMed]
- Van de Kamer, J.; Weijers, H.; Dicke, W. Coeliac disease. Some experiments on the cause of the harmful effect of wheat gliadin. Acta. Paediatr. Scand. 1953, 42, 223–231. [Google Scholar]
- Lee, A.R.; Ng, D.L.; Diamond, B.; Ciaccio, E.J.; Green, P.H. Living with coeliac disease: Survey results from the USA. J. Hum. Nutr. Diet 2012, 25, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Cranney, A.; Zarkadas, M.; Graham, I.D.; Switzer, C.; Case, S.; Molloy, M.; Warren, R.E.; Burrows, V.; Butzner, J.D. Celiac disease: Evaluation of the diagnosis and dietary compliance in Canadian children. Pediatrics 2005, 116, e754–e759. [Google Scholar] [CrossRef] [PubMed]
- Panzer, R.M.; Dennis, M.; Kelly, C.P.; Weir, D.; Leichtner, A.; Leffler, D.A. Navigating the gluten-free diet in college. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.; Berger, G.; Sinnreich, U.; Grylli, V.; Schober, E.; Huber, W.D.; Karwautz, A. Quality of life in adolescents with treated coeliac disease: Influence of compliance and age at diagnosis. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, E.; Taccari, L.M.; Ratsch, I.M.; di Giuseppe, S.; Coppa, G.V.; Catassi, C. Compliance with gluten-free diet in adolescents with screening-detected celiac disease: A 5-year follow-up study. J. Pediatr. 2000, 136, 841–843. [Google Scholar] [CrossRef]
- Anson, O.; Weizman, Z.; Zeevi, N. Celiac disease: Parental knowledge and attitudes of dietary compliance. Pediatrics 1990, 85, 98–103. [Google Scholar] [PubMed]
- Jadresin, O.; Misak, Z.; Sanja, K.; Sonicki, Z.; Zizic, V. Compliance with gluten-free diet in children with coeliac disease. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Mulder, C.J.; Wierdsma, N.J.; Berkenpas, M.; Jacobs, M.A.; Bouma, G. Preventing complications in celiac disease: Our experience with managing adult celiac disease. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Green, P.H.R.; Stavropoulos, S.N.; Panagi, S.G.; Goldstein, S.L.; Mcmahon, D.J.; Absan, H.; Neugut, A.I. Characteristics of adult celiac disease in the USA: Results of a national survey. Am. J. Gastroenterol. 2001, 96, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Farnetti, S.; Zocco, M.A.; Garcovich, M.; Gasbarrini, A.; Capristo, E. Functional and metabolic 876 disorders in celiac disease: New implications for nutritional treatment. J. Med. Food 2014, 17, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Hallert, C.; Grant, C.; Grehn, S.; Grännö, C.; Hultén, S.; Midhagen, G.; Strön, M.; Svensson, H.; Valdimarsson, T. Evidence of poor vitamin status in celiac patients on a gluten-free diet for 10 years. Aliment. Pharmacol. Ther. 2002, 16, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Dickey, W.; Kearney, N. Overweight in celiac disease: Prevalence, clinical characteristics and effect of a gluten-free diet. Am. J. Gastroenterol. 2006, 101, 2356–2359. [Google Scholar] [CrossRef] [PubMed]
- Valletta, E.; Fornaro, M.; Cipolli, M.; Conte, S.; Bissolo, F.; Danchielli, C. Celiac disease and obesity: Need for nutritional follow-up after diagnosis. Eur. J. Clin. Nutr. 2010, 64, 1371–1372. [Google Scholar] [CrossRef] [PubMed]
- Lanzini, A.; Lanzarotto, F.; Villanacci, V.; Mora, A.; Bertolazzi, S.; Turini, D.; Carella, G.; Malagoli, A.; Ferrante, G.; Cesana, B.M.; et al. Complete recovery of intestinal mucosa occurs very rarely in adult coeliac patients despite adherence to gluten-free diet. Aliment. Pharmacol. Ther. 2009, 29, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, B.; Granath, F.; Ekbom, A.; Smedby, K.E.; Murray, J.A.; Neugut, A.I.; Green, P.H.R.; Ludvigsson, J.F. Mucosal healing and risk for lymphoproliferative malignancy in celiac disease. Ann. Intern. Med. 2013, 159, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Vreugdenhil, A.C.; Wolters, V.M.; Adriaanse, M.P.; van den Neucker, A.M.; van Bijnen, A.A.; Houwen, R.; Buurman, W.A. Additional value of serum I-FABP levels for evaluating celiac disease activity in children. Scand. J. Gastroenterol. 2011, 46, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- McNicholl, B.; Egan-Mitchell, B.; Stevens, F.; Keane, R.; Baker, S.; McCarthy, C.F.; Fottrell, P.F. Mucosal recovery in treated childhood celiac disease (gluten-sensitive enteropathy). J. Pediatr. 1976, 89, 418–424. [Google Scholar] [CrossRef]
- Wahab, P.J.; Meijer, J.W.; Mulder, C.J. Histologic follow-up of people with celiac disease on a gluten-free diet: Slow and incomplete recovery. Am. J. Clin. Pathol. 2002, 118, 459–463. [Google Scholar] [PubMed]
- Bardella, M.T.; Velio, P.; Cesana, B.M.; Prampolini, L.; Casella, G.; Di Bella, C.; Lanzini, A.; Gambarotti, M.; Bassotti, G.; Villanacci, V. Coeliac disease: A histological follow-up study. Histopathology 2007, 50, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Tursi, A.; Brandimarte, G.; Giorgetti, G.M.; Elisei, W.; Inchingolo, C.D.; Monardo, E.; Aiello, F. Endoscopic and histological findings in the duodenum of adults with celiac disease before and after changing to a gluten-free diet: A 2-year prospective study. Endoscopy 2006, 38, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Biagi, F.; Vattiato, C.; Agazzi, S.; Balduzzi, D.; Schiepatti, A.; Gobbi, P.; Corazza, G.R. A second duodenal biopsy is necessary in the follow-up of adult coeliac patients. Ann. Med. 2014, 46, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Biagi, F.; Gobbi, P.; Marchese, A.; Borsotti, E.; Zingone, F.; Ciacci, C.; Volta, U.; Caio, G.; Carroccio, A.; Ambrosiano, G.; et al. Low incidence but poor prognosis of complicated coeliac disease: A retrospective multicentre study. Dig. Liver Dis. 2014, 46, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, B.; Granath, F.; Ekbom, A.; Montgomery, S.M.; Murray, J.A.; Rubio-Tapia, A.; Green, P.H.R.; Ludvigsson, J.F. Mucosal healing and mortality in coeliac disease. Alim. Pharmacol. Ther. 2013, 37, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Bruins, M.J. The clinical response to gluten challenge: A review of the literature. Nutrients 2013, 5, 4614–4641. [Google Scholar] [CrossRef] [PubMed]
- Dewar, D.H.; Donnelly, S.C.; Mc Laughlin, S.D.; Johnson, M.W.; Ellis, H.J.; Ciclitira, P.J. Celiac disease: Management of persistent symptoms in patients on a gluten-free diet. World J. Gastroenterol. 2012, 18, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Mooney, P.D.; Evans, K.E.; Singh, S.; Sanders, D.S. Treatment Failure in Coeliac Disease: A practical guide to investigation and treatment of non-responsive and refractory coeliac disease. J. Gastrointestin. Liver Dis. 2012, 21, 197–203. [Google Scholar] [PubMed]
- Mubarak, A.; Oudshoorn, J.H.; Kneepkens, C.M.F.; Butler, J.C.; Schreurs, M.W.J.; Mulder, C.J.; Houwen, R.H.J. A child with refractory coeliac disease. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 216–218. [Google Scholar] [CrossRef] [PubMed]
- Sigman, T.; Nguyen, V.H.; Costea, F.; Sant’Anna, A.; Seidman, E.G. Ulcerative jejunitis in a child with celiac disease. BMC Gastroenterol. 2014, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Van Wanrooij, R.L.J.; Müller, D.M.J.; Neefjes-Borst, E.A.; Meijer, J.; Koudstaal, L.G.; Heideman, D.A.M.; Bontkes, H.J.; von Blomberg, B.M.E.; Bouma, G.; Mulder, C.J.J. Optimal strategies to identify aberrant intra-epithelial lymphocytes in refractory coeliac disease. J. Clin. Immunol. 2014, 34, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Malamut, G.; Cellier, C. Refractory coeliac disease. Curr. Opin. Oncol. 2013, 25, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Biagi, F.; Lorenzini, P.; Corazza, G.R. Literature review on the clinical relationship between ulcerative jejunoileitis, coeliac disease, and enteropathy-associated T-cell. Scand. J. Gastroenterol. 2000, 35, 785–790. [Google Scholar] [PubMed]
- Woodward, J. The management of refractory coeliac disease. Ther. Adv. Chronic Dis. 2013, 4, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Corrao, G.; Corazza, G.R.; Bagnardi, V.; Brusco, G.; Ciacci, C.; Cottone, M.; Sategna Guidetti, C.; Usai, P.; Cesari, P.; Pelli, M.A.; et al. Mortality in patients with coeliac disease and their relatives: A cohort study. Lancet 2001, 358, 356–361. [Google Scholar] [CrossRef]
- Abdul Sultan, A.; Crooks, C.J.; Card, T.; Tata, L.J.; Fleming, K.M.; West, J. Causes of death in people with coeliac disease in England compared with the general population: A competing risk analysis. Gut 2015, 64, 1220–1226. [Google Scholar] [CrossRef] [PubMed]
- Biagi, F.; Corazza, G.R. Do different patients with coeliac disease have different mortality rates? Gut 2015, 64, 1187–1188. [Google Scholar] [CrossRef] [PubMed]
- Mäki, M.; Mustalahti, K.; Kokkonen, J.; Kulmala, P.; Haapalahti, M.; Karttunen, T.; Ilonen, J.; Laurila, K.; Dahlbom, I.; Hansson, T.; et al. Prevalence of celiac disease among children in Finland. N. Engl. J. Med. 2003, 348, 2517–2524. [Google Scholar] [CrossRef] [PubMed]
- Ivarsson, A.; Myléus, A.; Norström, F.; van der Pals, M.; Rosén, A.; Högberg, L.; Danielsson, L.; Halvarsson, B.; Hammarroth, S.; Hernell, O.; et al. Prevalence of childhood celiac disease and changes in infant feeding. Pediatrics 2013, 131, e687–e694. [Google Scholar] [CrossRef] [PubMed]
- David, T.J. Transition from the paediatric clinic to the adult service. JRSM 2001, 94, 373–374. [Google Scholar]
- Amaria, K.; Stinson, J.; Cullen-Dean, G.; Sappleton, K.; Kaufman, M. Tools for addressing systems issues in transition. Healthc. Q. 2011, 14, 72–76. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciccocioppo, R.; Kruzliak, P.; Cangemi, G.C.; Pohanka, M.; Betti, E.; Lauret, E.; Rodrigo, L. The Spectrum of Differences between Childhood and Adulthood Celiac Disease. Nutrients 2015, 7, 8733-8751. https://doi.org/10.3390/nu7105426
Ciccocioppo R, Kruzliak P, Cangemi GC, Pohanka M, Betti E, Lauret E, Rodrigo L. The Spectrum of Differences between Childhood and Adulthood Celiac Disease. Nutrients. 2015; 7(10):8733-8751. https://doi.org/10.3390/nu7105426
Chicago/Turabian StyleCiccocioppo, Rachele, Peter Kruzliak, Giuseppina C. Cangemi, Miroslav Pohanka, Elena Betti, Eugenia Lauret, and Luis Rodrigo. 2015. "The Spectrum of Differences between Childhood and Adulthood Celiac Disease" Nutrients 7, no. 10: 8733-8751. https://doi.org/10.3390/nu7105426
APA StyleCiccocioppo, R., Kruzliak, P., Cangemi, G. C., Pohanka, M., Betti, E., Lauret, E., & Rodrigo, L. (2015). The Spectrum of Differences between Childhood and Adulthood Celiac Disease. Nutrients, 7(10), 8733-8751. https://doi.org/10.3390/nu7105426






