Optimized Rapeseed Oils Rich in Endogenous Micronutrients Protect High Fat Diet Fed Rats from Hepatic Lipid Accumulation and Oxidative Stress
Abstract
:1. Introduction
2. Experimental Section
2.1. Oils Preparation
| mg/kg Oil | RRO | CPO | DCPO | MPCPO |
|---|---|---|---|---|
| Phenols (in equivalent, sinapic acid) | 8 | 34 | 43 | 645 |
| Phytosterols | 9592 | 9902 | 8834 | 11,027 |
| Tocopherol | 461 | 541 | 594 | 600 |
| Phospholipids | 22 | 600 | 590 | 1330 |
| Fatty Acid (Weight %) | RRO | CPO | DCPO | MPCPO |
|---|---|---|---|---|
| C16:0 | 2.413 | 2.500 | 2.485 | 2.440 |
| C18:0 | 3.667 | 3.769 | 3.846 | 3.548 |
| C18:1 | 66.19 | 66.075 | 66.287 | 66.561 |
| C18:2 | 16.822 | 16.798 | 17.088 | 16.447 |
| C18:3 | 9.146 | 9.281 | 8.463 | 9.431 |
2.2. Animals and Diets
2.3. Tissue Preparation
2.4. Lipid Content
2.5. Antioxidant Capacity and Lipid Peroxidation
2.5.1. Determination of SOD
2.5.2. Determination of GPx
2.5.3. Determination of CAT
2.5.4. Determination of GSH
2.5.5. Determination of TBARS
2.5.6. Assay of Protein Concentration
2.6. Western Blot Analysis
2.7. Quantitative Real-Time PCR
2.8. Statistical Analyses
3. Results
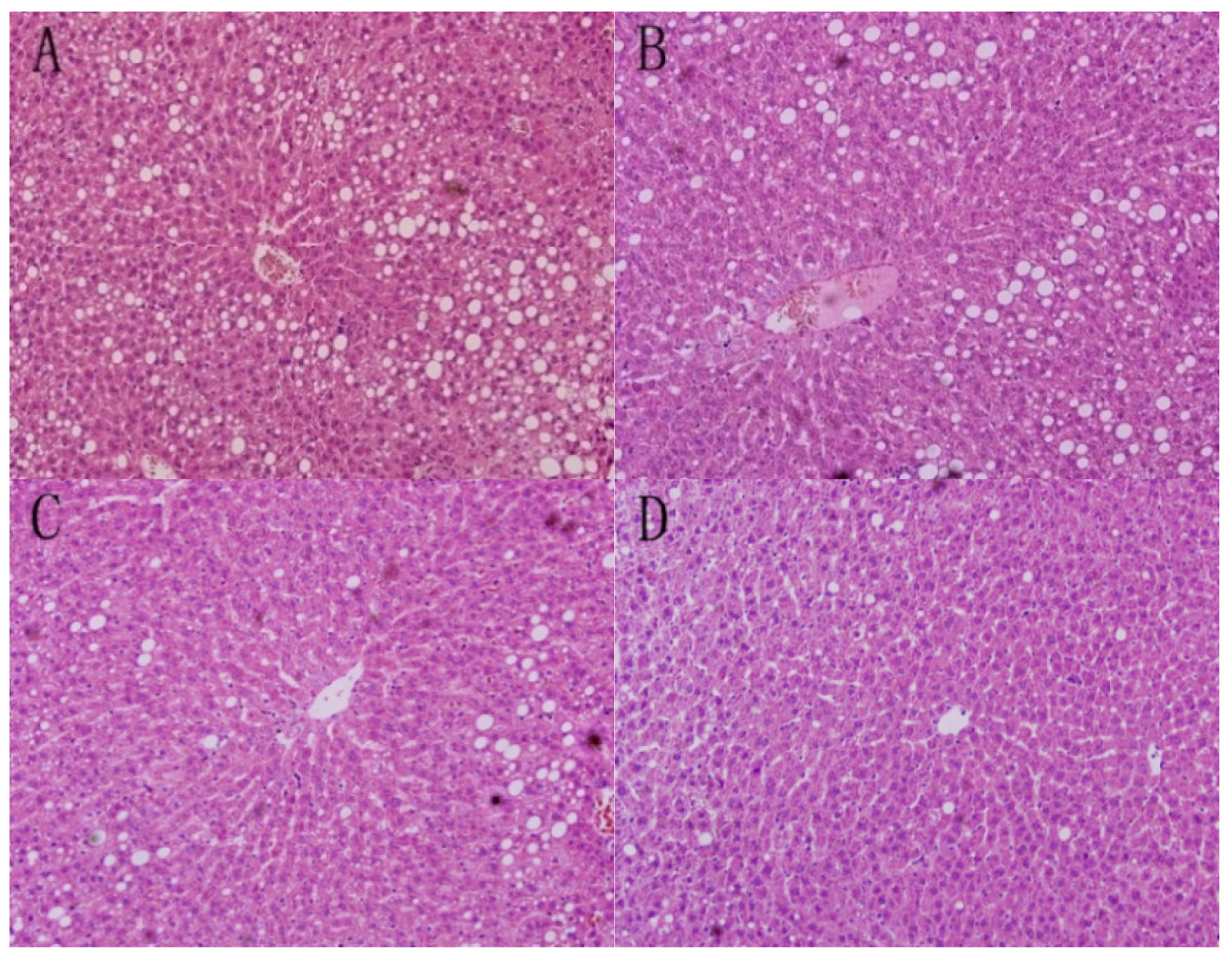
3.1. Liver Morphology
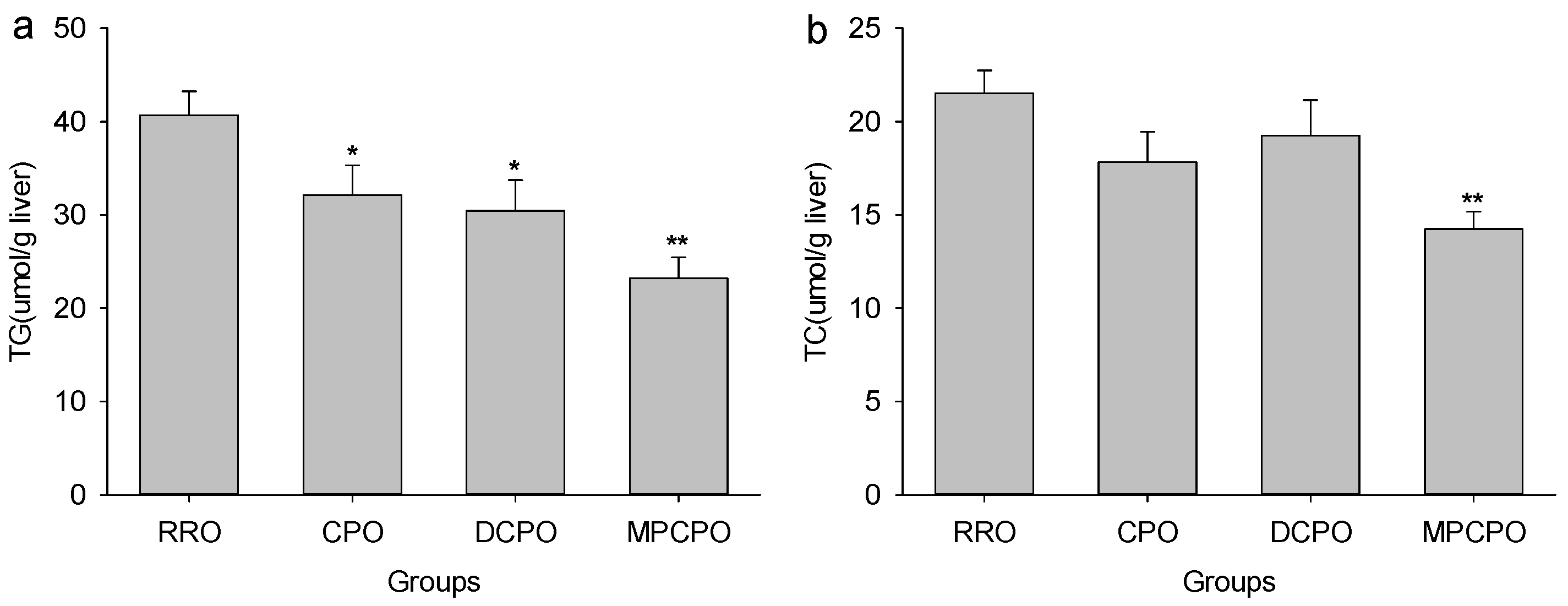
3.2. Liver Lipids Contents
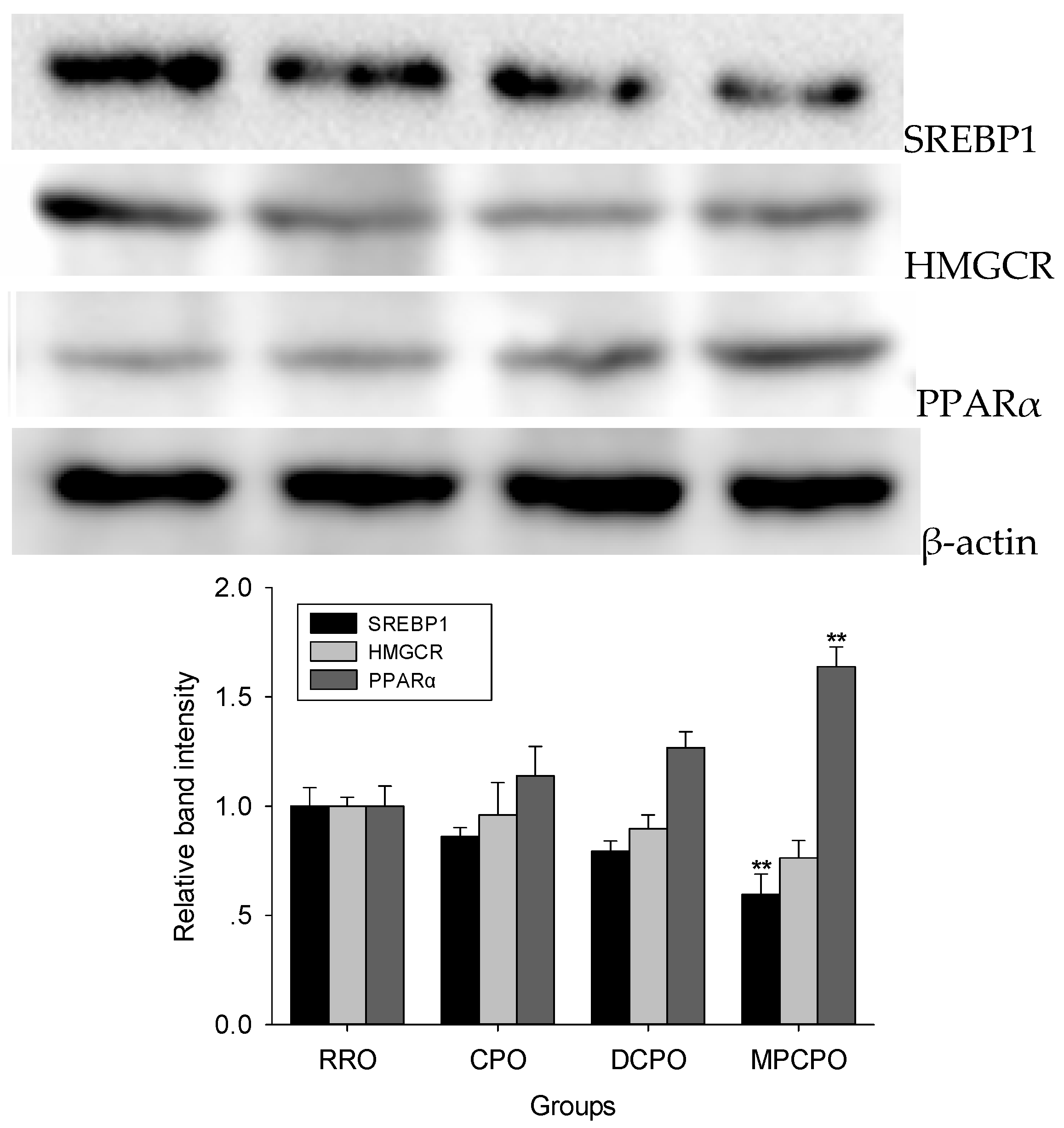
3.3. Liver Proteins Expressions
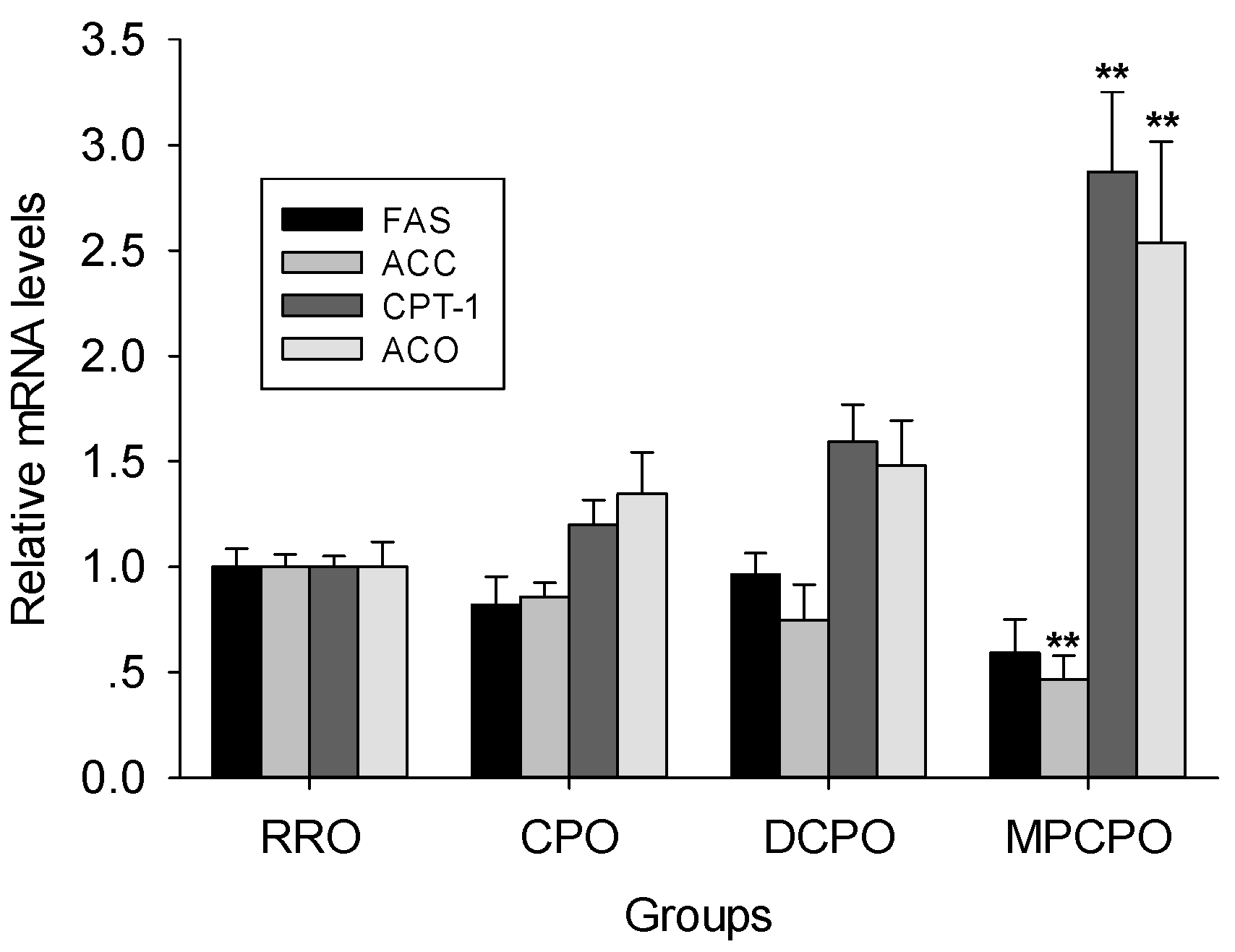
3.4. Liver mRNA Expressions
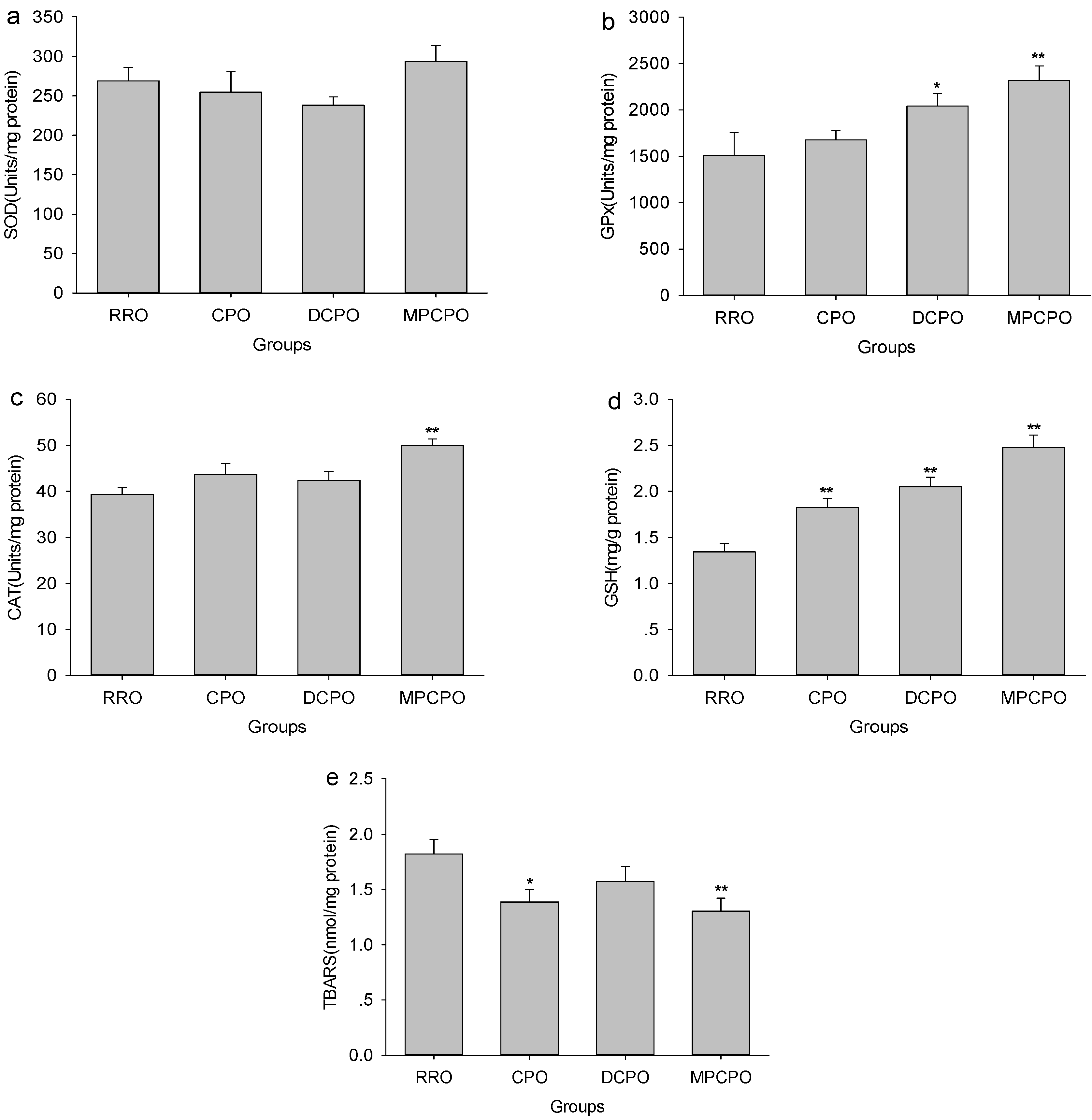
3.5. Liver Antioxidative Capacity and Lipid Peroxidation
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Farrell, G.C.; Chitturi, S.; Lau, G.K.; Sollano, J.D. Guidelines for the assessment and management of non-alcoholic fatty liver disease in the Asia-Pacific region: Executive summary. J. Gastroenterol. Hepatol. 2007, 22, 775–777. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.H.; Zhu, Y.X.; Lu, H.J.; Chen, H.F.; Li, Q.; Jiang, S.; Xiang, K.S.; Jia, W.P. Non-alcoholic fatty liver disease’s prevalence and impact on alanine aminotransferase associated with metabolic syndrome in the Chinese. J. Gastroenterol. Hepatol. 2011, 26, 722–730. [Google Scholar] [CrossRef] [PubMed]
- De Meijer, V.E.; Le, H.D.; Meisel, J.A.; Akhavan Sharif, M.R.; Pan, A.; Nose, V.; Puder, M. Dietary fat intake promotes the development of hepatic steatosis independently from excess caloric consumption in a murine model. Metabolism 2010, 59, 1092–1105. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.; Borlak, J. Molecular mechanisms and therapeutic targets in steatosis and steatohepatitis. Pharmacol. Rev. 2008, 60, 311–357. [Google Scholar] [CrossRef] [PubMed]
- Day, C.P.; James, O.F. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Li, C.; Yao, Y.; Zhao, G.; Cheng, W.; Liu, H.; Liu, C.; Shi, Z.; Chen, Y.; Wang, S. Comparison and analysis of fatty acids, sterols, and tocopherols in eight vegetable oils. J. Agric. Food Chem. 2011, 59, 12493–12498. [Google Scholar] [CrossRef] [PubMed]
- Catta-Preta, M.; Martins, M.A.; Cunha Brunini, T.M.; Mendes-Ribeiro, A.C.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. Modulation of cytokines, resistin, and distribution of adipose tissue in C57BL/6 mice by different high-fat diets. Nutrition 2012, 28, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Bose, M.; Lambert, J.D.; Ju, J.; Reuhl, K.R.; Shapses, S.A.; Yang, C.S. The major green tea polyphenol, (−)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice. J. Nutr. 2008, 138, 1677–1683. [Google Scholar] [PubMed]
- Ostlund, R.E., Jr. Phytosterols in human nutrition. Annu. Rev. Nutr. 2002, 22, 533–549. [Google Scholar] [CrossRef] [PubMed]
- Institute for Laboratory Animal Research (ILAR). Guide for the Care and Use of Laboratory Animals; National Academy Press: Washington, DC, USA, 2011. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Kono, Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch. Biochem. Biophys. 1978, 186, 189–195. [Google Scholar] [CrossRef]
- Sazuka, Y.; Tanizawa, H.; Takino, Y. Effect of adriamycin on the activities of superoxide dismutase, glutathione peroxidase and catalase in tissues of mice. Jpn. J. Cancer Res. 1989, 80, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Goth, L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta 1991, 196, 143–151. [Google Scholar] [CrossRef]
- Moron, M.S.; Depierre, J.W.; Mannervik, B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim. Biophys. Acta 1979, 582, 67–78. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Shimano, H. Sterol regulatory element-binding protein-1 as a dominant transcription factor for gene regulation of lipogenic enzymes in the liver. Trends Cardiovasc. Med. 2000, 10, 275–278. [Google Scholar] [CrossRef]
- Clemenz, M.; Frost, N.; Schupp, M.; Caron, S.; Foryst-Ludwig, A.; Bohm, C.; Hartge, M.; Gust, R.; Staels, B.; Unger, T.; et al. Liver-specific peroxisome proliferator-activated receptor α target gene regulation by the angiotensin type 1 receptor blocker telmisartan. Diabetes 2008, 57, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Fujita, T.; Usuda, N.; Cook, W.; Qi, C.; Peters, J.M.; Gonzalez, F.J.; Yeldandi, A.V.; Rao, M.S.; Reddy, J.K. Peroxisomal and mitochondrial fatty acid β-oxidation in mice nullizygous for both peroxisome proliferator-activated receptor α and peroxisomal fatty acyl-COA oxidase. Genotype correlation with fatty liver phenotype. J. Biol. Chem. 1999, 274, 19228–19236. [Google Scholar] [CrossRef] [PubMed]
- Iggman, D.; Gustafsson, I.B.; Berglund, L.; Vessby, B.; Marckmann, P.; Riserus, U. Replacing dairy fat with rapeseed oil causes rapid improvement of hyperlipidaemia: A randomized controlled study. J. Intern. Med. 2011, 270, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, E.; Doddivenaka, C.; Zhang, X.; Bommareddy, A.; Krishnan, P.; Matthees, D.P.; Dwivedi, C. Chemopreventive effects of dietary canola oil on colon cancer development. Nutr. Cancer 2011, 63, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Nguemeni, C.; Delplanque, B.; Rovere, C.; Simon-Rousseau, N.; Gandin, C.; Agnani, G.; Nahon, J.L.; Heurteaux, C.; Blondeau, N. Dietary supplementation of alpha-linolenic acid in an enriched rapeseed oil diet protects from stroke. Pharmacol. Res. 2010, 61, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Leyton, J.; Drury, P.J.; Crawford, M.A. Differential oxidation of saturated and unsaturated fatty acids in vivo in the rat. Br. J. Nutr. 1987, 57, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Bessesen, D.H.; Vensor, S.H.; Jackman, M.R. Trafficking of dietary oleic, linolenic, and stearic acids in fasted or fed lean rats. Am. J. Physiol. Endocrinol. MeTable 2000, 278, E1124–E1132. [Google Scholar]
- Sealls, W.; Gonzalez, M.; Brosnan, M.J.; Black, P.N.; DiRusso, C.C. Dietary polyunsaturated fatty acids (c18:2 omega6 and c18:3 omega3) do not suppress hepatic lipogenesis. Biochim. Biophys. Acta 2008, 1781, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhou, X.; Gao, H.; Chen, C.; Deng, Q.; Huang, Q.; Ma, J.; Wan, Z.; Yang, J.e.; Huang, F. Micronutrients-fortified rapeseed oil improves hepatic lipid accumulation and oxidative stress in rats fed a high-fat diet. Lipids Health Dis. 2013, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Ou, T.T.; Hsu, M.J.; Chan, K.C.; Huang, C.N.; Ho, H.H.; Wang, C.J. Mulberry extract inhibits oleic acid-induced lipid accumulation via reduction of lipogenesis and promotion of hepatic lipid clearance. J. Sci. Food Agric. 2011, 91, 2740–2748. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Lee, S.J.; Jang, S.H.; Ha, J.H.; Song, Y.M.; Ko, Y.G.; Kim, H.D.; Min, W.; Kang, S.N.; Cho, J.H. Sasa borealis stem extract attenuates hepatic steatosis in high-fat diet-induced obese rats. Nutrients 2014, 6, 2179–2195. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Chen, S.C.; Ou, T.T.; Chyau, C.C.; Chang, Y.C.; Wang, C.J. Mulberry leaf polyphenol extracts reduced hepatic lipid accumulation involving regulation of adenosine monophosphate activated protein kinase and lipogenic enzymes. J. Funct. Foods 2013, 5, 1620–1632. [Google Scholar] [CrossRef]
- Min, H.K.; Kapoor, A.; Fuchs, M.; Mirshahi, F.; Zhou, H.; Maher, J.; Kellum, J.; Warnick, R.; Contos, M.J.; Sanyal, A.J. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell MeTable 2012, 15, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Xie, P.; Zhao, F.; Zhang, L.; An, W.; Zhan, Y. Mechanism of the promotion of steatotic HEPG2 cell apoptosis by cholesterol. Int. J. Clin. Exp. Pathol. 2014, 7, 6807–6813. [Google Scholar] [PubMed]
- Moghadasian, M.H.; Nguyen, L.B.; Shefer, S.; Salen, G.; Batta, A.K.; Frohlich, J.J. Hepatic cholesterol and bile acid synthesis, low-density lipoprotein receptor function, and plasma and fecal sterol levels in mice: Effects of apolipoprotein E deficiency and probucol or phytosterol treatment. Metabolism 2001, 50, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Rideout, T.C.; Harding, S.V.; Jones, P.J. Consumption of plant sterols reduces plasma and hepatic triglycerides and modulates the expression of lipid regulatory genes and de novo lipogenesis in C57BL/6J mice. Mol. Nutr. Food Res. 2010, 54 (Suppl. 1), S7–S13. [Google Scholar] [CrossRef] [PubMed]
- Harding, S.V.; Rideout, T.C.; Jones, P.J. Hepatic nuclear sterol regulatory binding element protein 2 abundance is decreased and that of ABCG5 increased in male hamsters fed plant sterols. J. Nutr. 2010, 140, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Raso, G.M.; Esposito, E.; Iacono, A.; Pacilio, M.; Cuzzocrea, S.; Canani, R.B.; Calignano, A.; Meli, R. Comparative therapeutic effects of metformin and vitamin E in a model of non-alcoholic steatohepatitis in the young rat. Eur. J. Pharmacol. 2009, 604, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Kim do, Y.; Kim, J.; Ham, H.J.; Choue, R. Effects of d-α-tocopherol supplements on lipid metabolism in a high-fat diet-fed animal model. Nutr. Res. Pract. 2013, 7, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Jadeja, R.N.; Thounaojam, M.C.; Dandekar, D.S.; Devkar, R.V.; Ramachandran, A.V. Clerodendron glandulosum.Coleb extract ameliorates high fat diet/fatty acid induced lipotoxicity in experimental models of non-alcoholic steatohepatitis. Food Chem. Toxicol. 2010, 48, 3424–3431. [Google Scholar] [CrossRef] [PubMed]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.; Ferreira, I.C. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Piazzon, A.; Vrhovsek, U.; Masuero, D.; Mattivi, F.; Mandoj, F.; Nardini, M. Antioxidant activity of phenolic acids and their metabolites: Synthesis and antioxidant properties of the sulfate derivatives of ferulic and caffeic acids and of the acyl glucuronide of ferulic acid. J. Agric. Food Chem. 2012, 60, 12312–12323. [Google Scholar] [CrossRef] [PubMed]
- Frei, B.; Higdon, J.V. Antioxidant activity of tea polyphenols in vivo: Evidence from animal studies. J. Nutr. 2003, 133, 3275S–3284S. [Google Scholar] [PubMed]
- Lin, Y.L.; Chang, Y.Y.; Yang, D.J.; Tzang, B.S.; Chen, Y.C. Beneficial effects of noni (Morinda citrifolia L.) juice on livers of high-fat dietary hamsters. Food Chem. 2013, 140, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-Y.; Yang, D.-J.; Chiu, C.-H.; Lin, Y.-L.; Chen, J.-W.; Chen, Y.-C. Antioxidative and anti-inflammatory effects of polyphenol-rich litchi (Litchi chinensis Sonn.)-flower-water-extract on livers of high-fat-diet fed hamsters. J. Funct. Foods 2013, 5, 44–52. [Google Scholar] [CrossRef]
- Vivancos, M.; Moreno, J.J. β-sitosterol modulates antioxidant enzyme response in raw 264.7 macrophages. Free Radic. Biol. Med. 2005, 39, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Baskar, A.A.; Al Numair, K.S.; Gabriel Paulraj, M.; Alsaif, M.A.; Muamar, M.A.; Ignacimuthu, S. β-sitosterol prevents lipid peroxidation and improves antioxidant status and histoarchitecture in rats with 1,2-dimethylhydrazine-induced colon cancer. J. Med. Food 2012, 15, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Milbury, P.E.; Lapsley, K.; Blumberg, J.B. Flavonoids from almond skins are bioavailable and act synergistically with vitamins C and E to enhance hamster and human ldl resistance to oxidation. J. Nutr. 2005, 135, 1366–1373. [Google Scholar] [PubMed]
- Vivancos, M.; Moreno, J.J. Effect of resveratrol, tyrosol and beta-sitosterol on oxidised low-density lipoprotein-stimulated oxidative stress, arachidonic acid release and prostaglandin E2 synthesis by raw 264.7 macrophages. Br. J. Nutr. 2008, 99, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, S.; Leonard, S.; Traber, M.G.; Jialal, I. Gamma-tocopherol supplementation alone and in combination with α-tocopherol alters biomarkers of oxidative stress and inflammation in subjects with metabolic syndrome. Free Radic. Biol. Med. 2008, 44, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Attorri, L.; di Biase, A.; di Benedetto, R.; Rigato, P.; di Virgilio, A.; Salvati, S. Micronutrient-enriched rapeseed oils reduce cardiovascular disease risk factors in rats fed a high-fat diet. Atherosclerosis 2010, 213, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Salvati, S.; Attorri, L.; di Benedetto, R.; Fortuna, S.; di Biase, A. Micronutrient-enriched rapeseed oils improve the brain oxidant/antioxidant system in rats fed a high-fat diet. J. Agric. Food Chem. 2011, 59, 4483–4488. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ma, C.; Han, L.; Gao, H.; Zhou, Q.; Yang, M.; Chen, C.; Deng, Q.; Huang, Q.; Huang, F. Optimized rapeseed oils rich in endogenous micronutrients ameliorate risk factors of atherosclerosis in high fat diet fed rats. Lipids Health Dis. 2014, 13. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhou, X.; Deng, Q.; Huang, Q.; Yang, J.E.; Huang, F. Rapeseed oil fortified with micronutrients reduces atherosclerosis risk factors in rats fed a high-fat diet. Lipids Health Dis. 2011, 10. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Liu, X.; Gao, H.; Chen, C.; Deng, Q.; Huang, Q.; Ma, Z.; Huang, F. Optimized Rapeseed Oils Rich in Endogenous Micronutrients Protect High Fat Diet Fed Rats from Hepatic Lipid Accumulation and Oxidative Stress. Nutrients 2015, 7, 8491-8502. https://doi.org/10.3390/nu7105407
Xu J, Liu X, Gao H, Chen C, Deng Q, Huang Q, Ma Z, Huang F. Optimized Rapeseed Oils Rich in Endogenous Micronutrients Protect High Fat Diet Fed Rats from Hepatic Lipid Accumulation and Oxidative Stress. Nutrients. 2015; 7(10):8491-8502. https://doi.org/10.3390/nu7105407
Chicago/Turabian StyleXu, Jiqu, Xiaoli Liu, Hui Gao, Chang Chen, Qianchun Deng, Qingde Huang, Zhonghua Ma, and Fenghong Huang. 2015. "Optimized Rapeseed Oils Rich in Endogenous Micronutrients Protect High Fat Diet Fed Rats from Hepatic Lipid Accumulation and Oxidative Stress" Nutrients 7, no. 10: 8491-8502. https://doi.org/10.3390/nu7105407
APA StyleXu, J., Liu, X., Gao, H., Chen, C., Deng, Q., Huang, Q., Ma, Z., & Huang, F. (2015). Optimized Rapeseed Oils Rich in Endogenous Micronutrients Protect High Fat Diet Fed Rats from Hepatic Lipid Accumulation and Oxidative Stress. Nutrients, 7(10), 8491-8502. https://doi.org/10.3390/nu7105407




