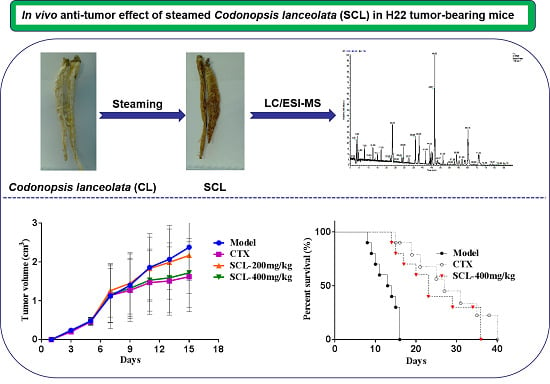
Anti-Tumor Effect of Steamed Codonopsis lanceolata in H22 Tumor-Bearing Mice and Its Possible Mechanism
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Reagents
2.2. Animals and Tumor Cell Lines
2.3. Sample Preparation
2.4. Animal Treatment and Experimental Design
2.5. Survival Assay
2.6. Assay of Cytokines
2.7. Hoechst 33258 Staining
2.8. Histological Analysis of Tumor Sections
2.9. Immunohistochemistry
2.10. LC/MS Analysis
2.11. Statistical Analysis
3. Results
3.1. Effect of SCL on Tumor Growth in H22 Tumor-Bearing Mice
| Groups | Dosage (mg/kg) | Weight (g) | Organ index (%) | Tumor weight (g) | Inhibitory rate (%) | |||
|---|---|---|---|---|---|---|---|---|
| Before | After | Thymus | Spleen | |||||
| model | - | 27.14 ± 1.43 | 33.96 ± 1.51 | 0.24 ± 0.11 | 0.69 ± 0.27 | 1.15 ± 0.85 | - | |
| CTX | 25 | 27.17 ± 1.41 | 34.03 ± 0.69 | 0.13 ± 0.10 * | 0.41 ± 0.07 ** | 0.21 ± 0.12 ** | 81.73 | |
| SCL | 200 | 27.34 ± 1.34 | 33.70 ± 2.84 | 0.20 ± 0.08 | 0.63 ± 0.15 | 0.62 ± 0.47 ** | 46.08 | |
| 400 | 26.71 ± 1.05 | 32.23 ± 2.12 | 0.22 ± 0.05 | 0.66 ± 0.08 | 0.45 ± 0.19 ** | 60.87 | ||
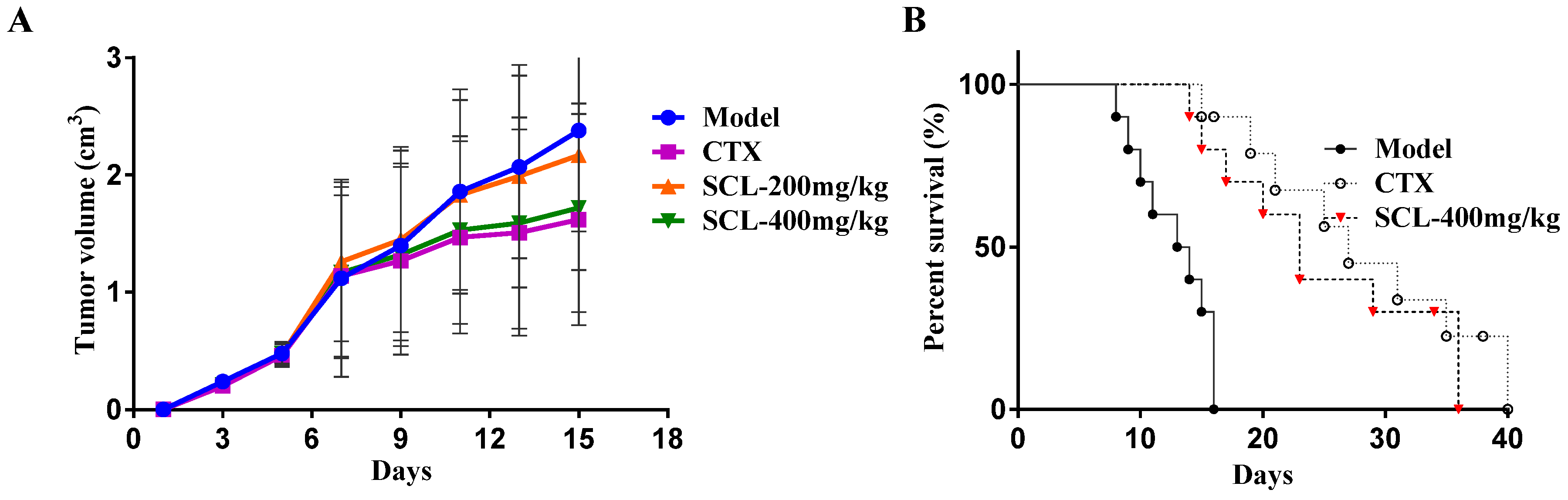
3.2. Effect of SCL on Life Extension in H22 Tumor-Bearing Mice
3.3. Effect of SCL on Body Weight and Organ Indices in Mice
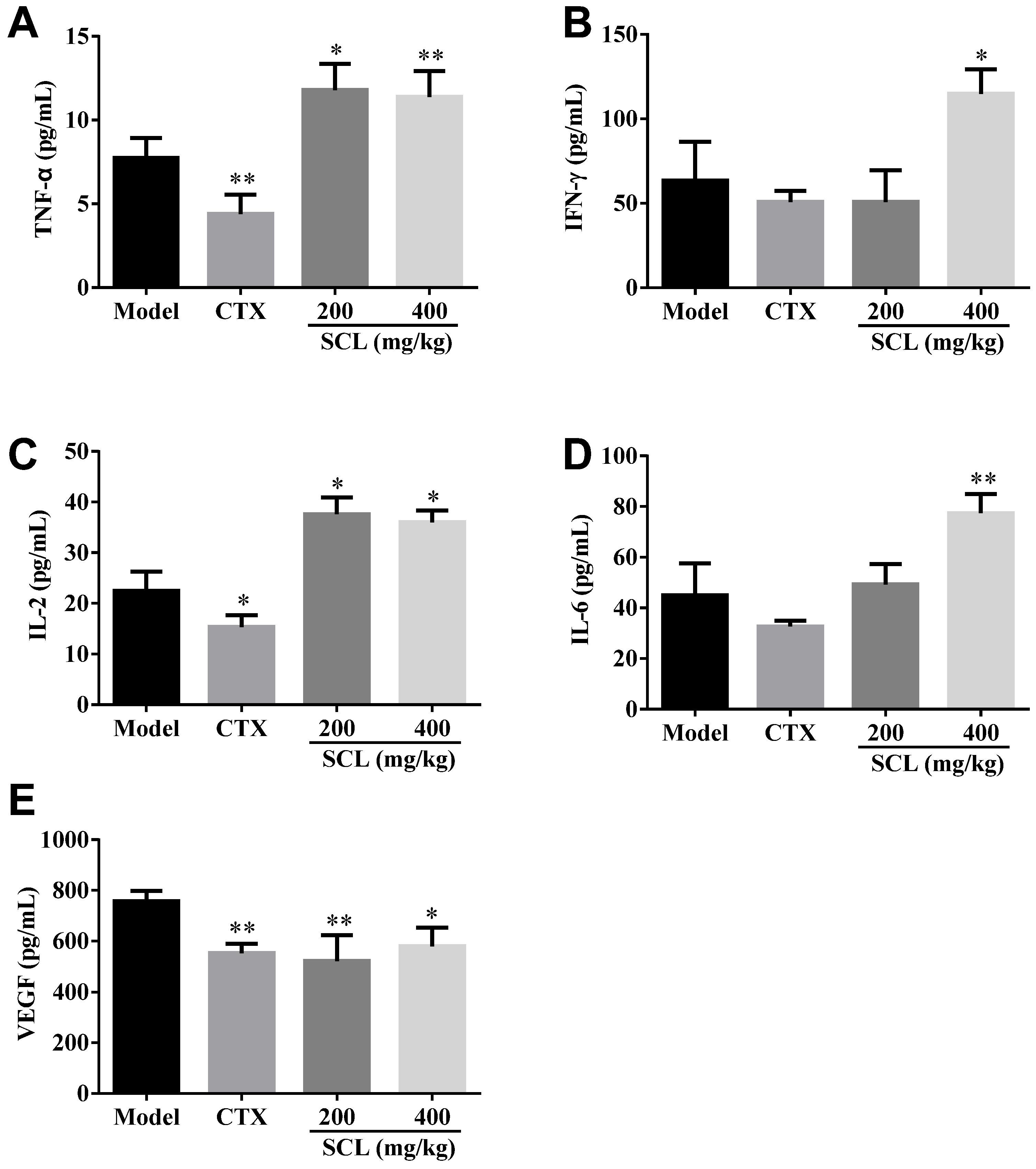
3.4. Effect of SCL on the Level of Serum Cytokines and VEGF
3.5. Morphological Change by Treatment of SCL
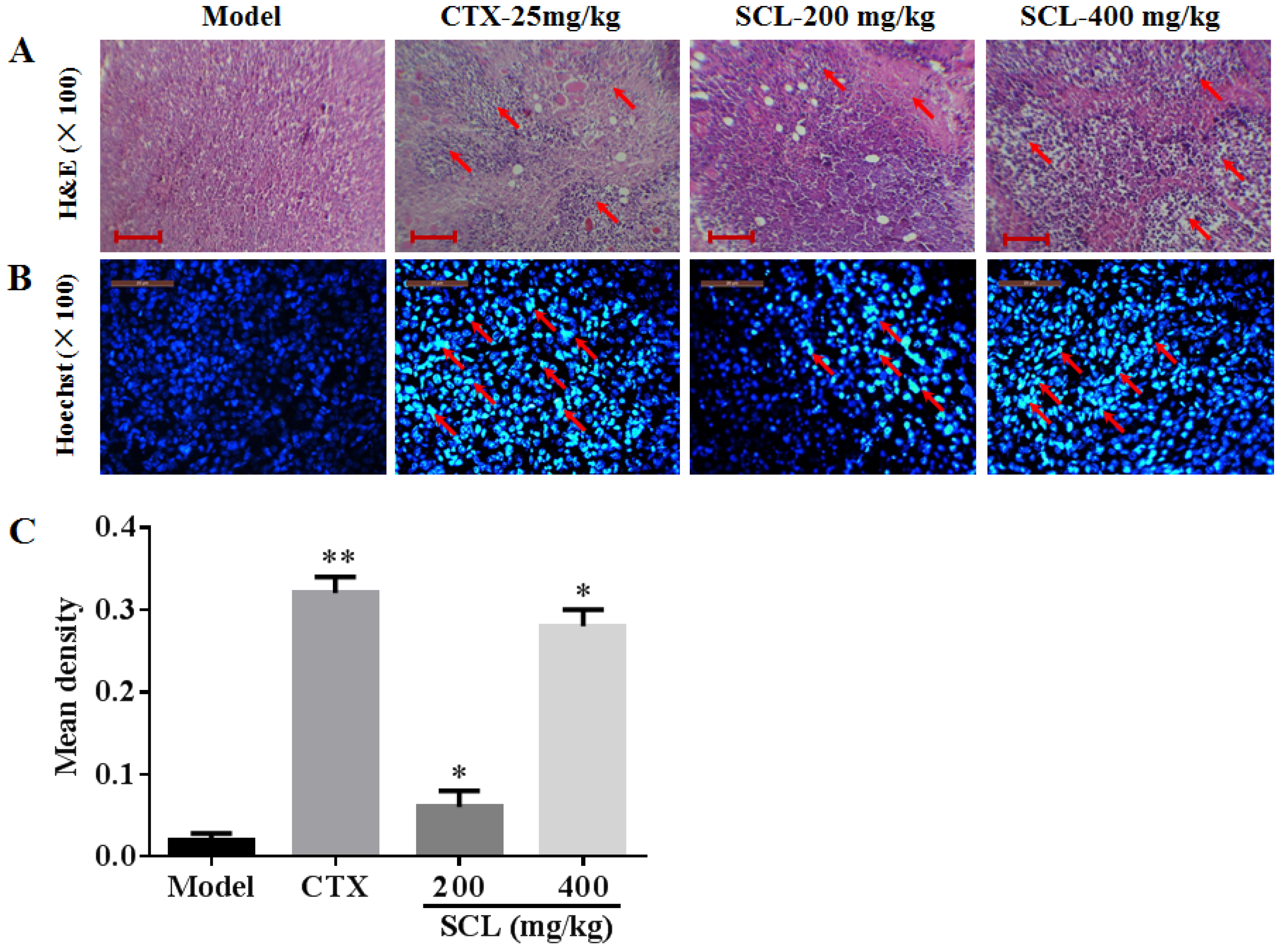
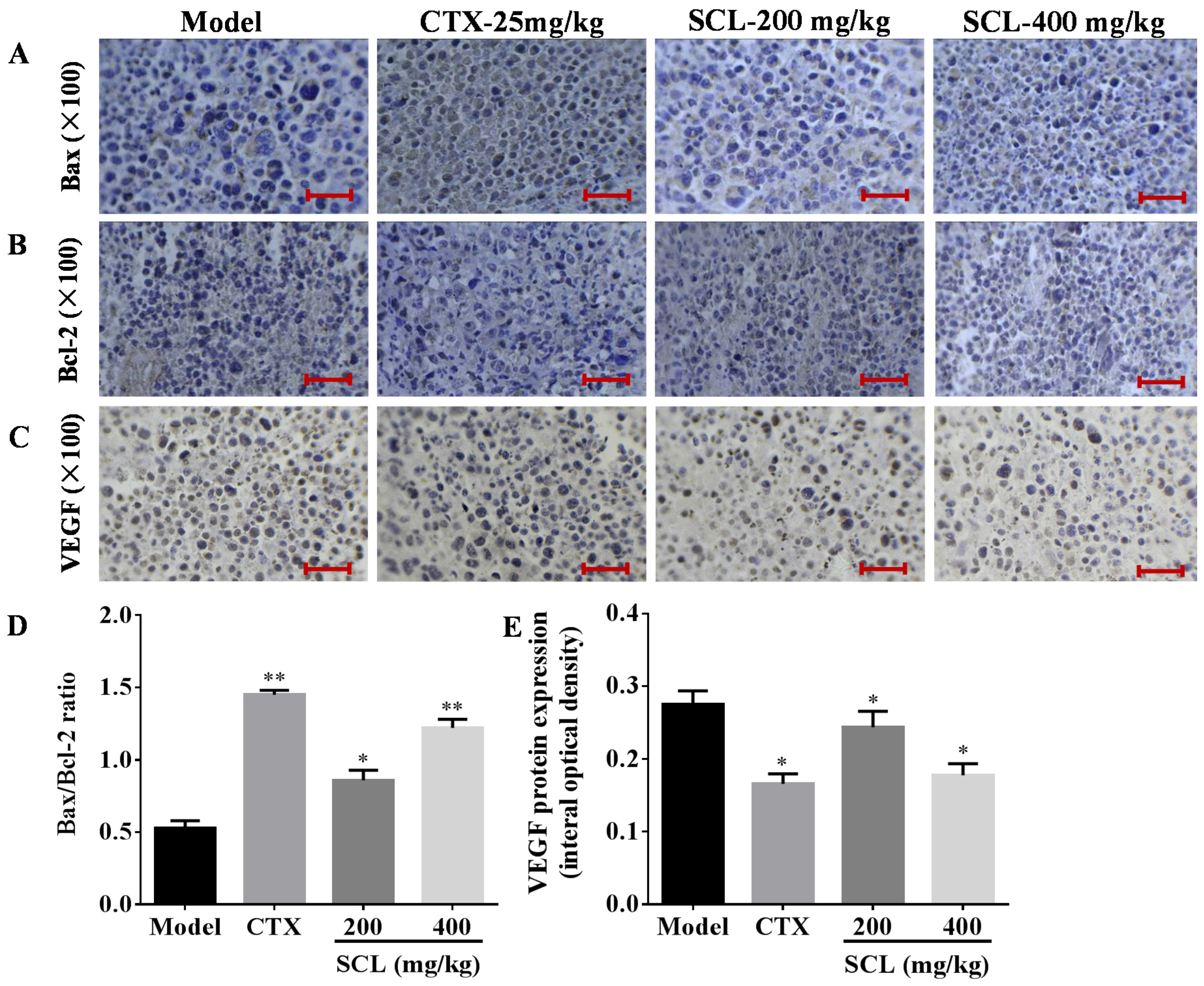
3.6. Effects of SCL on the Expression of Apoptosis-Related Proteins
3.7. Effects of SCL on the Expression of VEGF
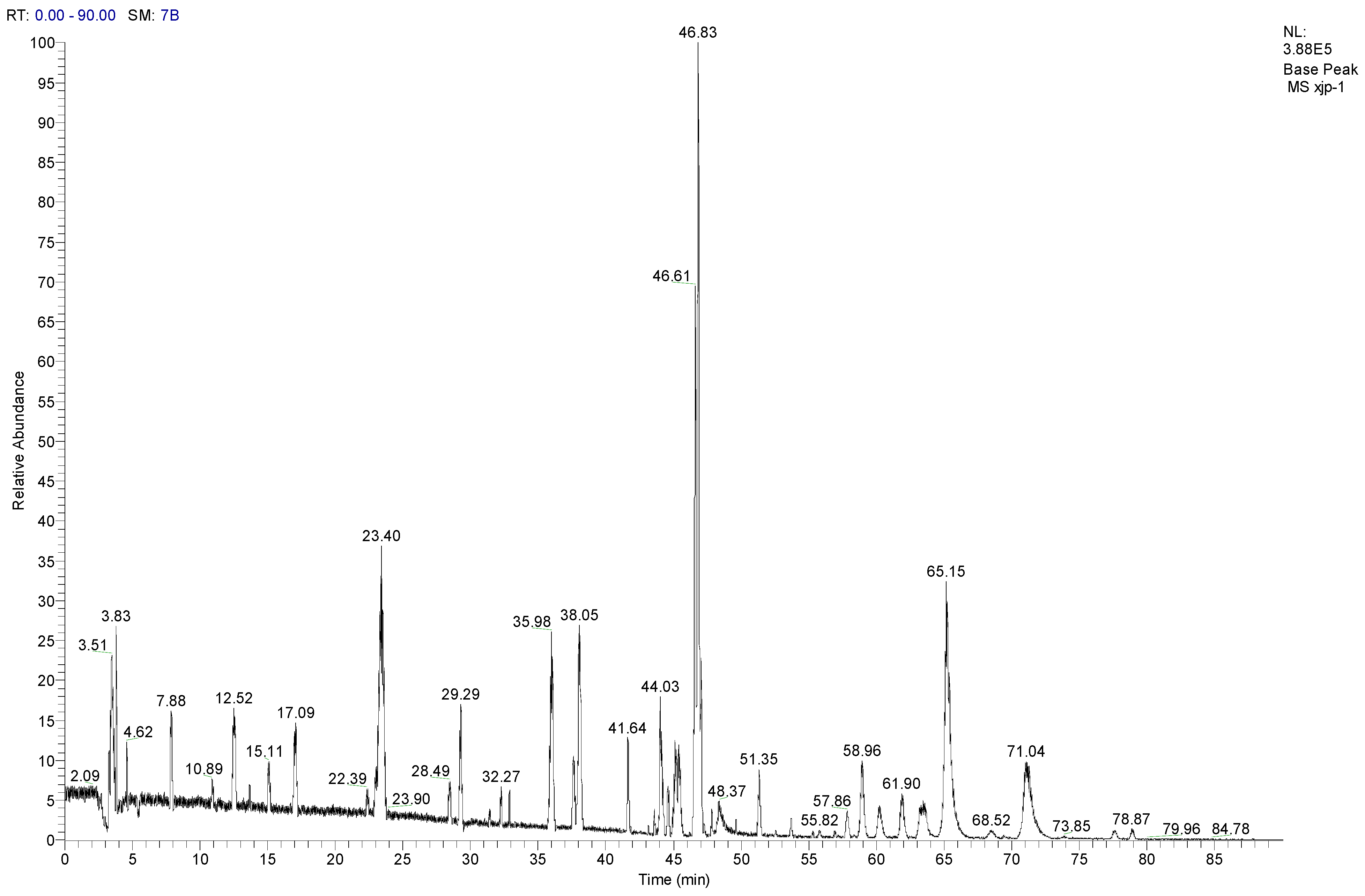
3.8. LC-MS/MS Analysis of SCL
| No. | Compounds | tR (min) | Parent ion (m/z) | Formula | Ion type | Mr. | Product ion (m/z) |
|---|---|---|---|---|---|---|---|
| 1 | Tangshenoside I | 23.4 | 696.9 | C29H42O18 | [M + NH4]+ | 678 | 193, 511, 513, 515, 678 |
| 2 | Lobetyolin | 28.49 | 414.2 | C20H28O8 | [M + NH4]+ | 396 | 295 |
| 3 | Lancemaside F | 43.61 | 1532.7 | C69H109O36 | [M + NH4]+ | 1514 | 267, 1371 |
| 4 | Lancemaside B | 44.14 | 1370.6 | C63H99O31 | [M + NH4]+ | 1352 | - |
| 5 | Lancemaside G | 44.21 | 1224.6 | C57H89O27 | [M + NH4]+ | 1206 | - |
| 6 | Lancemaside A | 45.14 | 1208.9 | C57H89O26 | [M + NH4]+ | 1190 | - |
| 7 | Codonoposide I | 47.85 | 1222.7 | C58H92O26 | [M + NH4]+ | 1204 | - |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lai, E.C.; Lau, W.Y. The continuing challenge of hepatic cancer in Asia. Surgeon 2005, 3, 210–215. [Google Scholar] [CrossRef]
- Karaman, B.; Battal, B.; Sari, S.; Verim, S. Hepatocellular carcinoma review: Current treatment, and evidence-based medicine. World J. Gastroentero 2014, 20, 18059–18060. [Google Scholar]
- Dobson, J. Reducing the side effects of cyclophosphamide chemotherapy in dogs. Vet. Rec. 2014, 174, 248–249. [Google Scholar] [CrossRef] [PubMed]
- Sitzia, J.; Huggins, L. Side effects of cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) chemotherapy for breast cancer. Cancer Pract. 1998, 6, 13–21. [Google Scholar] [PubMed]
- Wu, R.; Ru, Q.; Chen, L.; Ma, B.; Li, C. Stereospecificity of ginsenoside Rg3 in the promotion of cellular immunity in hepatoma H22-bearing mice. J. Food Sci. 2014, 79, H1430–H1435. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Nie, Z.K.; Zhou, Q.; Zhang, J.L.; Yin, J.J.; Xu, W.; Qiu, Y.; Ming, Y.L.; Liang, S. Antitumor efficacy in H22 tumor bearing mice and immunoregulatory activity on raw 264.7 macrophages of polysaccharides from talinum triangulare. Food Funct. 2014, 5, 2183–2193. [Google Scholar] [CrossRef] [PubMed]
- Weon, J.B.; Yun, B.R.; Lee, J.; Eom, M.R.; Ko, H.J.; Lee, H.Y.; Park, D.S.; Chung, H.C.; Chung, J.Y.; Ma, C.J. Neuroprotective effect of steamed and fermented Codonopsis lanceolata. Biomol. Ther. 2014, 22, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, M.; Ohta, S.; Komoto, N.; Ushijima, M.; Kodera, Y.; Hayama, M.; Shirota, O.; Sekita, S.; Kuroyanagi, M. Simultaneous determination of seven saponins in the roots of Codonopsis lanceolata by liquid chromatography-mass spectrometry. J. Nat. Med. 2009, 63, 52–57. [Google Scholar] [CrossRef]
- Hyam, S.R.; Jang, S.E.; Jeong, J.J.; Joh, E.H.; Han, M.J.; Kim, D.H. Echinocystic acid, a metabolite of lancemaside A, inhibits tnbs-induced colitis in mice. Int. Immunopharmacol. 2013, 15, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Yongxu, S.; Jicheng, L. Structural characterization of a water-soluble polysaccharide from the roots of Codonopsis lanceolata and its immunity activity. Int. J. Biol. Macromol. 2008, 43, 279–282. [Google Scholar] [CrossRef]
- Ushijima, M.; Komoto, N.; Sugizono, Y.; Mizuno, I.; Sumihiro, M.; Ichikawa, M.; Hayama, M.; Kawahara, N.; Nakane, T.; Shirota, O.; et al. Triterpene glycosides from the roots of Codonopsis lanceolata. Chem. Pharm. Bull. 2008, 56, 308–314. [Google Scholar] [PubMed]
- Cho, K.; Kim, S.J.; Park, S.H.; Kim, S.; Park, T. Protective effect of Codonopsis lanceolata root extract against alcoholic fatty liver in the rat. J. Med. Food 2009, 12, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, K.-J.; Kim, Y.-H.; Kim, D.-B.; Shin, G.-H.; Cho, J.-H.; Kim, B.K.; Lee, B.-Y.; Lee, O.-H. Codonopsis lanceolata extract prevents diet-induced obesity in C57BL/6 mice. Nutrients 2014, 6, 4663–4677. [Google Scholar] [CrossRef] [PubMed]
- Cha, A.; Choi, Y.; Jin, Y.; Sung, M.K.; Koo, Y.C.; Lee, K.W.; Park, T. Antilipogenic and anti-inflammatory activities of Codonopsis lanceolata in mice hepatic tissues after chronic ethanol feeding. J. Biomed. Biotechnol. 2012, 2012. [Google Scholar] [CrossRef]
- He, X.; Zou, Y.; Yoon, W.B.; Park, S.J.; Park, D.S.; Ahn, J. Effects of probiotic fermentation on the enhancement of biological and pharmacological activities of Codonopsis lanceolata extracted by high pressure treatment. J. Biosci. Bioeng. 2011, 112, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.S.; Qian, K.; Usami, Y.; Lin, L.; Itokawa, H.; Hsu, C.; Morris-Natschke, S.L.; Lee, K.H. 5-Hydroxymethyl-2-furfural, a clinical trials agent for sickle cell anemia, and its mono/di-glucosides from classically processed steamed rehmanniae radix. J. Nat. Med. 2008, 62, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.L.; Liu, Q.; Peng, Y.-B.; Qi, L.-W.; Li, P. Steamed ginger (Zingiber officinale): Changed chemical profile and increased anticancer potential. Food Chem. 2007, 129, 1785–1792. [Google Scholar] [CrossRef]
- Jiao, L.; Zhang, X.; Wang, M.; Li, B.; Liu, Z.; Liu, S. Chemical and antihyperglycemic activity changes of ginseng pectin induced by heat processing. Carbohyd. Polym. 2014, 114, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Cha, H.Y.; Seo, J.J.; Hong, J.T.; Han, K.; Oh, K.W. Anxiolytic-like effects of ginseng in the elevated plus-maze model: Comparison of red ginseng and sun ginseng. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 895–900. [Google Scholar] [PubMed]
- Weon, J.B.; Yun, B.R.; Lee, J.; Eom, M.R.; Ko, H.J.; Kim, J.S.; Lee, H.Y.; Park, D.S.; Chung, H.C.; Chung, J.Y.; et al. Effect of Codonopsis lanceolata with steamed and fermented process on scopolamine-induced memory impairment in mice. Biomol. Ther. 2013, 21, 405–410. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Weon, J.B.; Yun, B.R.; Lee, J.; Eom, M.R.; Ko, H.J.; Lee, H.Y.; Park, D.S.; Chung, H.C.; Chung, J.Y.; Ma, C.J. Cognitive-enhancing effect of steamed and fermented Codonopsis lanceolata: A behavioral and biochemical study. Evid. Based Complement. Alternat. Med. 2014, 2014. [Google Scholar] [CrossRef]
- Shin, J.A.; Kim, J.S.; Hong, I.S.; Cho, S.D. Bak is a key molecule in apoptosis induced by methanol extracts of Codonopsis lanceolata and tricholoma matsutake in HSC-2 human oral cancer cells. Oncol. Lett. 2012, 4, 1379–1383. [Google Scholar] [PubMed]
- Wang, L.; Xu, M.L.; Hu, J.H.; Rasmussen, S.K.; Wang, M.H. Codonopsis lanceolata extract induces G0/G1 arrest and apoptosis in human colon tumor HT-29 cells-involvement of ros generation and polyamine depletion. Food Chem. Toxicol. 2011, 49, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, X.; Xue, Y.; Wang, N.; Liu, W. Anti-hepatoma activity and mechanism of corn silk polysaccharides in H22 tumor-bearing mice. Int. J. Biol. Macromol. 2014, 64, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Shu, G.; Chen, L.; Mi, X.; Mei, Z.; Deng, X. A flavonoid component from Docynia delavayi (franch.) schneid represses transplanted H22 hepatoma growth and exhibits low toxic effect on tumor-bearing mice. Food Chem. Toxicol. 2012, 50, 3166–3173. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Miao, L.; Zhang, H.; Yang, O.; Ge, H.; Li, Y.; Wang, L. Induction of interleukin 2 expression in the liver for the treatment of H22 hepatoma in mice. Dig. Liver Dis. 2013, 45, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, H.; Li, J.; Lu, C.; Chen, G.; Zhang, G.; Lu, A.; He, X. Triptolide downregulates treg cells and the level of IL-10, TGF-beta, and VEGF in melanoma-bearing mice. Planta Med. 2013, 79, 1401–1407. [Google Scholar]
- Ishida, S.; Okasaka, M.; Ramos, F.; Kashiwada, Y.; Takaishi, Y.; Kodzhimatov, O.K.; Ashurmetov, O. New alkaloid from the aerial parts of Codonopsis lanceolata. J. Nat. Med. 2008, 62, 236–238. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.T.; Choi, J.; Jung, W.T.; Nam, J.H.; Jung, H.J.; Park, H.J. Structure of a new echinocystic acid bisdesmoside isolated from Codonopsis lanceolata roots and the cytotoxic activity of prosapogenins. J. Agri. Food Chem. 2002, 50, 4190–4193. [Google Scholar] [CrossRef]
- Xu, L.P.; Wang, H.; Yuan, Z. Triterpenoid saponins with anti-inflammatory activity from Codonopsis lanceolata. Planta Med. 2008, 74, 1412–1415. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Jung, H.J.; Park, H.J.; Kim, D.G.; Lee, J.Y.; Lee, K.T. β-d-xylopyranosyl-(1→3)-β-d-glucuronopyranosyl echinocystic acid isolated from the roots of Codonopsis lanceolata induces caspase-dependent apoptosis in human acute promyelocytic leukemia HL-60 cells. Biol. Pharm. Bull. 2005, 28, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Weon, J.B.; Yun, B.R.; Lee, J.; Eom, M.R.; Kim, J.S.; Lee, H.Y.; Park, D.S.; Chung, H.C.; Chung, J.Y.; Ma, C.J. The ameliorating effect of steamed and fermented Codonopsis lanceolata on scopolamine-induced memory impairment in mice. Evid. Based Complement. Alternat. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Chen, C.; Cowles, V.E.; Sweeney, M.; Stolyarov, I.D.; Illarioshkin, S.N. Pharmacokinetics and pharmacodynamics of gastroretentive delivery of levodopa/carbidopa in patients with parkinson disease. Clin. Neuropharmacol. 2012, 35, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Rajmani, R.S.; Singh, P.K.; Ravi Kumar, G.; Saxena, S.; Singh, L.V.; Kumar, R.; Sahoo, A.P.; Gupta, S.K.; Chaturvedi, U.; Tiwari, A.K. In-vitro characterization and evaluation of apoptotic potential of bicistronic plasmid encoding hn gene of newcastle disease virus and human TNF-alpha. Anim. Biotechnol. 2015, 26, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Lejeune, F.J.; Lienard, D.; Matter, M.; Ruegg, C. Efficiency of recombinant human TNF in human cancer therapy. Cancer Immun. 2006, 6, 6. [Google Scholar] [PubMed]
- Gansbacher, B.; Bannerji, R.; Daniels, B.; Zier, K.; Cronin, K.; Gilboa, E. Retroviral vector-mediated gamma-interferon gene transfer into tumor cells generates potent and long lasting antitumor immunity. Cancer Res. 1990, 50, 7820–7825. [Google Scholar] [PubMed]
- Wang, G.; Zhao, J.; Liu, J.; Huang, Y.; Zhong, J.J.; Tang, W. Enhancement of IL-2 and IFN-γ expression and NK cells activity involved in the anti-tumor effect of ganoderic acid me in vivo. Int. Immunopharmacol. 2007, 7, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.G.; Kim, J.Y.; Lee, J.Y.; Byeon, S.E.; Hong, E.K.; Lee, J.; Rhee, M.H.; Park, H.J.; Cho, J.Y. Regulatory effects of Codonopsis lanceolata on macrophage-mediated immune responses. J. Ethnopharmacol. 2007, 112, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.L.; Kuo, P.L.; Liu, C.F.; Lin, C.C. Acacetin-induced cell cycle arrest and apoptosis in human non-small cell lung cancer A549 cells. Cancer Lett. 2004, 212, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Wouters, B.G. Apoptosis, p53, and tumor cell sensitivity to anticancer agents. Cancer Res. 1999, 59, 1391–1399. [Google Scholar] [PubMed]
- Gogvadze, V.; Orrenius, S.; Zhivotovsky, B. Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochim. Biophys. Acta 2006, 1757, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Ghobrial, I.M.; Witzig, T.E.; Adjei, A.A. Targeting apoptosis pathways in cancer therapy. CA Cancer J. Clin. 2005, 55, 178–194. [Google Scholar] [CrossRef] [PubMed]
- Gapany, C.; Zhao, M.; Zimmermann, A. The apoptosis protector, Bcl-2 protein, is downregulated in bile duct epithelial cells of human liver allografts. J. Hepatol. 1997, 26, 535–542. [Google Scholar] [CrossRef]
- Li, J.T.; Zhang, J.L.; He, H.; Ma, Z.L.; Nie, Z.K.; Wang, Z.Z.; Xu, X.G. Apoptosis in human hepatoma HepG2 cells induced by corn peptides and its anti-tumor efficacy in H22 tumor bearing mice. Food Chem. Toxicol 2013, 51, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Li, J.; Zhu, J.; Wang, D.; Chen, S.; Bai, X. Hydroxysafflor yellow a inhibits angiogenesis of hepatocellular carcinoma via blocking ERK/MAPK and NF-kB signaling pathway in H22 tumor-bearing mice. Eur. J. Pharmacol. 2015, 754, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Li, Y.; Yi, Q.; Xie, F.; Du, B.; Feng, L.; Qiu, L. Total saponins from Albizia julibrissin inhibit vascular endothelial growth factor-mediated angiogenesis in vitro and in vivo. Mol. med. Rep. 2015, 11, 3405–3413. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Xu, Q.; He, Y.-F.; Liu, Y.; Yang, S.-B.; Wang, Z.; Zhang, J.; Zhao, L.-C. Anti-Tumor Effect of Steamed Codonopsis lanceolata in H22 Tumor-Bearing Mice and Its Possible Mechanism. Nutrients 2015, 7, 8294-8307. https://doi.org/10.3390/nu7105395
Li W, Xu Q, He Y-F, Liu Y, Yang S-B, Wang Z, Zhang J, Zhao L-C. Anti-Tumor Effect of Steamed Codonopsis lanceolata in H22 Tumor-Bearing Mice and Its Possible Mechanism. Nutrients. 2015; 7(10):8294-8307. https://doi.org/10.3390/nu7105395
Chicago/Turabian StyleLi, Wei, Qi Xu, Yu-Fang He, Ying Liu, Shu-Bao Yang, Zi Wang, Jing Zhang, and Li-Chun Zhao. 2015. "Anti-Tumor Effect of Steamed Codonopsis lanceolata in H22 Tumor-Bearing Mice and Its Possible Mechanism" Nutrients 7, no. 10: 8294-8307. https://doi.org/10.3390/nu7105395
APA StyleLi, W., Xu, Q., He, Y.-F., Liu, Y., Yang, S.-B., Wang, Z., Zhang, J., & Zhao, L.-C. (2015). Anti-Tumor Effect of Steamed Codonopsis lanceolata in H22 Tumor-Bearing Mice and Its Possible Mechanism. Nutrients, 7(10), 8294-8307. https://doi.org/10.3390/nu7105395