Fucoidan Supplementation Improves Exercise Performance and Exhibits Anti-Fatigue Action in Mice
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials, Animals, and Experiment Design
2.2. Forelimb Grip Strength Test
2.3. Swimming Exercise Performance Test
2.4. Determination of Fatigue-Associated Biochemical Variables
2.5. Clinical Biochemical Profiles
2.6. Histological Staining of Tissues
2.7. Statistical Analysis
3. Results and Discussion
3.1. Effects of FCD on Forelimb Grip Strength
3.2. Effects of FCD on Exercise Performance in a Weight-Loaded Swimming Test
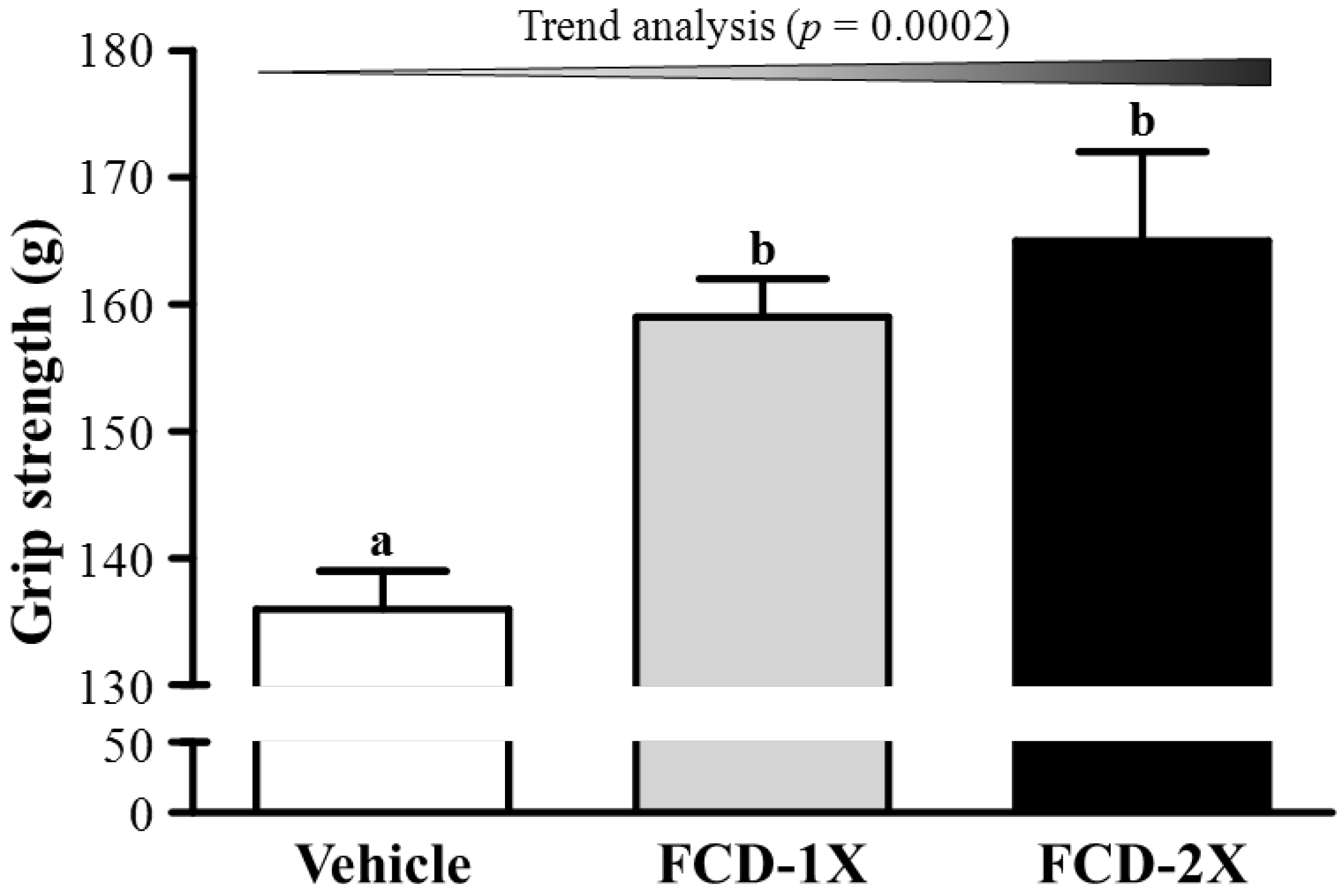
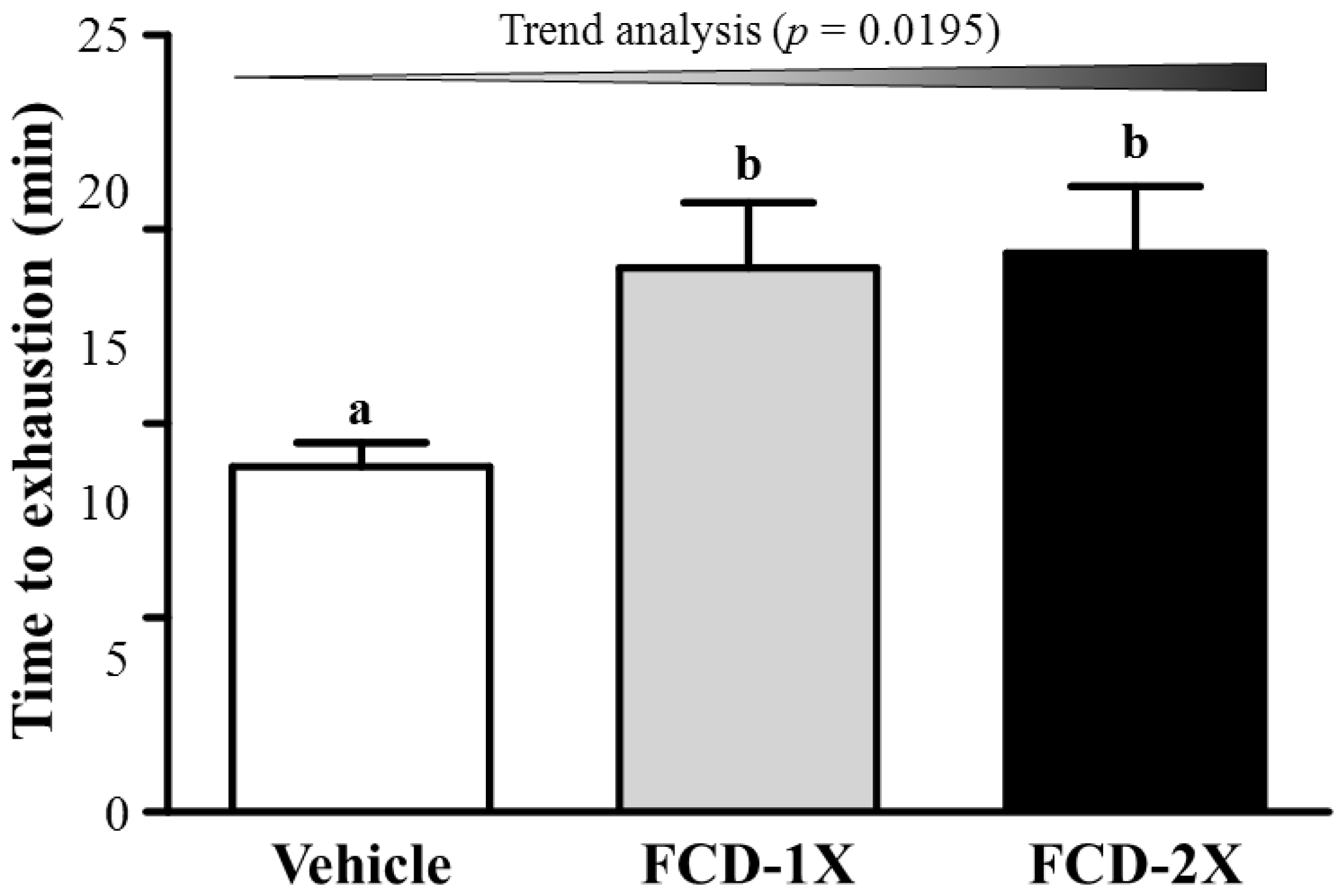
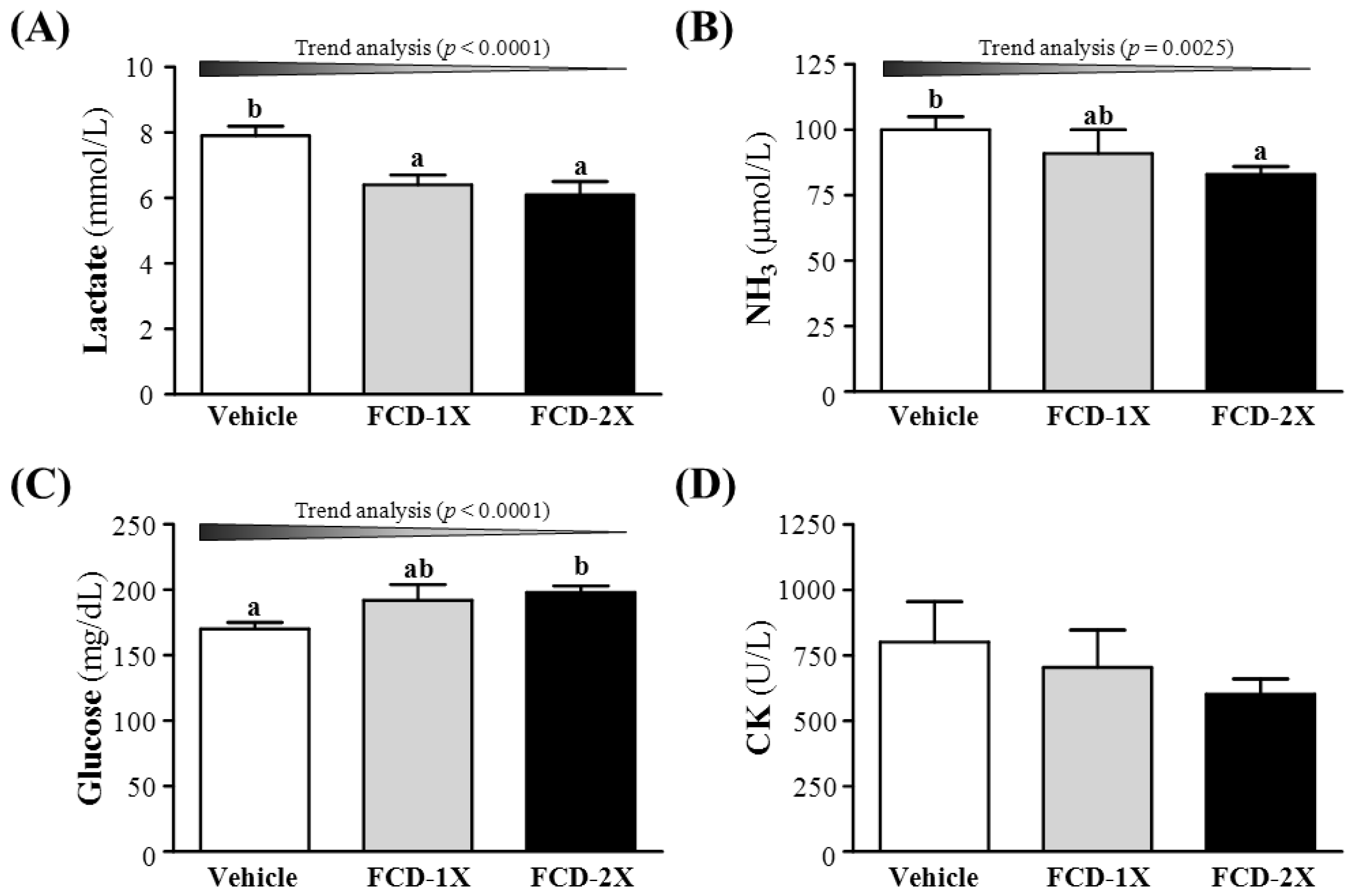
3.3. Effect of FCD Supplementation on Serum Lactate, Ammonia, Glucose and CK Levels after Acute Exercise Challenge
3.4. Subchronic Toxicity Evaluation of FCD Supplementation
| Characteristic | Vehicle | FCD-1X | FCD-2X | Trend Analysis |
|---|---|---|---|---|
| Initial BW (g) | 31.9 ± 0.6 | 32.7 ± 0.4 | 32.8 ± 0.5 | 0.1167 |
| Final BW (g) | 35.1 ± 0.7 a | 36.3 ± 0.5 ab | 38.1 ± 0.6 b | <0.0003 (↑) |
| Food intake (g/day) | 6.1 ± 0.0 a | 6.6 ± 0.1 b | 6.6 ± 0.1 b | <0.0001 (↑) |
| Water intake (mL/day) | 7.6 ± 0.1 a | 8.5 ± 0.2 b | 8.8 ± 0.2 b | <0.0001 (↑) |
| Weight (g) | ||||
| Liver | 1.98 ± 0.08 a | 2.01 ± 0.06 ab | 2.20 ± 0.04 b | 0.0073 (↑) |
| Muscle | 0.35 ± 0.01 a | 0.35 ± 0.01 ab | 0.38 ± 0.01 b | 0.0089 (↑) |
| Heart | 0.21 ± 0.01 | 0.21 ±0.01 | 0.22 ± 0.01 | 0.8012 |
| Lung | 0.38 ± 0.03 | 0.39 ± 0.02 | 0.43 ± 0.03 | 0.0901 |
| Kidney | 0.62 ± 0.05 | 0.61 ± 0.02 | 0.63 ± 0.02 | 0.4339 |
| EFP | 0.49 ± 0.04 | 0.58 ± 0.07 | 0.56 ± 0.03 | 0.2890 |
| BAT | 0.159 ± 0.009 a | 0.167 ± 0.007 ab | 0.184 ± 0.006 b | 0.0081 (↑) |
| Relative Weight (%) | ||||
| Liver | 5.64 ± 0.16 | 5.54 ± 0.10 | 5.79 ± 0.08 | 0.2432 |
| Muscle | 0.99 ± 0.02 | 0.97 ± 0.01 | 0.99 ± 0.01 | 0.9784 |
| Heart | 0.61 ± 0.03 | 0.59 ± 0.03 | 0.59 ± 0.03 | 0.6053 |
| Lung | 1.08 ± 0.10 | 1.08 ±0.07 | 1.13 ± 0.07 | 0.5161 |
| Kidney | 1.76 ± 0.12 | 1.68 ± 0.07 | 1.65 ± 0.04 | 0.7710 |
| EFP | 1.40 ± 0.11 | 1.58 ± 0.18 | 1.47 ± 0.09 | 0.5741 |
| BAT | 0.45 ± 0.02 | 0.46 ± 0.02 | 0.48 ± 0.02 | 0.1330 |
3.5. Effect of FCD Supplementation on Biochemical Analyses at the End of the Experiment
| Parameter | Vehicle | FCD-1X | FCD-2X | Trend Analysis |
|---|---|---|---|---|
| AST (U/L) | 70 ± 5 b | 57 ± 3 a | 60 ± 4 ab | 0.1327 |
| ALT (U/L) | 51 ± 2 | 42 ± 2 | 45 ± 5 | 0.1072 |
| ALP (U/L) | 283 ± 12 ab | 263 ± 19 a | 311 ± 13 b | 0.1086 |
| LDH (U/L) | 328 ± 17 | 342 ± 26 | 350 ± 29 | 0.4820 |
| CK (U/L) | 170 ± 23 b | 101 ± 16 a | 124 ± 15 ab | 0.1381 |
| Albumin (g/dL) | 3.5 ± 0.0 a | 3.7 ± 0.0 b | 3.6 ± 0.0 ab | 0.5587 |
| TBIL (μg/dL) | 62 ± 6 | 58 ± 5 | 69 ± 4 | 0.2365 |
| TP (g/dL) | 5.8 ± 0.1 a | 6.2 ± 0.1 b | 6.1 ± 0.1 b | 0.0297 (↑) |
| BUN (mg/dL) | 29.0 ± 1.0 b | 24.7 ± 0.7 a | 26.0 ± 0.7 a | 0.0320 (↓) |
| Creatinine (mg/dL) | 0.32 ± 0.01 | 0.31 ± 0.01 | 0.32 ± 0.01 | 0.7530 |
| UA (mg/dL) | 1.00 ± 0.05 | 0.98 ± 0.11 | 1.11 ± 0.11 | 3.664 |
| TC (mg/dL) | 167 ± 8 | 161 ± 9 | 152 ± 10 | 0.5126 |
| TG (mg/dL) | 167 ± 17 c | 119 ± 12 b | 69 ± 8 a | <0.0001 (↓) |
| Glucose (mg/dL) | 204 ± 6 | 191 ± 11 | 195 ± 9 | 0.4642 |

3.6. Effect of FCD Supplementation on Histological Examinations at the End of the Experiment
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and Bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Q.; Zhang, Z.; Zhang, H.; Niu, X. Structural studies on a novel fucogalactan sulfate extracted from the brown seaweed Laminaria japonica. Int. J. Biol. Macromol. 2010, 47, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Rocha, H.A.; Franco, C.R.; Trindade, E.S.; Veiga, S.S.; Leite, E.L.; Nader, H.B.; Dietrich, C.P. Fucan inhibits Chinese hamster ovary cell (CHO) adhesion to fibronectin by binding to the extracellular matrix. Planta Med. 2005, 71, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Aisa, Y.; Miyakawa, Y.; Nakazato, T.; Shibata, H.; Saito, K.; Ikeda, Y.; Kizaki, M. Fucoidan induces apoptosis of human HS-Sultan cells accompanied by activation of caspase-3 and down-regulation of ERK Pathways. Am. J. Hematol. 2005, 78, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Nishino, T.; Nagumo, T. Structure characterization of a new anticoagulant fucan sulfate from the brown seaweed Ecklonia kurome. Carbohydr. Res. 1991, 211, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Berteau, O.; Mulloy, B. Sulfated fucans, fresh perspective: Structure, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 2003, 13, 29–40. [Google Scholar]
- Duo, C.C.; Gong, F.Y.; He, X.Y.; Li, Y.M.; Wang, J.; Zhang, J.P.; Gao, X.M. Soluble calreticulin induces tumor necrosis factor-α (TNF-α) and interleukin (IL)-6 production by macrophages through Mitogen-Activated Protein Kinase (MAPK) and NFκB signaling pathways. Int. J. Mol. Sci. 2014, 15, 2916–2928. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kim, J.H.; Jin, H.J.; Lee, S.Y. Antimicrobial activity of ethanol extracts of Laminaria japonica against oral microorganisms. Anaerobe 2013, 21, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Q.; Zhang, Z.; Li, Z. Antioxidant activity of sulfated polysaccharide fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2008, 42, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Liu, M.; Fang, Z.; Zhang, Q. In vitro antioxidant effects and cytotoxicity of polysaccharides extracted from Laminaria japonica. Int. J. Biol. Macromol. 2012, 50, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.J.; Rice, C.L. Fatigue-induced reductions of torque and shortening velocity are muscle dependent. Med. Sci. Sports Exerc. 2010, 42, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.H.; Blomqvist, G. Maximal oxygen uptake. N. Engl. J. Med. 1971, 284, 1018. [Google Scholar] [CrossRef] [PubMed]
- Noakes, T.D. Time to move beyond a brainless exercise physiology: The evidence for complex regulation of human exercise performance. Appl. Physiol. Nutr. Metab. 2011, 36, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.B. Nitric oxide, reactive oxygen species, and skeletal muscle contraction. Med. Sci. Sports Exerc. 2001, 33, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Hsu, M.C.; Huang, W.C.; Yang, H.R.; Hou, C.C. Triterpenoid-rich extract from Antrodia camphorata improves physical fatigue and exercise performance in mice. Evid. Based Complement. Alternat. Med. 2012. [Google Scholar] [CrossRef]
- Su, K.Y.; Yu, C.Y.; Chen, Y.W.; Huang, Y.T.; Chen, C.T.; Wu, H.F.; Chen, Y.L. Rutin, a flavonoid and principal component of Saussurea involucrata, attenuates physical fatigue in a forced swimming mouse model. Int. J. Med. Sci. 2014, 11, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.E.; Huang, W.C.; Liao, C.C.; Chang, Y.K.; Kan, N.W.; Huang, C.C. Resveratrol protects against physical fatigue and improves exercise performance in mice. Molecules 2013, 18, 4689–4702. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.A.; Lee, S.H.; Ko, C.I.; Cha, S.H.; Kang, M.C.; Kang, S.M.; Jeon, Y.J. Protective effect of fucoidan against AAPH-induced oxidative stress in zebrafish model. Carbohydr. Polym. 2014, 102, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Huang, X.; Cheng, L.; Bu, Y.; Liu, G.; Yi, F.; Song, F. Evaluation of antioxidant and immune activity of Phellinus ribis glucan in mice. Food Chem. 2009, 115, 581–584. [Google Scholar] [CrossRef]
- Wang, S.Y.; Huang, W.C.; Liu, C.C.; Wang, M.F.; Ho, C.S.; Huang, W.P.; Hou, C.C.; Chuang, H.L.; Huang, C.C. Pumpkin (Cucurbita moschata) fruit extract improves physical fatigue and exercise performance in mice. Molecules 2012, 17, 11864–11876. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Huang, W.C.; Chiu, C.C.; Chang, Y.K.; Huang, C.C. Whey protein improves exercise performance and biochemical profiles in trained mice. Med. Sci. Sports Exerc. 2014, 46, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Chen, Y.M.; Kan, N.W.; Chao, H.L.; Ho, C.S.; Hsu, M.C. Cornu cervi pantotrichum supplementation improves exercise performance and protects against physical fatigue in mice. Molecules 2014, 19, 4669–4680. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Lin, C.I.; Chiu, C.C.; Lin, Y.T.; Huang, W.K.; Huang, H.Y.; Huang, C.C. Chicken essence improves exercise performance and ameliorates physical fatigue. Nutrients 2014, 6, 2681–2696. [Google Scholar] [CrossRef] [PubMed]
- Strojnik, V.; Komi, P.V. Fatigue after submaximal intensive stretch-shortening cycle exercise. Med. Sci. Sports Exerc. 2000, 32, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, M.; González-Izal, M.; Navarro-Amezqueta, I.; Calbet, J.A.; Ibañez, J.; Malanda, A.; Mallor, F.; Häkkinen, K.; Kraemer, W.J.; Gorostiaga, E.M. Effects of strength training on muscle fatigue mapping from surface EMG and blood metabolites. Med. Sci. Sports Exerc. 2011, 43, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Green, H.; Halestrap, A.; Mockett, C.; O’Toole, D.; Grant, S.; Ouyang, J. Increases in muscle MCT are associated with reductions in muscle lactate after a single exercise session in humans. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E154–E160. [Google Scholar] [PubMed]
- Hobson, R.M.; Saunders, B.; Ball, G.; Harris, R.C.; Sale, C. Effects of β-alanine supplementation on exercise performance: A meta-analysis. Amino Acids 2012, 43, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.P.; Storey, K.B. A quantitative evaluation of the effect of enzyme complexes on the glycolytic rate in vivo: Mathematical modeling of the glycolytic complex. J. Theor. Biol. 1991, 149, 361–375. [Google Scholar] [CrossRef] [PubMed]
- McConell, G.K.; Shinewell, J.; Stephens, T.J.; Stathis, C.G.; Canny, B.J.; Snow, R.J. Creatine supplementation reduces muscle inosine monophosphate during endurance exercise in humans. Med. Sci. Sports Exerc. 2005, 37, 2054–2061. [Google Scholar] [CrossRef] [PubMed]
- Coyle, E.F.; Hagberg, J.M.; Hurley, B.F.; Martin, W.H.; Ehsani, A.A.; Holloszy, J.O. Carbohydrate feeding during prolonged strenuous exercise can delay fatigue. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1983, 55, 230–235. [Google Scholar] [PubMed]
- Zou, D.; Chen, K.; Liu, P.; Chang, H.; Zhu, J.; Mi, M. Dihydromyricetin improves physical performance under simulated high altitude. Med. Sci. Sports Exerc. 2014, 46, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Kanemaki, T.; Kitade, H.; Kaibori, M.; Sakitani, K.; Hiramatsu, Y.; Kamiyama, Y.; Ito, S.; Okumura, T. Interleukin 1β and interleukin 6, but not tumor necrosis factor α, inhibit insulin-stimulated glycogen synthesis in rat hepatocytes. Hepatology 1998, 27, 1296–1303. [Google Scholar] [CrossRef] [PubMed]
- Uchida, M.; Oyanagi, E.; Kawanishi, N.; Iemitsu, M.; Miyachi, M.; Kremenik, M.J.; Yano, H. Exhaustive exercise increases the TNF-α production in response to flagellin via the upregulation of toll-like receptor 5 in the large intestine in mice. Immunol. Lett. 2014, 158, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Han, M.H.; Park, C.; Jin, C.Y.; Kim, G.Y.; Choi, I.W.; Kim, N.D.; Nam, T.J.; Kwon, T.K.; Choi, Y.H. Anti-inflammatory effects of fucoidan through inhibition of NF-κB, MAPK and Akt activation in lipopolysaccharide-induced BV2 microglia cells. Food Chem. Toxicol. 2011, 49, 1745–1752. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.I.; Raghavendran, H.R.; Sung, N.Y.; Kim, J.H.; Chun, B.S.; Ahn, D.H.; Choi, H.S.; Kang, K.W.; Lee, J.W. Effect of fucoidan on aspirin-induced stomach ulceration in rats. Chem. Biol. Interact. 2010, 183, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, L.; Rider, C.C. Selective and differential binding of interleukin (IL)-1α, IL-1β, IL-2 and IL-6 to glycosaminoglycans. Eur. J. Immunol. 1992, 22, 3027–3031. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Q.; Zhang, Z.; Song, H.; Li, P. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2010, 46, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, F.; Yun, H.; Zhang, H.; Zhang, Q. Effect and mechanism of fucoidan derivatives from Laminaria japonica in experimental adenine-induced chronic kidney disease. J. Ethnopharmacol. 2012, 139, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Gallaher, D.D.; Gallaher, C.M.; Mahrt, G.J.; Carr, T.P.; Hollingshead, C.H.; Hesslink, R., Jr.; Wise, J. A glucomannan and chitosan fiber supplement decreases plasma cholesterol and increases cholesterol excretion in overweight normocholesterolemic humans. J. Am. Coll. Nutr. 2002, 21, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wen, K.; Gao, X.; Liu, Y. Hypolipidemic effect of fucoidan from Laminaria japonica in hyperlipidemic rats. Pharm. Biol. 2010, 48, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, Q.; Song, J. Toxicological evaluation of fucoidan extracted from Laminaria japonica in Wistar rats. Food Chem. Toxicol. 2005, 43, 421–426. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-M.; Tsai, Y.-H.; Tsai, T.-Y.; Chiu, Y.-S.; Wei, L.; Chen, W.-C.; Huang, C.-C. Fucoidan Supplementation Improves Exercise Performance and Exhibits Anti-Fatigue Action in Mice. Nutrients 2015, 7, 239-252. https://doi.org/10.3390/nu7010239
Chen Y-M, Tsai Y-H, Tsai T-Y, Chiu Y-S, Wei L, Chen W-C, Huang C-C. Fucoidan Supplementation Improves Exercise Performance and Exhibits Anti-Fatigue Action in Mice. Nutrients. 2015; 7(1):239-252. https://doi.org/10.3390/nu7010239
Chicago/Turabian StyleChen, Yi-Ming, Yi-Hsin Tsai, Tsung-Yu Tsai, Yen-Shuo Chiu, Li Wei, Wen-Chyuan Chen, and Chi-Chang Huang. 2015. "Fucoidan Supplementation Improves Exercise Performance and Exhibits Anti-Fatigue Action in Mice" Nutrients 7, no. 1: 239-252. https://doi.org/10.3390/nu7010239
APA StyleChen, Y.-M., Tsai, Y.-H., Tsai, T.-Y., Chiu, Y.-S., Wei, L., Chen, W.-C., & Huang, C.-C. (2015). Fucoidan Supplementation Improves Exercise Performance and Exhibits Anti-Fatigue Action in Mice. Nutrients, 7(1), 239-252. https://doi.org/10.3390/nu7010239





