Heart Rate Variability and Cognitive Function Following a Multi-Vitamin and Mineral Supplementation with Added Guarana (Paullinia cupana)
Abstract
:1. Introduction
2. Experimental Section
2.1. Participants
2.2. Procedure
| Active | Units | Per Day | European RDA | |
|---|---|---|---|---|
| Males | Females | |||
| Vitamin C | 180 mg | 180 mg | 90 mg | 75 mg |
| Vitamin B1 | 0.705 mg | 0.705 mg | 1.2 mg | 1.1 mg |
| Vitamin B2 | 0.798 mg | 0.798 mg | 1.3 mg | 1.1 mg |
| Vitamin B3 | 9005 mg | 9005 mg | 16 mg | 14 mg |
| Vitamin B6 | 1003 mg | 1003 mg | 1.7 mg | 1.5 mg |
| Zinc | 7490 mg | 7490 mg | 11 mg | 8 mg |
| Guaraná ES | 300 mg | 300 mg | NA | NA |
| Ginseng ES | 100 mg | 100 mg | NA | NA |
2.3. Cognitive Performance
2.3.1. Go/No-Go Task
2.3.2. Simple Reaction Time (SRT) Task
2.4. Heart Rate Measure
2.5. Statistics
3. Results
3.1. Cognitive Performance
| Go/No-Go | Condition | Time (min) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 15 | 30 | 45 | 60 | 90 | 120 | 150 | 180 | |||
| reaction time (ms) | Ac | mean | 329 | 329 | 317 * | 314 *,§ | 315 *,§ | 325 *,§ | 345 | 347 * | 349 * |
| SD | 16 | 13 | 24 | 18 | 25 | 19 | 14 | 17 | 20 | ||
| Pl | mean | 330 | 331 | 334 | 339 | 345 | 352 ‡ | 359 ‡ | 358 ‡ | 358 ‡ | |
| SD | 18 | 25 | 32 | 46 | 33 | 25 | 26 | 21 | 13 | ||
| C | mean | 327 | 329 | 329 | 333 | 335 | 340 | 350 † | 355 † | 356 † | |
| SD | 17 | 19 | 28 | 32 | 29 | 22 | 20 | 19 | 16 | ||
| Go/no-go errors (%) | Ac | mean | 1 | 0.6 | 0.8 | 0.7 | 1.3 | 0.5 | 0.7 | 1.2 | 0.5 |
| SD | 0.7 | 0.7 | 0.4 | 1.5 | 0.3 | 0.6 | 0.4 | 0.8 | 0.8 | ||
| Pl | mean | 1 | 0.6 | 0.0 | 0.9 | 0.6 | 0.5 | 0.4 | 0.8 | 0.6 | |
| SD | 0.5 | 0.0 | 1.0 | 0.3 | 0.3 | 0.4 | 0.4 | 0.3 | 0.5 | ||
| C | mean | 0 | 0.6 | 0.4 | 0.8 | 0.9 | 0.5 | 1.3 | 1.0 | 0.6 | |
| SD | 0.6 | 0.3 | 0.7 | 0.9 | 0.3 | 0.5 | 0.4 | 0.5 | 0.7 | ||
| Simple reaction time (ms) | Ac | mean | 230 | 224 | 227 | 256 * | 247 * | 251 * | 250 * | 242 * | 246 * |
| SD | 11 | 9 | 17 | 13 | 18 | 13 | 10 | 12 | 14 | ||
| Pl | mean | 228 | 233 | 242 | 264 ‡ | 253 ‡ | 259 ‡ | 249 ‡ | 245 ‡ | 253 ‡ | |
| SD | 12 | 18 | 22 | 32 | 23 | 17 | 18 | 15 | 9 | ||
| C | mean | 227 | 227 | 233 | 258 † | 248 † | 253 † | 248 † | 242 † | 248 † | |
| SD | 12 | 13 | 19 | 22 | 20 | 15 | 14 | 13 | 12 | ||
| RMSSD (ms) | Ac | mean | 64.1 | 66.6 | 63.3 | 63.6 § | 60.6 *,§ | 60.7 *,§ | 57.3 * | 56.2 * | 54.5 * |
| SD | 8.9 | 10.5 | 12.7 | 4.3 | 4.1 | 5.8 | 5.7 | 7.4 | 7.8 | ||
| Pl | mean | 67.0 | 66.2 | 61.4 ‡ | 59.8 ‡ | 56.5 ‡ | 54.8 ‡ | 54.1 ‡ | 53.3 ‡ | 54.6 ‡ | |
| SD | 10.2 | 11.6 | 15.5 | 8.3 | 2.4 | 4.5 | 2.2 | 3.8 | 11.2 | ||
| C | mean | 65.2 | 66.0 | 64.3 | 61.4 † | 58.2 † | 57.4 † | 55.4 † | 54.5 † | 54.3 † | |
| SD | 9.5 | 11.0 | 14.0 | 6.3 | 3.3 | 5.1 | 8.9 | 5.6 | 9.4 | ||
| HF (n.u.) | Ac | mean | 41.1 | 40.7 | 40.6 | 39.7 § | 38.7 *,§ | 36.9 * | 35.5 * | 36.3 * | 35.6 * |
| SD | 2.8 | 4.5 | 5.8 | 8.1 | 5.9 | 8.4 | 2.1 | 5.6 | 3.7 | ||
| Pl | mean | 41.6 | 40.5 | 36.6 ‡ | 35.6 ‡ | 35.5 ‡ | 35.4 ‡ | 35.8 ‡ | 36.1 ‡ | 35.8 ‡ | |
| SD | 9.8 | 3.2 | 3.7 | 6.3 | 3.2 | 3.6 | 5.1 | 4.1 | 4.8 | ||
| C | mean | 41.4 | 40.5 | 39.9 | 37.5 † | 36.6 † | 36.0 † | 35.9 † | 36.5 † | 36.0 † | |
| SD | 6.2 | 3.8 | 4.7 | 7.1 | 4.5 | 6.0 | 3.6 | 3.9 | 5.1 | ||
3.1.1. Go/No-Go Task
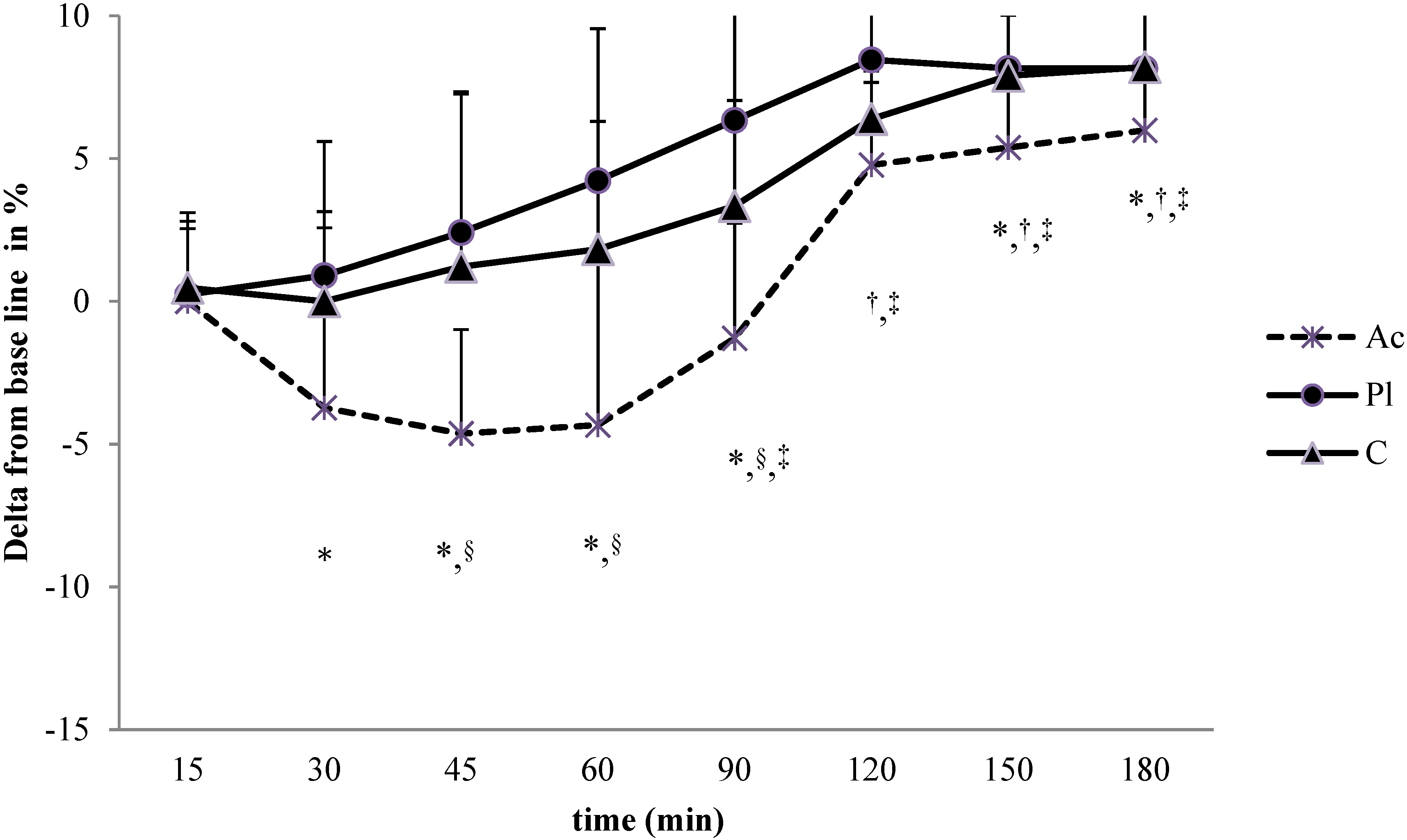
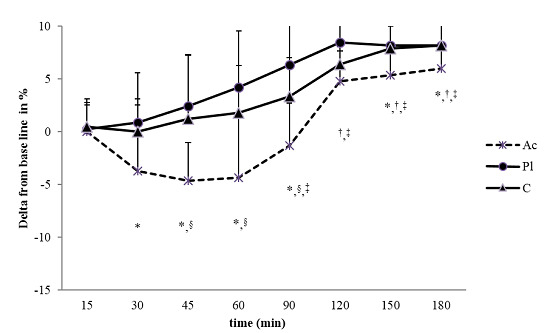
3.1.2. Simple Reaction Time (SRT) Task
3.2. Heart Rate Variability (HRV)

4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interests
References
- De Oliveira Campos, M.P.; Riechelmann, R.; Martins, L.C.; Hassan, B.J.; Casa, F.B.; del Giglio, A. Guaraná (Paullinia cupana) improves fatigue in breast cancer patients undergoing systemic chemotherapy. J. Altern. Complement. Med. 2011, 17, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.P.; Hassan, B.J.; Riechelmann, R.; del Giglio, A. Cancer-related fatigue: A review. Rev. Assoc. Med. Bras. 2011, 57, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Haskell, C.F.; Robertson, B.; Reay, J.; Brewster-Maund, C.; Luedemann, J.; Maggini, S.; Ruf, M.; Zangara, A.; Scholey, A.B. Improved cognitive performance and mental fatigue following a multi-vitamin and mineral supplement with added Guaraná (Paullinia cupana). Appetite 2008, 50, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Galduroz, J.C.; Carlini, E.A. The effects of long-term administration of Guaraná on the cognition of normal, elderly volunteers. Sao Paulo Med. J. 1996, 114, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Haskell, C.F.; Wesnes, K.A.; Scholey, A.B. Improved cognitive performance in human volunteers following administration of Guaraná (Paullinia cupana) extract: Comparison and interaction with Panax ginseng. Pharmacol. Biochem. Behav. 2004, 79, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Haskell, C.F.; Kennedy, D.O.; Wesnes, K.A.; Milne, A.L.; Scholey, A.B. A double-blind, placebo-controlled, multi-dose evaluation of the acute behavioural effects of Guaraná in humans. J. Psychopharmacol. 2007, 21, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Scholey, A.; Bauer, I.; Neale, C.; Savage, K.; Camfield, D.; White, D.; Maggini, S.; Pipingas, A.; Stough, C.; Hughes, M. Acute effects of different multivitamin mineral preparations with and without Guaraná on mood, cognitive performance and functional brain activation. Nutrients 2013, 5, 3589–3604. [Google Scholar] [CrossRef] [PubMed]
- Opala, T.; Rzymski, P.; Pischel, I.; Wilczak, M.; Wozniak, J. Efficacy of 12 weeks supplementation of a botanical extract-based weight loss formula on body weight, body composition and blood chemistry in healthy, overweight subjects—A randomised double-blind placebo-controlled clinical trial. Eur. J. Med. Res. 2006, 11, 343–350. [Google Scholar] [PubMed]
- Lima, W.P.; Carnevali, L.C.; Eder, R., Jr.; Costa Rosa, L.F.; Bacchi, E.M.; Seelaender, M.C. Lipid metabolism in trained rats: Effect of guarana (Paullinia cupana Mart.) supplementation. Clin. Nutr. 2005, 24, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Yamaguti-Sasaki, E.; Ito, L.A.; Canteli, V.C.; Ushirobira, T.M.; Ueda-Nakamura, T.; Dias Filho, B.P.; Nakamura, C.V.; de Mello, J.C. Antioxidant capacity and in vitro prevention of dental plaque formation by extracts and condensed tannins of Paullinia cupana. Molecules 2007, 12, 1950–1963. [Google Scholar] [CrossRef] [PubMed]
- Scholey, A.; Haskell, C. Neurocognitive effects of guaraná plant extract. Drugs Fut. 2008, 33. [Google Scholar] [CrossRef]
- Espinola, E.B.; Dias, R.F.; Mattei, R.; Carlini, E.A. Pharmacological activity of Guarana (Paullinia cupana Mart.) in laboratory animals. J. Ethnopharmacol. 1997, 55, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Mattei, R.; Dias, R.F.; Espínola, E.B.; Carlini, E.A.; Barros, S.B. Guarana (Paullinia cupana): Toxic behavioral effects in laboratory animals and antioxidants activity in vitro. J. Ethnopharmacol. 1998, 60, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Scholey, A.B.; Kennedy, D.O.; Zangara, A.; Robertson, B.C.; Reay, J.; Luedemann, J.; Maggini, S.; Ruf, M. A multivitamin–mineral preparation with guaraná positively effects cognitive performance and reduces mental fatigue during sustained mental demand. Appetite 2008, 50. [Google Scholar] [CrossRef]
- Mednick, S.C.; Cai, D.J.; Kanady, J.; Drummond, S.P. Comparing the benefits of caffeine, naps and placebo on verbal, motor and perceptual memory. Behav. Brain Res. 2008, 193, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Clauson, K.A.; Shields, K.M.; McQueen, C.E.; Persad, N. Safety issues associated with commercially available energy drinks. J. Am. Pharm. Assoc. (2003) 2008, 48, 55–67. [Google Scholar] [CrossRef]
- Brisswalter, J.; Collardeau, M.; Rene, A. Effects of acute physical exercise characteristics on cognitive performance. Sports Med. 2002, 32, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Luft, C.D.; Takase, E.; Darby, D. Heart rate variability and cognitive function: Effects of physical effort. Biol. Psychol. 2009, 82, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Duschek, S.; Muckenthaler, M.; Werner, N.; del Paso, G.A. Relationships between features of autonomic cardiovascular control and cognitive performance. Biol. Psychol. 2009, 81, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.L.; Johnsen, B.H.; Thayer, J.F. Vagal influence on working memory and attention. Int. J. Psychophysiol. 2003, 48, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Porges, S.W. Vagal tone: A physiologic marker of stress vulnerability. Pediatrics 1992, 90, 498–504. [Google Scholar] [PubMed]
- Miu, A.C.; Heilman, R.M.; Miclea, M. Reduced heart rate variability and vagal tone in anxiety: Trait versus state, and the effects of autogenic training. Auton. Neurosci. 2009, 145, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.R.; Higgins, S.T.; Bickel, W.K.; Hunt, W.K.; Fenwick, J.W.; Gulliver, S.B.; Mireault, G.C. Caffeine self-administration, withdrawal, and adverse effects among coffee drinkers. Arch. Gen. Psychiatry 1991, 48, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Sollers, J.J.; Ruiz-Padial, E.; Vila, J. Estimating respiratory frequency from autoregressive spectral analysis of heart period. IEEE Eng. Med. Biol. Mag. 2002, 21, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Greenhouse, S.W.; Geisser, S. On methods in the analysis of profile data. Psychometrika 1959, 24, 95–112. [Google Scholar] [CrossRef]
- Winer, B.J.; Brown, D.R.; Michels, K.M. Statistical Principles in Experimental Design; McGraw-Hill: New York, NY, USA, 1991. [Google Scholar]
- Campos, A.R.; Barros, A.I.; Albuquerque, F.A.; Leal, L.K.A.M.; Rao, V.S. Acute effects of Guaraná (Paullinia cupana Mart.) on mouse behaviour in forced swimming and open field tests. Phytother. Res. 2005, 19, 441–443. [Google Scholar] [CrossRef] [PubMed]
- Durga, J.; van Boxtel, M.P.; Schouten, E.G.; Bots, M.L.; Kok, F.J.; Verhoef, P. Folate and the methylenetetrahydrofolate reductase 677C→T mutation correlate with cognitive performance. Neurobiol. Aging 2006, 27, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Balk, E.M.; Raman, G.; Tatsioni, A.; Chung, M.; Lau, J.; Rosenberg, I.H. Vitamin B6, B12, and folic acid supplementation and cognitive function: A systematic review of randomized trials. Arch. Intern. Med. 2007, 167, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Huskisson, E.; Maggini, S.; Ruf, M. The influence of micronutrients on cognitive function and performance. J. Int. Med. Res. 2007, 35, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Attwood, A.; Terry, P.; Higgs, S. Conditioned effects of caffeine on performance in humans. Physiol. Behav. 2010, 99, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, H.R.; Tharion, W.J.; Shukitt-Hale, B.; Speckman, K.L.; Tulley, R. Effects of caffeine, sleep loss, and stress on cognitive performance and mood during U.S. Navy SEAL training. Psychopharmacology 2002, 164, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Friedman, B.H. An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biol. Psychol. 2007, 74, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.L.; Johnsen, B.H.; Sollers, J.J.; Stenvik, K.; Thayer, J.F. Heart rate variability and its relation to prefrontal cognitive function: The effects of training and detraining. Eur. J. Appl. Physiol. 2004, 93, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Elliot, A.J.; Payen, V.; Brisswalter, J.; Cury, F.; Thayer, J.F. A subtle threat cue, heart rate variability, and cognitive performance. Psychophysiology 2011, 48, 1340–1345. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Lane, R.D. A model of neurovisceral integration in emotion regulation and dysregulation. J. Affect. Disord. 2000, 61, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Jorna, P.G. Spectral analysis of heart rate and psychological state: A review of its validity as a workload index. Biol. Psychol. 1992, 34, 237–257. [Google Scholar] [CrossRef] [PubMed]
- Tattersall, A.J.; Hockey, G.R. Level of operator control and changes in heart rate variability during simulated flight maintenance. Hum. Factors 1995, 37, 682–698. [Google Scholar] [CrossRef] [PubMed]
- Croizet, J.C.; Despres, G.; Gauzins, M.E.; Huguet, P.; Leyens, J.P.; Meot, A. Stereotype threat undermines intellectual performance by triggering a disruptive mental load. Pers. Soc. Psychol. Bull. 2004, 30, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.L.; Johnsen, B.H.; Thayer, J.F. Relationship between heart rate variability and cognitive function during threat of shock. Anxiety Stress Coping 2009, 22, 77–89. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pomportes, L.; Davranche, K.; Brisswalter, I.; Hays, A.; Brisswalter, J. Heart Rate Variability and Cognitive Function Following a Multi-Vitamin and Mineral Supplementation with Added Guarana (Paullinia cupana). Nutrients 2015, 7, 196-208. https://doi.org/10.3390/nu7010196
Pomportes L, Davranche K, Brisswalter I, Hays A, Brisswalter J. Heart Rate Variability and Cognitive Function Following a Multi-Vitamin and Mineral Supplementation with Added Guarana (Paullinia cupana). Nutrients. 2015; 7(1):196-208. https://doi.org/10.3390/nu7010196
Chicago/Turabian StylePomportes, Laura, Karen Davranche, Ioanna Brisswalter, Arnaud Hays, and Jeanick Brisswalter. 2015. "Heart Rate Variability and Cognitive Function Following a Multi-Vitamin and Mineral Supplementation with Added Guarana (Paullinia cupana)" Nutrients 7, no. 1: 196-208. https://doi.org/10.3390/nu7010196
APA StylePomportes, L., Davranche, K., Brisswalter, I., Hays, A., & Brisswalter, J. (2015). Heart Rate Variability and Cognitive Function Following a Multi-Vitamin and Mineral Supplementation with Added Guarana (Paullinia cupana). Nutrients, 7(1), 196-208. https://doi.org/10.3390/nu7010196