Coffee Consumption and Risk of Gastric Cancer: A Large Updated Meta-Analysis of Prospective Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search and Selection
2.2. Data Extraction
2.3. Data Synthesis and Analyses
3. Results
3.1. Study Characteristics

3.2. Overall Analysis
3.3. Stratified, Sensitivity and Dose-Response Analyses
| Study | Country | Duration, Years | No. of Cases | No. of Subjects | Sex | Highest vs. Lowest Consumption | Range of Consumption | RR (95% CI) | Variables Adjusted for |
|---|---|---|---|---|---|---|---|---|---|
| Jacobsen, 1986 [8] | Norway | 11.5 | 147 | 16555 | M/F | ≥7 vs. ≤2 cups/day | 6.5 cups/day | 1.46 (0.84–2.55) a | Age, sex, and residence. |
| Nomura, 1986 [11] | USA | 15 | 106 | 7355 | M | ≥5 vs. 0 | 5 cups/day | 1.18 (0.61–2.26) b | Age |
| Stensvold, 1994 [13] | Norway | 10.1 | 80 | 42973 | M/F | ≥7 vs. ≤2 cups/day | 4.5 cups/day | 0.50 (0.21–1.19) (M) a 0.50 (0.17–1.52) (F) a | Age, smoking, and residence. |
| van Loon, 1998 [15] | Netherlands | 4.3 | 146 | 1525 (sub-cohort) | M | >4 vs. ≤4 cups/day | 4 cups/day | 1.43 (0.93–2.19) b | - |
| Galanis, 1998 [7] | USA | 14.8 | 108 | 11907 | M/F | ≥2 vs. 0 cups/day | 3 cups/day | 1.80 (1.00–3.30) | Age, sex, smoking (M), education and place of birth. |
| Tsubono, 2001 [14] | Japan | 9 | 419 | 26311 | M/F | ≥3 vs. 0 cups/day | 3.5 cups/day | 1.00 (0.60–1.60) | Age, sex, smoking, consumption of tea, alcohol, rice, meat, vegetables, fruits and bean-past soup, and type of health insurance. |
| Khan, 2004 [17] | Japan | 13.8 (M) 14.8 (F) | 51 | 3155 | M/F | ≥several times/week vs. ≤several times/month | <1 cups/day | 1.00 (0.50–2.00) (M) 0.30 (0.10–1.40) (F) | Age, smoking, health status (F), health education (F), and health screening (F). |
| Larsson, 2006 [9] | Sweden | 15.7 | 160 | 61433 | F | ≥4 vs. ≤1 cups/day | 4 cups/day | 1.86 (1.07–3.25) | Age, calendar year, education, and consumption of tea andm alcohol. |
| Nilsson, 2010 [10] | Sweden | 6 | 70 | 64603 | M/F | ≥4 vs. <1 cups/day | 6.5 cups/day | 0.99 (0.44–2.21) | Age, sex, BMI, smoking, education, and physical activity. |
| Ren, 2010 [12] | USA | 5.4 | 455 | 481563 | M/F | >3 vs. <1 cups/day | 3 cups/day | 1.57 (1.03–2.39) c 1.06 (0.68–1.64) d | Age, sex, BMI, smoking, education, ethnicity, physical activity, and consumption of alcohol, fruits, vegetables, red meat, white meat and calories. |
| Bidel, 2013 [6] | Finland | 18 | 299 | 60041 | M/F | ≥10 vs. 0 cups/day | 11 cups/day | 0.75 (0.40–1.41) | Age, sex, BMI, study year, education, smoking, physical activity, history of diabetes, and consumption of tea and alcohol. |
| Ainslie-Wal dman, 2014 [16] | Singapore | 14.7 | 647 | 63257 | M/F | ≥4 cups/d vs. never/monthly | 4.5 cups/day | 0.93 (0.49–1.07) | Age, BMI, gender, interview year, dialect, education, smoking, intakes of caffeine and total energy intake. |
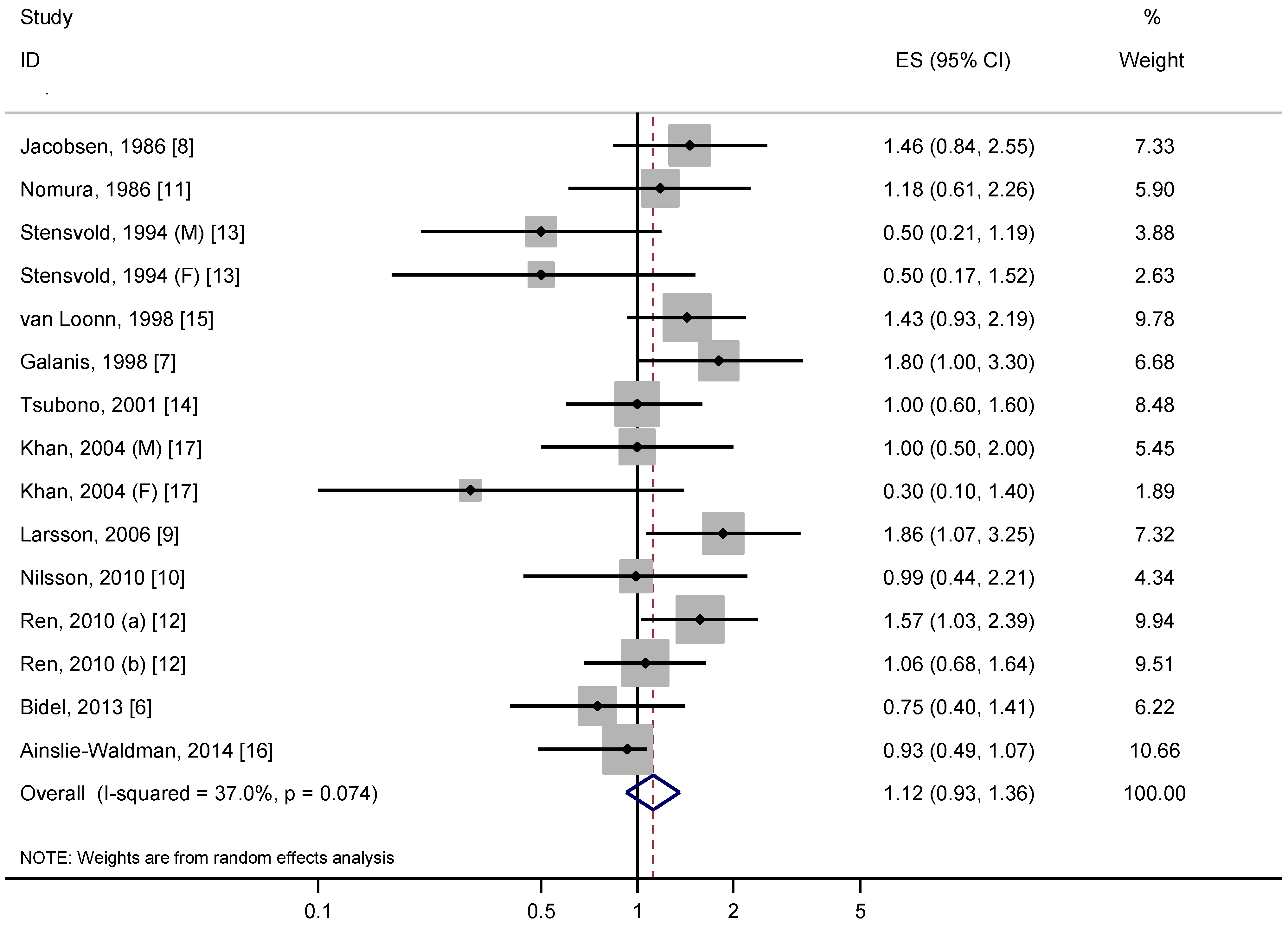
3.4. Stratified, Sensitivity and Dose-Response Analyses
| Subgroups | N | RR (95% CI) | Heterogeneity Test | |
|---|---|---|---|---|
| P | I2 (%) | |||
| Areas | ||||
| USA | 3 | 1.36 (1.06–1.74) | 0.44 | 0.0 |
| Europe | 6 | 1.08 (0.76–1.53) | 0.06 | 50.9 |
| Japan | 3 | 0.92 (0.70–1.20) | 0.40 | 0.0 |
| Duration of follow-up | ||||
| ≥10 years | 8 | 1.02 (0.76–1.38) | 0.03 | 50.4 |
| <10 years | 4 | 1.24 (1.00–1.54) | 0.53 | 0.0 |
| No. of cases | ||||
| ≥200 | 4 | 1.07 (0.85–1.34) | 0.29 | 19.7 |
| <200 | 8 | 1.14 (0.85–1.53) | 0.06 | 44.8 |
| Sex | ||||
| Men | 8 | 0.98 (0.70–1.36) | 0.08 | 44.9 |
| Women | 6 | 1.04 (0.58–1.89) | 0.06 | 53.1 |
| Range of consumption | ||||
| ≥5 cups/day | 4 | 1.09 (0.79–1.51) | 0.47 | 0.0 |
| <5 cups/day | 8 | 1.12 (0.88–1.42) | 0.03 | 49.0 |
| Adjustment | ||||
| Smoking, yes | 8 | 0.99 (0.78–1.25) | 0.10 | 38.2 |
| no | 4 | 1.48 (1.13–1.93) | 0.77 | 0.0 |
| Alcohol, yes | 4 | 1.21 (0.90–1.62) | 0.14 | 42.1 |
| no | 8 | 1.05 (0.81–1.37) | 0.10 | 39.3 |
| BMI, yes | 4 | 1.07 (0.84–1.37) | 0.29 | 19.0 |
| no | 8 | 1.14 (0.86–1.50) | 0.05 | 46.1 |
| Education, yes | 6 | 1.22 (0.95–1.57) | 0.12 | 40.1 |
| no | 6 | 0.99 (0.73–1.35) | 0.12 | 39.3 |
| Physical activity, yes | 3 | 1.12 (0.82–1.54) | 0.25 | 27.4 |
| no | 9 | 1.11 (0.87–1.43) | 0.05 | 44.8 |
| Dietary factors, yes | 5 | 1.14 (0.89–1.47) | 0.14 | 39.9 |
| no | 7 | 1.06 (0.78–1.45) | 0.08 | 42.5 |
3.5. Publication Bias
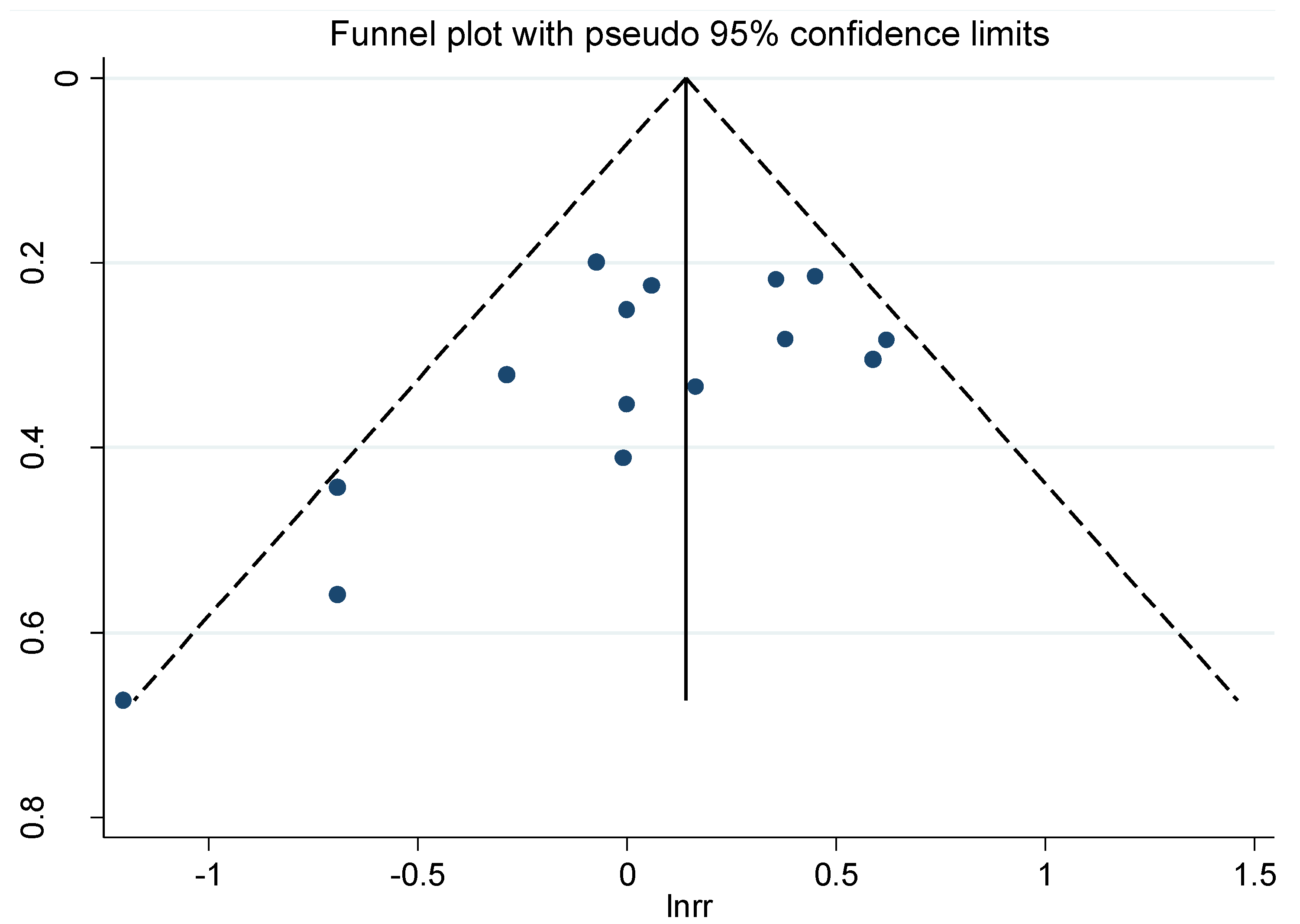
4. Discussion
5. Conclusions
Supplementary Files
Supplementary File 1Author Contributions
Conflicts of Interest
References
- Brenner, H.; Rothenbacher, D.; Arndt, V. Epidemiology of stomach cancer. Methods Mol. Biol. 2009, 472, 467–477. [Google Scholar]
- McMichael, A.J.; McCall, M.G.; Hartshorne, J.M.; Woodings, T.L. Patterns of gastro-intestinal cancer in european migrants to australia: The role of dietary change. Int. J. Cancer 1980, 25, 431–437. [Google Scholar] [CrossRef]
- Malerba, S.; Turati, F.; Galeone, C.; Pelucchi, C.; Verga, F.; La Vecchia, C.; Tavani, A. A meta-analysis of prospective studies of coffee consumption and mortality for all causes, cancers and cardiovascular diseases. Eur. J. Epidemiol. 2013, 28, 527–539. [Google Scholar] [CrossRef]
- Yu, X.; Bao, Z.; Zou, J.; Dong, J. Coffee consumption and risk of cancers: A meta-analysis of cohort studies. BMC Cancer 2011, 11, 96. [Google Scholar] [CrossRef]
- Botelho, F.; Lunet, N.; Barros, H. Coffee and gastric cancer: Systematic review and meta-analysis. Cad. Saude Publica 2006, 22, 889–900. [Google Scholar] [CrossRef]
- Bidel, S.; Hu, G.; Jousilahti, P.; Pukkala, E.; Hakulinen, T.; Tuomilehto, J. Coffee consumption and risk of gastric and pancreatic cancer—A prospective cohort study. Int. J. Cancer 2013, 132, 1651–1659. [Google Scholar] [CrossRef]
- Galanis, D.J.; Kolonel, L.N.; Lee, J.; Nomura, A. Intakes of selected foods and beverages and the incidence of gastric cancer among the japanese residents of hawaii: A prospective study. Int. J. Epidemiol. 1998, 27, 173–180. [Google Scholar] [CrossRef]
- Jacobsen, B.K.; Bjelke, E.; Kvale, G.; Heuch, I. Coffee drinking, mortality, and cancer incidence: Results from a norwegian prospective study. J. Natl. Cancer Inst. 1986, 76, 823–831. [Google Scholar]
- Larsson, S.C.; Giovannucci, E.; Wolk, A. Coffee consumption and stomach cancer risk in a cohort of swedish women. Int. J. Cancer 2006, 119, 2186–2189. [Google Scholar] [CrossRef]
- Nilsson, L.M.; Johansson, I.; Lenner, P.; Lindahl, B.; van Guelpen, B. Consumption of filtered and boiled coffee and the risk of incident cancer: A prospective cohort study. Cancer Causes Control 2010, 21, 1533–1544. [Google Scholar] [CrossRef]
- Nomura, A.; Heilbrun, L.K.; Stemmermann, G.N. Prospective study of coffee consumption and the risk of cancer. J. Natl. Cancer Inst. 1986, 76, 587–590. [Google Scholar]
- Ren, J.S.; Freedman, N.D.; Kamangar, F.; Dawsey, S.M.; Hollenbeck, A.R.; Schatzkin, A.; Abnet, C.C. Tea, coffee, carbonated soft drinks and upper gastrointestinal tract cancer risk in a large united states prospective cohort study. Eur. J. Cancer 2010, 46, 1873–1881. [Google Scholar] [CrossRef]
- Stensvold, I.; Jacobsen, B.K. Coffee and cancer: A prospective study of 43,000 norwegian men and women. Cancer Causes Control 1994, 5, 401–408. [Google Scholar] [CrossRef]
- Tsubono, Y.; Nishino, Y.; Komatsu, S.; Hsieh, C.C.; Kanemura, S.; Tsuji, I.; Nakatsuka, H.; Fukao, A.; Satoh, H.; Hisamichi, S.; et al. Green tea and the risk of gastric cancer in japan. N. Engl. J. Med. 2001, 344, 632–636. [Google Scholar] [CrossRef]
- Van Loon, A.J.; Goldbohm, R.A.; van den Brandt, P.A. Socioeconomic status and stomach cancer incidence in men: Results from the netherlands cohort study. J. Epidemiol. Community Health 1998, 52, 166–171. [Google Scholar] [CrossRef]
- Ainslie-Waldman, C.E.; Koh, W.P.; Jin, A.; Yeoh, K.G.; Zhu, F.; Wang, R.; Yuan, J.M.; Butler, L.M. Coffee intake and gastric cancer risk: The singapore chinese health study. Cancer Epidemiol. Biomark. Prev. 2014, 23, 638–647. [Google Scholar] [CrossRef]
- Khan, M.M.; Goto, R.; Kobayashi, K.; Suzumura, S.; Nagata, Y.; Sonoda, T.; Sakauchi, F.; Washio, M.; Mori, M. Dietary habits and cancer mortality among middle aged and older japanese living in hokkaido, japan by cancer site and sex. Asian Pac. J. Cancer Prev. 2004, 5, 58–65. [Google Scholar]
- Ladeiras-Lopes, R.; Pereira, A.K.; Nogueira, A.; Pinheiro-Torres, T.; Pinto, I.; Santos-Pereira, R.; Lunet, N. Smoking and gastric cancer: Systematic review and meta-analysis of cohort studies. Cancer Causes Control 2008, 19, 689–701. [Google Scholar] [CrossRef]
- Abioye, A.I.; Odesanya, M.O.; Ibrahim, N.A. Physical activity and risk of gastric cancer: A meta-analysis of observational studies. Br. J. Sports Med. 2014. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L.; Wang, X.; Wang, J.; Yan, Z.; Cheng, J.; Gong, G.; Li, G. Body mass index and risk of gastric cancer: A meta-analysis of a population with more than ten million from 24 prospective studies. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1395–1408. [Google Scholar] [CrossRef]
- Tramacere, I.; Negri, E.; Pelucchi, C.; Bagnardi, V.; Rota, M.; Scotti, L.; Islami, F.; Corrao, G.; La Vecchia, C.; Boffetta, P.; et al. A meta-analysis on alcohol drinking and gastric cancer risk. Ann. Oncol. 2012, 23, 28–36. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Greenland, S.; Longnecker, M.P. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am. J. Epidemiol. 1992, 135, 1301–1309. [Google Scholar]
- D’Elia, L.; Galletti, F.; Strazzullo, P. Dietary salt intake and risk of gastric cancer. Cancer Treat Res. 2014, 159, 83–95. [Google Scholar]
- Yoon, J.M.; Son, K.Y.; Eom, C.S.; Durrance, D.; Park, S.M. Pre-existing diabetes mellitus increases the risk of gastric cancer: A meta-analysis. World J. Gastroenterol. 2013, 19, 936–945. [Google Scholar] [CrossRef]
- Hu, M.L. Dietary polyphenols as antioxidants and anticancer agents: More questions than answers. Chang Gung Med. J. 2011, 34, 449–460. [Google Scholar]
- Galati, G.; O’Brien, P.J. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef]
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar]
- Abraham, S.K.; Stopper, H. Anti-genotoxicity of coffee against n-methyl-n-nitro-n-nitrosoguanidine in mouse lymphoma cells. Mutat. Res. 2004, 561, 23–33. [Google Scholar] [CrossRef]
- Wolfrom, D.; Welsch, C.W. Caffeine and the development of normal, benign and carcinomatous human breast tissues: A relationship? J. Med. 1990, 21, 225–250. [Google Scholar]
- Farah, A.; de Paulis, T.; Trugo, L.C.; Martin, P.R. Effect of roasting on the formation of chlorogenic acid lactones in coffee. J. Agric. Food Chem. 2005, 53, 1505–1513. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xie, F.; Wang, D.; Huang, Z.; Guo, Y. Coffee Consumption and Risk of Gastric Cancer: A Large Updated Meta-Analysis of Prospective Studies. Nutrients 2014, 6, 3734-3746. https://doi.org/10.3390/nu6093734
Xie F, Wang D, Huang Z, Guo Y. Coffee Consumption and Risk of Gastric Cancer: A Large Updated Meta-Analysis of Prospective Studies. Nutrients. 2014; 6(9):3734-3746. https://doi.org/10.3390/nu6093734
Chicago/Turabian StyleXie, Feiyue, Dan Wang, Zhifang Huang, and Yajun Guo. 2014. "Coffee Consumption and Risk of Gastric Cancer: A Large Updated Meta-Analysis of Prospective Studies" Nutrients 6, no. 9: 3734-3746. https://doi.org/10.3390/nu6093734
APA StyleXie, F., Wang, D., Huang, Z., & Guo, Y. (2014). Coffee Consumption and Risk of Gastric Cancer: A Large Updated Meta-Analysis of Prospective Studies. Nutrients, 6(9), 3734-3746. https://doi.org/10.3390/nu6093734




