Oligonol Supplementation Affects Leukocyte and Immune Cell Counts after Heat Loading in Humans
Abstract
:1. Introduction
2. Experimental Section
2.1. Subjects
2.2. Supplements
2.3. Heat Load
2.4. Blood Sampling
2.5. Analysis of Serum IL-1ß and IL-6
2.6. Full Blood Counts
2.7. Assessment of Lymphocyte Subsets
2.8. Tympanic Temperature Measurement
2.9. Statistical Analysis
3. Results
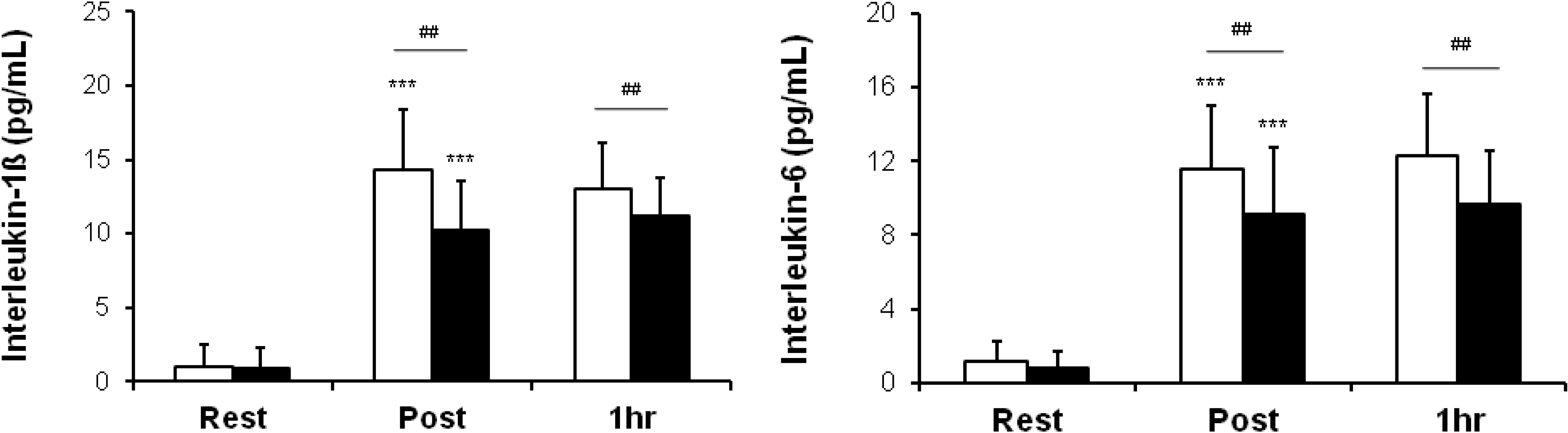
3.1. Serum Concentrations of IL-1ß and IL-6
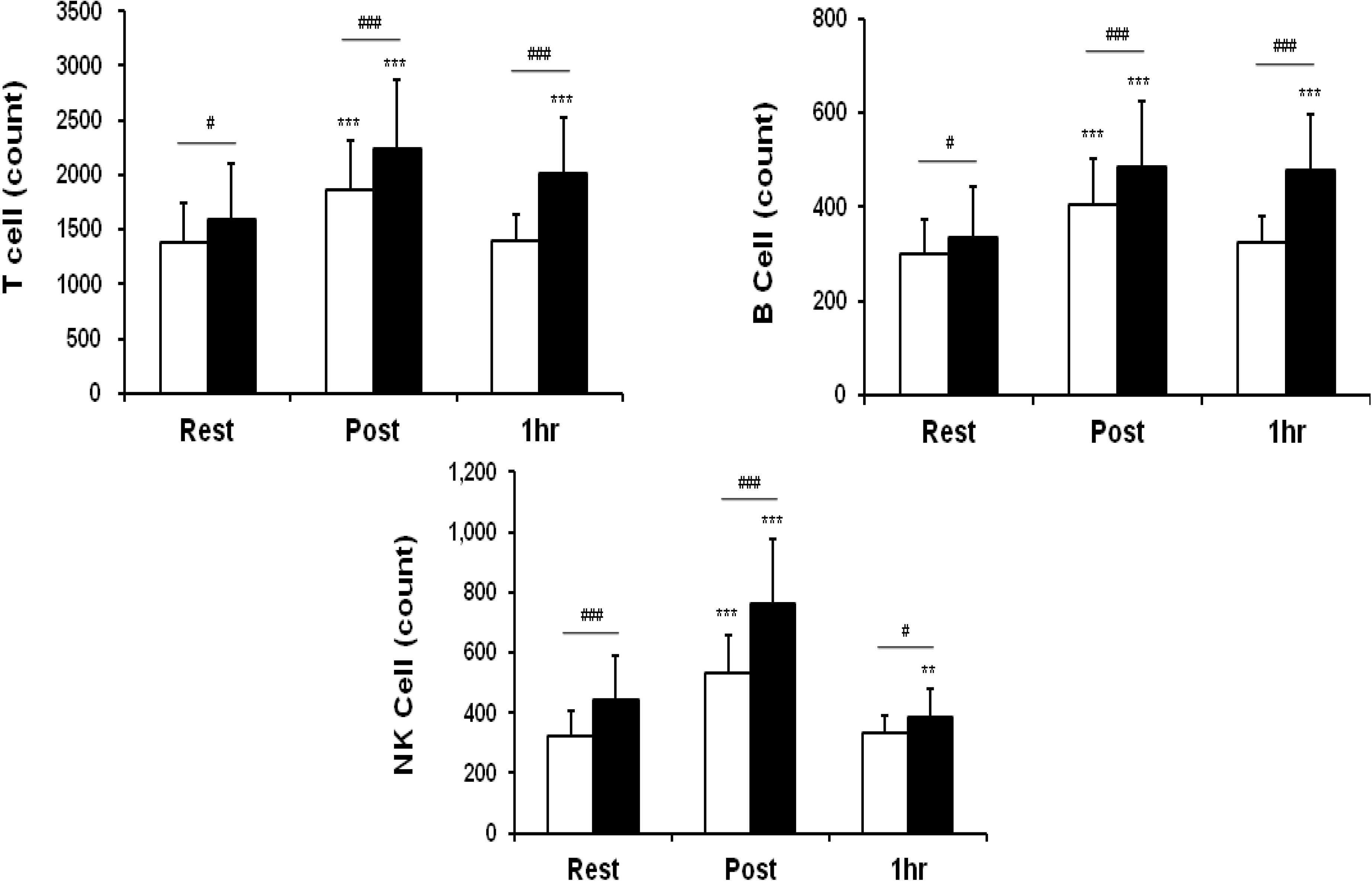
3.2. Leukocytes, Lymphocytes, and Cell Subsets
3.3. Tympanic Temperature

4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brambilla, D.; Mancuso, C.; Scuderi, M.R.; Bosco, P.; Cantarella, G.; Lempereur, L.; di Benedetto, G.; Pezzino, S.; Bernardini, R. The role of antioxidant supplement in immune system, neoplastic, and neurodegenerative disorders: A point of view for an assessment of the risk/benefit profile. Nutr. J. 2008, 7, 29. [Google Scholar] [CrossRef]
- Meydani, S.N.; Meydani, M.; Blumberg, J.B.; Leka, L.S.; Siber, G.; Loszewski, R.; Thompson, C.; Pedrosa, M.C.; Diamond, R.D.; Stollar, B.D. Vitamin E supplementation and in vivo immune response in healthy elderly subjects. A randomized controlled trial. Jama 1997, 277, 1380–1386. [Google Scholar] [CrossRef]
- Wintergerst, E.S.; Maggini, S.; Hornig, D.H. Contribution of selected vitamins and trace elements to immune function. Ann. Nutr. Metab. 2007, 51, 301–323. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Sun, B.; Fujii, H.; Neergheen, V.S.; Bahorun, T.; Kang, K.S.; Sung, M.K. Low molecular proanthocyanidin dietary biofactor oligonol: Its modulation of oxidative stress, bioefficacy, neuroprotection, food application and chemoprevention potentials. Biofactors 2006, 27, 245–265. [Google Scholar] [CrossRef]
- Lee, J.B.; Shin, Y.O.; Min, Y.K.; Yang, H.M. The effect of oligonol intake on cortisol and related cytokines in healthy young men. Nutr. Res. Pract. 2010, 4, 203–207. [Google Scholar] [CrossRef]
- Shin, Y.-O.; Lee, J.-B.; Min, Y.-K.; Yang, H.-M. Effect of oligonol intake on cortisol and cytokines, and body temperature after leg immersion into hot water. Food Sci. Biotechnol. 2011, 20, 659–663. [Google Scholar]
- Noh, J.S.; Park, C.H.; Yokozawa, T. Treatment with oligonol, a low-molecular polyphenol derived from lychee fruit, attenuates diabetes-induced hepatic damage through regulation of oxidative stress and lipid metabolism. Br. J. Nutr. 2011, 106, 1013–1022. [Google Scholar]
- Kundu, J.K.; Chang, E.J.; Fujii, H.; Sun, B.; Surh, Y.J. Oligonol inhibits UVB-induced cox-2 expression in HR-1 hairless mouse skin—AP-1 and C/EBP as potential upstream targets. Photochem. Photobiol. 2008, 84, 399–406. [Google Scholar]
- Severs, Y.; Brenner, I.; Shek, P.N.; Shephard, R.J. Effects of heat and intermittent exercise on leukocyte and sub-population cell counts. Eur. J. Appl. Physiol. Occup. Physiol. 1996, 74, 234–245. [Google Scholar] [CrossRef]
- Conti, B.; Tabarean, I.; Andrei, C.; Bartfai, T. Cytokines and fever. Front. Biosci. 2004, 9, 1433–1449. [Google Scholar] [CrossRef]
- Fujii, H.; Sun, B.; Nishioka, H.; Hirose, A.; Aruoma, O.I. Evaluation of the safety and toxicity of the oligomerized polyphenol oligonol. Food Chem. Toxicol. 2007, 45, 378–387. [Google Scholar]
- Sobieska, M.; Stratz, T.; Samborski, W.; Hrycaj, P.; Mennet, P.; Müller, W. Interleukin-6 (IL-6) after Whole Body Cryotherapy and Local Hot Mud Pack Treatment. Eur. J. Phys. Med. Rehabil. 1993, 3, 205. [Google Scholar]
- Olszewski, W.L.; Grzelak, I.; Ziolkowska, A.; Engeset, A. Effect of local hyperthermia on lymph immune cells and lymphokines of normal human skin. J. Surg. Oncol. 1989, 41, 109–116. [Google Scholar] [CrossRef]
- Metz, J.R.; Huising, M.O.; Leon, K.; Verburg-van Kemenade, B.M.; Flik, G. Central and peripheral interleukin-1 beta and interleukin-1 receptor I expression and their role in the acute stress response of common carp, cyprinus carpio l. J. Endocrinol. 2006, 191, 25–35. [Google Scholar] [CrossRef]
- O’Connor, K.A.; Johnson, J.D.; Hansen, M.K.; Wieseler Frank, J.L.; Maksimova, E.; Watkins, L.R.; Maier, S.F. Peripheral and central proinflammatory cytokine response to a severe acute stressor. Brain Res. 2003, 991, 123–132. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin-1 and interleukin-1 antagonism. Blood 1991, 77, 1627–1652. [Google Scholar]
- Li, S.; Wang, Y.; Matsumura, K.; Ballou, L.R.; Morham, S.G.; Blatteis, C.M. The febrile response to lipopolysaccharide is blocked in cyclooxygenase-2−/−, but not in cyclooxygenase-1−/− mice. Brain Res. 1999, 825, 86–94. [Google Scholar] [CrossRef]
- Shin, Y.O.; Lee, J.B.; Song, Y.J.; Min, Y.K.; Yang, H.M. Oligonol supplementation attenuates body temperature and the circulating levels of prostaglandin E2 and cyclooxygenase-2 after heat stress in humans. J. Med. Food 2013, 16, 318–323. [Google Scholar] [CrossRef]
- Lange, U.; Muller-Ladner, U.; Schmidt, K.L. Balneotherapy in rheumatic diseases—An overview of novel and known aspects. Rheumatol. Int. 2006, 26, 497–499. [Google Scholar] [CrossRef]
- Bouchama, A.; Al Hussein, K.; Adra, C.; Rezeig, M.; Al Shail, E.; Al Sedairy, S. Distribution of peripheral blood leukocytes in acute heatstroke. J. Appl. Physiol. 1992, 73, 405–409. [Google Scholar]
- Kappel, M.; Kharazmi, A.; Nielsen, H.; Gyhrs, A.; Pedersen, B.K. Modulation of the counts and functions of neutrophils and monocytes under in vivo hyperthermia conditions. Int. J. Hyperth. 1994, 10, 165–173. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Kappel, M.; Klokker, M.; Nielsen, H.B.; Secher, N.H. The immune system during exposure to extreme physiologic conditions. Int. J. Sports Med. 1994, 15 (Suppl. 3), S116–S121. [Google Scholar] [CrossRef]
- Atanackovic, D.; Nierhaus, A.; Neumeier, M.; Hossfeld, D.K.; Hegewisch-Becker, S. 41.8 Degrees C whole body hyperthermia as an adjunct to chemotherapy induces prolonged T cell activation in patients with various malignant diseases. Cancer Immunol. Immunother. 2002, 51, 603–613. [Google Scholar] [CrossRef]
- Ostapenko, V.V.; Tanaka, H.; Miyano, M.; Nishide, T.; Ueda, H.; Nishide, I.; Tanaka, Y.; Mune, M.; Yukawa, S. Immune-related effects of local hyperthermia in patients with primary liver cancer. Hepato Gastroenterol. 2005, 52, 1502–1506. [Google Scholar]
- Tomiyama-Miyaji, C.; Watanabe, M.; Ohishi, T.; Kanda, Y.; Kainuma, E.; Bakir, H.Y.; Shen, J.; Ren, H.; Inoue, M.; Tajima, K.; et al. Modulation of the endocrine and immune systems by well-controlled hyperthermia equipment. Biomed. Res. 2007, 28, 119–125. [Google Scholar]
- Huang, Y.H.; Haegerstrand, A.; Frostegard, J. Effects of in vitro hyperthermia on proliferative responses and lymphocyte activity. Clin. Exp. Immunol. 1996, 103, 61–66. [Google Scholar]
- Downing, J.F.; Taylor, M.W. The effect of in vivo hyperthermia on selected lymphokines in man. Lymphokine Res. 1987, 6, 103–109. [Google Scholar]
- Shen, R.N.; Hornback, N.B.; Shidnia, H.; Shupe, R.E.; Brahmi, Z. Whole-body hyperthermia decreases lung metastases in lung tumor-bearing mice, possibly via a mechanism involving natural killer cells. J. Clin. Immunol. 1987, 7, 246–253. [Google Scholar] [CrossRef]
- Jiang, Q.; Detolla, L.; Singh, I.S.; Gatdula, L.; Fitzgerald, B.; van Rooijen, N.; Cross, A.S.; Hasday, J.D. Exposure to febrile temperature upregulates expression of pyrogenic cytokines in endotoxin-challenged mice. Am. J. Physiol. 1999, 276, R1653–R1660. [Google Scholar]
- Kluger, M.J.; Rudolph, K.; Soszynski, D.; Conn, C.A.; Leon, L.R.; Kozak, W.; Wallen, E.S.; Moseley, P.L. Effect of heat stress on LPS-induced fever and tumor necrosis factor. Am. J. Physiol. 1997, 273, R858–R863. [Google Scholar]
- Tilg, H.; Dinarello, C.A.; Mier, J.W. IL-6 and APPs: Anti-inflammatory and immunosuppressive mediators. Immunol. Today 1997, 18, 428–432. [Google Scholar] [CrossRef]
- Hegde, S.; Pahne, J.; Smola-Hess, S. Novel immunosuppressive properties of interleukin-6 in dendritic cells: Inhibition of NF-kappab binding activity and CCR7 expression. FASEB J. 2004, 18, 1439–1441. [Google Scholar]
- Rhind, S.G.; Gannon, G.A.; Shek, P.N.; Brenner, I.K.; Severs, Y.; Zamecnik, J.; Buguet, A.; Natale, V.M.; Shephard, R.J.; Radomski, M.W. Contribution of exertional hyperthermia to sympathoadrenal-mediated lymphocyte subset redistribution. J. Appl. Physiol. 1999, 87, 1178–1185. [Google Scholar]
- Elenkov, I.J. Neurohormonal-cytokine interactions: Implications for inflammation, common human diseases and well-being. Neurochem. Int. 2008, 52, 40–51. [Google Scholar] [CrossRef]
- Monteiro, M.M.; Franca-Silva, M.S.; Alves, N.F.; Porpino, S.K.; Braga, V.A. Quercetin improves baroreflex sensitivity in spontaneously hypertensive rats. Molecules 2012, 17, 12997–13008. [Google Scholar] [CrossRef]
- Giusti, M.F.; Sato, M.A.; Cardoso, L.M.; Braga, V.A.; Colombari, E. Central antioxidant therapy inhibits parasympathetic baroreflex control in conscious rats. Neurosci. Lett. 2011, 489, 115–118. [Google Scholar] [CrossRef]
- Manzella, D.; Barbieri, M.; Ragno, E.; Paolisso, G. Chronic administration of pharmacologic doses of vitamin E improves the cardiac autonomic nervous system in patients with type 2 diabetes. Am. J. Clin. Nutr. 2001, 73, 1052–1057. [Google Scholar]
- Saeki, Y.; Nagai, N.; Hishinuma, M. Effects of footbathing on autonomic nerve and immune function. Complement. Ther. Clin. Pract. 2007, 13, 158–165. [Google Scholar] [CrossRef]
- Dos Santos, M.D.; Almeida, M.C.; Lopes, N.P.; de Souza, G.E. Evaluation of the anti-inflammatory, analgesic and antipyretic activities of the natural polyphenol chlorogenic acid. Biol. Pharm. Bull. 2006, 29, 2236–2240. [Google Scholar] [CrossRef]
- Muhammad, N.; Saeed, M.; Khan, H. Antipyretic, analgesic and anti-inflammatory activity of viola betonicifolia whole plant. BMC Complement. Altern. Med. 2012, 12, 59. [Google Scholar] [CrossRef]
- Shilpi, J.A.; Islam, M.E.; Billah, M.; Islam, K.M.; Sabrin, F.; Uddin, S.J.; Nahar, L.; Sarker, S.D. Antinociceptive, anti-inflammatory, and antipyretic activity of mangrove plants: A mini review. Adv. Pharmacol. Sci. 2012, 2012, 576086. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lee, J.B.; Shin, Y.O. Oligonol Supplementation Affects Leukocyte and Immune Cell Counts after Heat Loading in Humans. Nutrients 2014, 6, 2466-2477. https://doi.org/10.3390/nu6062466
Lee JB, Shin YO. Oligonol Supplementation Affects Leukocyte and Immune Cell Counts after Heat Loading in Humans. Nutrients. 2014; 6(6):2466-2477. https://doi.org/10.3390/nu6062466
Chicago/Turabian StyleLee, Jeong Beom, and Young Oh Shin. 2014. "Oligonol Supplementation Affects Leukocyte and Immune Cell Counts after Heat Loading in Humans" Nutrients 6, no. 6: 2466-2477. https://doi.org/10.3390/nu6062466
APA StyleLee, J. B., & Shin, Y. O. (2014). Oligonol Supplementation Affects Leukocyte and Immune Cell Counts after Heat Loading in Humans. Nutrients, 6(6), 2466-2477. https://doi.org/10.3390/nu6062466



