Distribution and Determinants of Plasma Homocysteine Levels in Rural Chinese Twins across the Lifespan
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Participants
2.2. Ethics Statement
2.3. Epidemiological Information
2.4. Anthropomorphic Measures
2.5. Blood Pressure Measurements and Definition of High Blood Pressure
2.6. Laboratory Assay
2.7. Statistical Methods
| Characteristics | No. (%) * | |||
|---|---|---|---|---|
| Child (<21 Years) | Adult (≥21 Years) | |||
| Male | Female | Male | Female | |
| Number of Participants | 87 | 58 | 570 | 402 |
| Alcohol Use | 0 (0) | 0 (0) | 232 (40.7) | 11 (2.7) |
| Cigarette Use | 0 (0) | 0 (0) | 371 (65.1) | 14 (3.5) |
| High Fasting Glucose Level (≥5.6 mmol/L) | 16 (18.4) | 13 (22.4) | 144 (25.3) | 101 (25.1) |
| High Homocysteine Level (>10 μmol/L) | 20 (23.0) | 9 (15.5) | 245 (43.0) | 45 (11.2) |
| Low High-Density Lipoprotein (HDL) Cholesterol Level (<1.03 mmol/L for male and <1.29 mmol/L for female) | 15 (17.2) | 15 (25.9) | 82 (14.4) | 45 (11.2) |
| High Triglyceride Level (≥1.69 mmol/L) | 8 (9.2) | 5 (8.6) | 63 (11.1) | 42 (10.4) |
| High Systolic Blood Pressure (≥130 mmHg) | 8 (9.2) | 5 (8.6) | 63 (11.1) | 43 (10.8) |
| High Diastolic Blood Pressure (≥85 mmHg) | 3 (3.4) | 5 (8.6) | 52 (9.2) | 42 (10.5) |
| Middle School And Higher Education | 38 (43.7) | 22 (37.9) | 259 (45.4) | 81 (20.1) |
| Farmers | 5 (5.8) | 0 (0) | 247 (48.1) | 234 (58.2) |
| Mean (SD) | ||||
| Child (<21 Years) | Adult (≥21 Years) | |||
| Male | Female | Male | Female | |
| Age, year | 13.1 (2.5) | 12.6 (1.9) | 42.0 (10.9) | 39.1 (8.3) |
| Plasma Lipids, mmol/L | ||||
| High-Density Lipoprotein (HDL) Cholesterol | 1.5 (0.4) | 1.4 (0.3) | 1.7 (0.5) | 1.6 (0.5) |
| Triglycerides | 0.6 (0.3) | 0.8 (0.3) | 1.0 (0.7) | 1.0 (0.6) |
| Blood Pressure, mmHg | ||||
| Systolic | 104.3 (9.7) | 101.9 (8.4) | 113.6 (15.6) | 108.2 (17.0) |
| Diastolic | 59.5 (8.6) | 56.1 (7.8) | 72.4 (10.8) | 68.6 (11.4) |
| Waist Circumference | 57.9 (6.0) | 55.6 (4.9) | 73.4 (8.1) | 72.5 (7.5) |
| Body mass index, kg/m2 | 16.4 (2.0) | 15.6 (2.1) | 21.6 (2.5) | 22.2 (2.7) |
| Median and Interquartile of Plasma Total Homocysteine, μmol/L | 7.5 (6.5–9.7) | 6.5 (5.4–8.2) | 9.6 (7.7–11.7) | 7.3 (6.0–8.6) |
3. Results
3.1. Characteristics of the Participants
3.2. Age, Gender and Period Effects

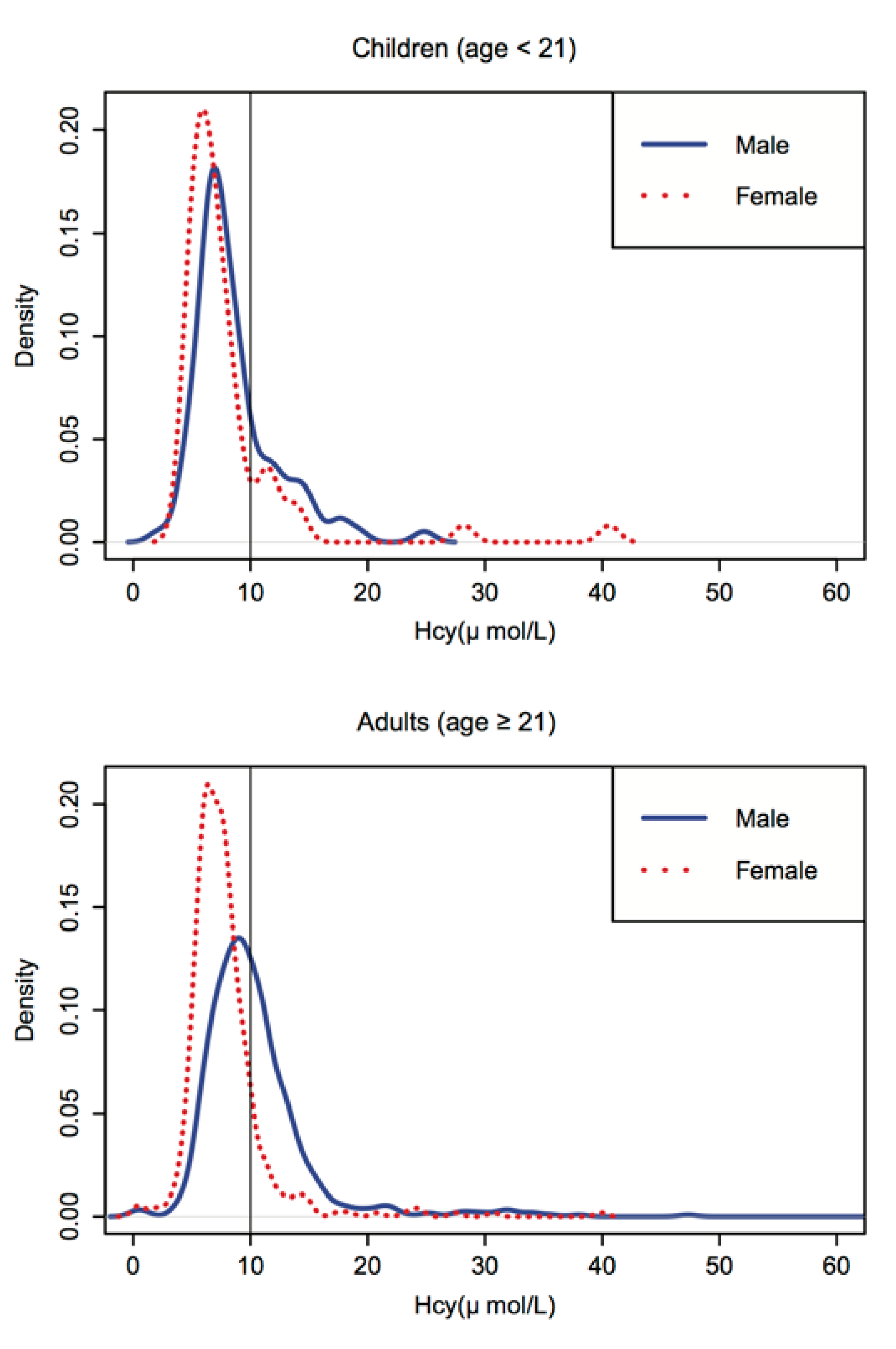
3.3. The Effects of Genetic and Environment Factors on Hcy Concentration
| β | Standard Error | p-Value | |
|---|---|---|---|
| Both Genders | |||
| Follow-up (baseline as ref) | 0.37 | 0.47 | 0.430 |
| Male (female as ref) | 2.64 | 0.52 | 0.000 *** |
| Alcohol drinking (non-drinking as ref) | −0.19 | 0.52 | 0.720 |
| Cigarette smoking (non-smoking as ref) | 0.85 | 0.48 | 0.080 |
| Middle school or higher (lower than middle school as ref) | −0.30 | 0.46 | 0.510 |
| Farmer (non-farmer as ref) | 0.38 | 0.38 | 0.330 |
| Male | |||
| Follow-up (baseline as ref) | 0.56 | 0.66 | 0.396 |
| Alcohol drinking (non-drinking as ref) | −0.19 | 0.52 | 0.715 |
| Cigarette smoking (non-smoking as ref) | 0.87 | 0.52 | 0.093 |
| Middle school or higher (lower than middle school as ref) | −0.77 | 0.59 | 0.194 |
| Farmer (non-farmer as ref) | 1.21 | 0.65 | 0.063 |
| Female | |||
| Follow-up (baseline as ref) | 0.01 | 0.55 | 0.986 |
| Alcohol drinking (non-drinking as ref) | 0.82 | 0.69 | 0.231 |
| Cigarette smoking (non-smoking as ref) | 0.01 | 0.79 | 0.989 |
| Middle school or higher (lower than middle school as ref) | 0.60 | 0.68 | 0.377 |
| Farmer (non-farmer as ref) | −0.44 | 0.34 | 0.196 |
| Age Group | Inter-Pair Correlation | Heritability † | |||
|---|---|---|---|---|---|
| MZ | DZ | ||||
| N | R | N | R | ||
| <21 Year | |||||
| Overall § | 33 | 0.80 | 37 | 0.54 | 0.52 |
| ≥21 Year | |||||
| Overall | 299 | 0.58 | 173 | 0.36 | 0.44 |
| Male | 172 | 0.53 | 107 | 0.36 | 0.36 |
| Female | 127 | 0.55 | 66 | 0.20 | 0.69 |
| MTHFR C677T Genotypes | Cigarette Use | No. | β | SE | p-Value |
|---|---|---|---|---|---|
| CC/CT | 370 | reference | |||
| TT | 36 | 5.41 | 2.36 | 0.022 * | |
| no | 288 | reference | |||
| yes | 118 | 1.35 | 0.67 | 0.043 * | |
| CC/CT | no | 262 | reference | ||
| TT | no | 26 | 5.43 | 1.17 | 0.000 *** |
| CC/CT | yes | 108 | 1.30 | 0.81 | 0.110 |
| TT | yes | 10 | 9.19 | 2.22 | 0.000 *** |
| P for Interaction | 0.365 | ||||

4. Discussion
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Castelli, W.P. Lipids, risk factors and ischaemic heart disease. Atherosclerosis 1996, 124, S1–S9. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, H.C. Glucose: A continuous risk factor for cardiovascular disease. Diabet. Med. J. Br. Diabet. Assoc. 1997, 14, S25–S31. [Google Scholar] [CrossRef]
- Wald, D.S.; Wald, N.J.; Morris, J.K.; Law, M. Folic acid, homocysteine, and cardiovascular disease: Judging causality in the face of inconclusive trial evidence. BMJ 2006, 333, 1114–1117. [Google Scholar] [CrossRef] [PubMed]
- Vaya, A.; Rivera, L.; Hernandez-Mijares, A.; de la Fuente, M.; Sola, E.; Romagnoli, M.; Alis, R.; Laiz, B. Homocysteine levels in morbidly obese patients. Its association with waist circumference and insulin resistance. Clin. Hemorheol. Microcirc. 2012, 52, 49–56. [Google Scholar]
- Ferretti, G.; Bacchetti, T.; Marotti, E.; Curatola, G. Effect of homocysteinylation on human high-density lipoproteins: A correlation with paraoxonase activity. Metab. Clin. Exp. 2003, 52, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.H.; Lim, S.; Jang, H.C.; Park, H.K.; Metzger, B.E. Elevated homocysteine as a risk factor for the development of diabetes in women with a previous history of gestational diabetes mellitus: A 4-year prospective study. Diabet. Care 2005, 28, 2750–2755. [Google Scholar] [CrossRef]
- Refsum, H.; Ueland, P.M.; Nygard, O.; Vollset, S.E. Homocysteine and cardiovascular disease. Ann. Rev. Med. 1998, 49, 31–62. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Lu, Z.; Tan, M.; Liu, H.; Lu, D. A meta-analysis of association between C677T polymorphism in the methylenetetrahydrofolate reductase gene and hypertension. Eur. J. Hum. Genet. EJHG 2007, 15, 1239–1245. [Google Scholar] [CrossRef]
- Holmes, M.V.; Newcombe, P.; Hubacek, J.A.; Sofat, R.; Ricketts, S.L.; Cooper, J.; Breteler, M.M.; Bautista, L.E.; Sharma, P.; Whittaker, J.C.; et al. Effect modification by population dietary folate on the association between mthfr genotype, homocysteine, and stroke risk: A meta-analysis of genetic studies and randomised trials. Lancet 2011, 378, 584–594. [Google Scholar]
- Qin, X.; Huo, Y.; Xie, D.; Hou, F.; Xu, X.; Wang, X. Homocysteine-lowering therapy with folic acid is effective in cardiovascular disease prevention in patients with kidney disease: A meta-analysis of randomized controlled trials. Clin. Nutr. 2013, 32, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, M.A.; Cooper, C.; Thornburg, K.L. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 2008, 359, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. The fetal and infant origins of adult disease. BMJ 1990, 301, 1111. [Google Scholar] [CrossRef] [PubMed]
- Bremer, A.A.; Mietus-Snyder, M.; Lustig, R.H. Toward a unifying hypothesis of metabolic syndrome. Pediatrics 2012, 129, 557–570. [Google Scholar] [CrossRef] [PubMed]
- McGill, H.C. Morphologic Development of the Atherosclerotic Plaque; Raven Press: New York, NY, USA, 1980. [Google Scholar]
- Kannel, W.B.; Dawber, T.R. Atherosclerosis as a pediatric problem. J. Pediatr. 1972, 80, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Jaaskelainen, P.; Magnussen, C.G.; Pahkala, K.; Mikkila, V.; Kahonen, M.; Sabin, M.A.; Fogelholm, M.; Hutri-Kahonen, N.; Taittonen, L.; Telama, R.; et al. Childhood nutrition in predicting metabolic syndrome in adults: The cardiovascular risk in young finns study. Diabet. Care 2012, 35, 1937–1943. [Google Scholar]
- Osganian, S.K.; Stampfer, M.J.; Spiegelman, D.; Rimm, E.; Cutler, J.A.; Feldman, H.A.; Montgomery, D.H.; Webber, L.S.; Lytle, L.A.; Bausserman, L.; et al. Distribution of and factors associated with serum homocysteine levels in children: Child and adolescent trial for cardiovascular health. JAMA 1999, 281, 1189–1196. [Google Scholar]
- Tamai, Y.; Wada, K.; Tsuji, M.; Nakamura, K.; Sahashi, Y.; Watanabe, K.; Yamamoto, K.; Ando, K.; Nagata, C. Dietary intake of vitamin B12 and folic acid is associated with lower blood pressure in japanese preschool children. Am. J. Hypertens. 2011, 24, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Bostom, A.G.; Garber, C. Endpoints for homocysteine-lowering trials. Lancet 2000, 355, 511–512. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Ma, J.; Zhu, J.; Stampfer, M.J.; Tian, Y.; Willett, W.C.; Li, Z. Vitamin B-12 deficiency is prevalent in 35- to 64-year-old chinese adults. J. Nutr. 2007, 137, 1278–1285. [Google Scholar] [PubMed]
- Hao, L.; Ma, J.; Zhu, J.; Stampfer, M.J.; Tian, Y.; Willett, W.C.; Li, Z. High prevalence of hyperhomocysteinemia in chinese adults is associated with low folate, vitamin B-12, and vitamin B-6 status. J. Nutr. 2007, 137, 407–413. [Google Scholar] [PubMed]
- Qin, X.; Li, J.; Cui, Y.; Liu, Z.; Zhao, Z.; Ge, J.; Guan, D.; Hu, J.; Wang, Y.; Zhang, F.; et al. Effect of folic acid intervention on the change of serum folate level in hypertensive chinese adults: Do methylenetetrahydrofolate reductase and methionine synthase gene polymorphisms affect therapeutic responses? Pharmacogenet. Genomics 2012, 22, 421–428. [Google Scholar]
- Ouyang, F.; Christoffel, K.K.; Brickman, W.J.; Zimmerman, D.; Wang, B.; Xing, H.; Zhang, S.; Arguelles, L.M.; Wang, G.; Liu, R.; et al. Adiposity is inversely related to insulin sensitivity in relatively lean chinese adolescents: A population-based twin study. Am. J. Clin. Nutr. 2010, 91, 662–671. [Google Scholar]
- Liu, R.; Liu, X.; Arguelles, L.M.; Patwari, P.P.; Zee, P.C.; Chervin, R.D.; Ouyang, F.; Christoffel, K.K.; Zhang, S.; Hong, X.; et al. A population-based twin study on sleep duration and body composition. Obesity 2012, 20, 192–199. [Google Scholar]
- Alberti, K.G.; Zimmet, P.; Shaw, J. The metabolic syndrome—A new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef] [PubMed]
- Christian, J.C.; Norton, J.A., Jr.; Sorbel, J.; Williams, C.J. Comparison of analysis of variance and maximum likelihood based path analysis of twin data: Partitioning genetic and environmental sources of covariance. Genet. Epidemiol. 1995, 12, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, C.; Xiao, S.H.; Wan, B. Association of tagging snps in the mthfr gene with risk of type 2 diabetes mellitus and serum homocysteine levels in a chinese population. Dis. Markers 2014. [CrossRef]
- Jiang, S.; Chen, Q.; Venners, S.A.; Zhong, G.; Hsu, Y.H.; Xing, H.; Wang, X.; Xu, X. Effect of simvastatin on plasma homocysteine levels and its modification by MTHFR C677T polymorphism in chinese patients with primary hyperlipidemia. Cardiovasc. Ther. 2013, 31, e27–e33. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Li, J.; Cui, Y.; Liu, Z.; Zhao, Z.; Ge, J.; Guan, D.; Hu, J.; Wang, Y.; Zhang, F.; et al. MTHFR C677T and MTR A2756G polymorphisms and the homocysteine lowering efficacy of different doses of folic acid in hypertensive chinese adults. Nutr. J. 2012, 11, 2. [Google Scholar]
- Wang, X.; Qin, X.; Demirtas, H.; Li, J.; Mao, G.; Huo, Y.; Sun, N.; Liu, L.; Xu, X. Efficacy of folic acid supplementation in stroke prevention: A meta-analysis. Lancet 2007, 369, 1876–1882. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Y.; Kong, X.; Wang, G.; Hong, X.; Xu, X.; Chen, Z.; Bartell, T.; Xu, X.; Tang, G.; Hou, F.; et al. Distribution and Determinants of Plasma Homocysteine Levels in Rural Chinese Twins across the Lifespan. Nutrients 2014, 6, 5900-5914. https://doi.org/10.3390/nu6125900
Ji Y, Kong X, Wang G, Hong X, Xu X, Chen Z, Bartell T, Xu X, Tang G, Hou F, et al. Distribution and Determinants of Plasma Homocysteine Levels in Rural Chinese Twins across the Lifespan. Nutrients. 2014; 6(12):5900-5914. https://doi.org/10.3390/nu6125900
Chicago/Turabian StyleJi, Yuelong, Xiangyi Kong, Guoying Wang, Xiumei Hong, Xin Xu, Zhu Chen, Tami Bartell, Xiping Xu, Genfu Tang, Fanfan Hou, and et al. 2014. "Distribution and Determinants of Plasma Homocysteine Levels in Rural Chinese Twins across the Lifespan" Nutrients 6, no. 12: 5900-5914. https://doi.org/10.3390/nu6125900
APA StyleJi, Y., Kong, X., Wang, G., Hong, X., Xu, X., Chen, Z., Bartell, T., Xu, X., Tang, G., Hou, F., Huo, Y., Wang, X., & Wang, B. (2014). Distribution and Determinants of Plasma Homocysteine Levels in Rural Chinese Twins across the Lifespan. Nutrients, 6(12), 5900-5914. https://doi.org/10.3390/nu6125900




